
Correlation between the neutrophil-to-lymphocyte ratio and clinicopathological parameters in epithelial ovarian cancer patients and its effect on prognosis—a retrospective cohort study
Introduction
Ovarian cancer is one of the most common malignancies of the female reproductive system, and due to its high degree of malignancy, the mortality rate is as high as 30% or more (1,2). Factors influencing death in patients with ovarian cancer include clinical staging, lymph node metastasis, and tumor biology (3,4). Identifying risk factors for postoperative recurrence, metastasis, or death in ovarian cancer patients is beneficial for improving treatment measures to prolong the survival and predict the prognosis of these patients. In recent years, the relationship between the neutrophil-to-lymphocyte ratio (NLR) and malignant tumors has gradually attracted increasing attention from scholars, and studies have confirmed that NLR can predict patient prognosis in breast, liver, lung, and bladder cancers, these studies showed that a higher level of NLR was associated with a poor prognosis (5-8). Studies have also shown that NLR is associated with the efficacy of neoadjuvant chemotherapy in patients with ovarian cancer, a higher level of NLR is associated with a poor efficacy of neoadjuvant chemotherapy (9,10), but whether this change in efficacy translates into a long-term mortality benefit is unclear. Therefore, we designed this study to explore the correlation between the NLR and clinicopathological parameters in patients with epithelial ovarian cancer and its effect on prognosis, so as to promote the application of NLR in patients with ovarian cancer. We present the following article in accordance with the REMARK reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-413/rc).
Methods
General information
A total of 168 epithelial ovarian cancer patients admitted to the Pu Ren Hospital in Wuhan City and Shandong Qingdao Hospital of Integrated Traditional and Western Medicine from January 2015 to January 2017 were continuously included in this retrospective cohort study. All of the patients underwent surgical treatment. The patients were equally divided into high NLR (n=84, NLR >3.5) and low NLR (n=84, NLR ≤3.5) groups according to their preoperative NLR levels.
The inclusion criteria were as follows: (I) patients with epithelial ovarian cancer (pathological diagnosis); (II) patients who received surgical treatment; (III) patients who did not receive other special treatment before surgery; (IV) age ≥18 years; and (V) those with complete clinical data. The exclusion criteria were as follows: (I) concomitant with other malignant tumors; (II) cases of death due to other reasons during the follow-up period; (III) patients with serious cardiovascular and cerebrovascular diseases; (IV) patients with liver and kidney dysfunction; (V) patients who did not complete the postoperative treatment as required; (VI) patients with co-infectious diseases at the time of admission; (VII) failure to undergo follow-up; and (VIII) patients with IV stage epithelial ovarian cancer.
All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Pu Ren Hospital in Wuhan City (No. 20210182) and Shandong Qingdao Hospital of Integrated Traditional and Western Medicine [No. (2002)001], and the requirement for individual informed consent for this retrospective analysis was waived.
Observation data
The observation data were as follows: Clinicopathological parameters: age, Federation International of Gynecology and Obstetrics (FIGO) stage, pathological type, histological grade, tumor size, ascites, lymph node metastasis, vascular tumor thrombus, carbohydrate antigen 125 (CA125). Prognosis outcomes: 5 years progression-free survival (PFS) and 5-year mortality rate. All patients were followed up for 5 years by telephone or clinic visits.
Detection method
- 5-year PFS was defined as the time from the day after surgery to the recurrence, metastasis, or death of ovarian cancer.
- 5-year mortality: all patients were followed up for 5 years to calculate the mortality rate.
- Vascular tumor thrombus and lymph node metastases: we obtained a sample of the patient’s tumor tissue during surgery, and then performed pathological examinations to determine whether a vascular tumor thrombus had formed.
- CA125: we took 5 mL of venous blood under fasting conditions in the early morning before surgery, centrifuged it at 3,000 r/min to obtain the upper serum, and immediately sent it for testing using a Roche CobasE601 automatic electrochemiluminescence immunoassay analyzer (Roche, Switzerland) to detect serum CA125.
- Neutrophils and lymphocytes: under preoperative fasting conditions, 5 mL venous blood was taken and immediately sent for examination. The BC-6800 automatic blood cell analyzer (Shanghai Jumu Medical Instrument Co., Ltd., Shanghai, China) was used to determine the levels of neutrophils and lymphocytes.
Statistical analysis
Data analysis in this study was completed using SPSS26.0 (IBM, Chicago, USA), and the difference was considered statistically significant when P<0.05 (two-sided). The measurement data of the two groups were expressed as mean ± standard deviation, and the differences between the two groups were analyzed by independent sample t-tests. Receiver operating characteristic (ROC) curves were used to analyze the predictive value of the NLR in the prognosis of patients with ovarian cancer. Differences in PFS and mortality in the high and low NLR groups were analyzed using Kaplan-Meier (KM) survival curves.
Results
Correlation between the NLR and the clinicopathological features of ovarian cancer patients
The preoperative NLR levels were associated with FIGO staging, ascites, and lymph node metastasis in patients with ovarian cancer (P<0.05, Table 1).
Table 1
| Group | High NLR group (n=84) | Low NLR group (n=84) | χ2 value | P value |
|---|---|---|---|---|
| Age, n (%) | 2.914 | 0.088 | ||
| >60 years | 43 (51.19) | 32 (38.10) | ||
| ≤60 years | 41 (48.81) | 52 (61.90) | ||
| FIGO stage, n (%) | 4.350 | 0.037 | ||
| I stage | 47 (55.95) | 60 (71.43) | ||
| II or III stage | 37 (44.05) | 24 (28.57) | ||
| Pathological type, n (%) | 0.025 | 0.874 | ||
| Serous | 52 (61.90) | 51 (60.71) | ||
| Other types | 32 (38.10) | 33 (39.29) | ||
| Histological grade, n (%) | 0.215 | 0.643 | ||
| I grade | 42 (50.00) | 45 (53.57) | ||
| II or III grade | 42 (50.00) | 39 (46.43) | ||
| Tumor size >5 cm, n (%) | 2.085 | 0.149 | ||
| Yes | 35 (41.67) | 26 (30.95) | ||
| No | 49 (58.33) | 58 (69.05) | ||
| Ascites, n (%) | 5.376 | 0.020 | ||
| Yes | 47 (55.95) | 32 (38.10) | ||
| No | 37 (44.05) | 52 (61.90) | ||
| Lymph node metastasis, n (%) | 4.725 | 0.030 | ||
| Yes | 26 (30.95) | 14 (16.67) | ||
| No | 58 (69.05) | 70 (83.33) | ||
| Vascular tumor thrombus, n (%) | 1.538 | 0.215 | ||
| Yes | 50 (59.52) | 42 (50.00) | ||
| No | 34 (40.48) | 42 (50.00) | ||
| CA125 >35 U/mL, n (%) | 0.024 | 0.877 | ||
| Yes | 45 (53.57) | 46 (54.76) | ||
| No | 39 (46.43) | 38 (45.24) |
NLR, neutrophil-to-lymphocyte ratio; FIGO, Federation International of Gynecology and Obstetrics.
Predictive value of the NLR for recurrence or metastasis in ovarian cancer patients within 5 years after surgery
The area under the curve of NLR in predicting recurrence or metastasis within 5 years after surgery in patients with ovarian cancer was 0.675 [95% confidence interval (CI): 0.594–0.757, P=0.000]. See Figure 1.
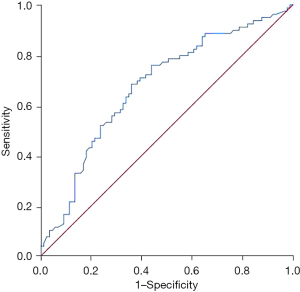
Predictive value of NLR for deaths within 5 years after surgery in patients with ovarian cancer
The area under the curve of NLR in predicting death within 5 years after surgery in patients with ovarian cancer was 0.785 (95% CI: 0.717–0.853, P=0.000). See Figure 2.
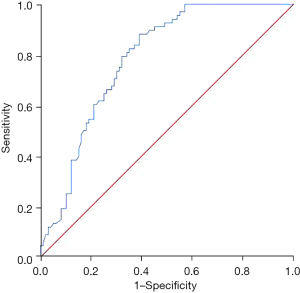
Comparison of PFS between the two groups
Compared with the low NLR group, the PFS of the patients in the high NLR group was significantly reduced (P=0.000). See Figure 3.
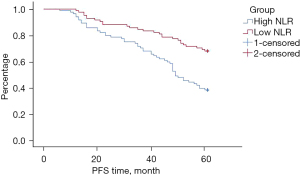
Comparison of 5-year mortality rates between the two groups
The 5-year mortality rate in the high NLR group was markedly higher than that of the low NLR group (P=0.000). See Figure 4.
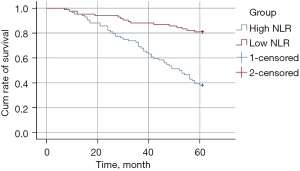
Discussion
There is growing evidence that cancer cells can induce tumor angiogenesis by activating systemic inflammation, which ultimately promotes tumor cell proliferation and metastasis (11-13). The systemic inflammatory response is closely related to the onset, development, and metastasis of cancer. Thus, inflammatory markers, including the NLR, have been shown to be associated with cancer mortality and are utilized as useful prognostic indicators in many solid tumors (14,15). In our study, the relationship between the NLR and ovarian cancer patients’ clinical-pathological characteristics, postoperative PFS, and mortality rate was explored. We observed that high NLR was related to lymph node metastasis, ascites, and FIGO staging in ovarian cancer patients. Also, the postoperative PFS was shortened and the mortality rate was increased in ovarian cancer patients with high NLR.
Neutrophils are a kind of inflammatory cell. Following the activation of systemic inflammation by the tumor, the level of neutrophils increases, and the systemic inflammation leads to local angiogenesis of the tumor tissue, proliferation and growth of tumor cells, and eventually results in tumor cell metastasis. Lymphocytes are the core of the immune response and can be divided into three categories, namely T cells, B cells, and natural killer (NK) cells. T cells are thymus-dependent lymphocytes that are differentiated from bone marrow-derived lymphocytes in the thymus and can be divided into three subpopulations according to their function in the immune response: cytotoxic T cells, helper T cells, and regulatory T cells. The full name of B cells is bone marrow-dependent lymphocytes. These cells are derived from the bone marrow and can be increased to differentiate into effector B cells after being stimulated by antigens. B cells synthesize and secrete antibodies, and exert immune effects. NK cells are differentiated from lymphoid stem cells in the bone marrow and can directly kill virus-infected cells, tumor cells, and allogeneic cells. Thus, lymphocytes are the basis of the body’s ability to kill tumor cells (16-18).
An elevated NLR indicates that the body’s systemic inflammatory state is activated and lymphocytes are inhibited; in this case, the body’s ability to kill tumor cells is diminished, and the proliferation and metastasis of tumor cells increases, which can lead to a poor prognosis (19,20). Our study showed that a high NLR may promote the proliferation and metastasis of ovarian cancer cells, manifested by an increased FIGO staging, lymph node metastasis, and ascites, which ultimately lead to a poor prognosis. These findings have been confirmed by several previous studies (21,22). In addition, studies have also shown that a high NLR is associated with the sensitivity of chemotherapy drugs in patients with ovarian cancer, which is manifested by patients with a high NLR reacting less after receiving chemotherapy (9,10). This may also be a factor in the poor prognosis of patients with ovarian cancer caused by a high NLR.
Limitations
There were some limitations in our study that should be noted. Firstly, this was a retrospectively clinical study. In addition, we failed to study the mechanism of NLR leading to ovarian cancer cell metastasis.
Conclusions
High NLR in patients with ovarian cancer is related to FIGO staging, lymph node metastasis, ascites, etc., which has good value for predicting poor prognosis in ovarian cancer patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-413/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-413/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-413/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Pu Ren Hospital in Wuhan City (No. 20210182) and Shandong Qingdao Hospital of Integrated Traditional and Western Medicine [No. (2002)001], and the requirement for individual informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shim SH, Lim MC, Lee D, et al. Cause-specific mortality rate of ovarian cancer in the presence of competing risks of death: a nationwide population-based cohort study. J Gynecol Oncol 2022;33:e5. [Crossref] [PubMed]
- Wang Z, Guo E, Yang B, et al. Trends and age-period-cohort effects on mortality of the three major gynecologic cancers in China from 1990 to 2019: Cervical, ovarian and uterine cancer. Gynecol Oncol 2021;163:358-63. [Crossref] [PubMed]
- Foote J, Lopez-Acevedo M, Samsa G, et al. Predicting 6- and 12-Month Risk of Mortality in Patients With Platinum-Resistant Advanced-Stage Ovarian Cancer: Prognostic Model to Guide Palliative Care Referrals. Int J Gynecol Cancer 2018;28:302-7. [Crossref] [PubMed]
- Trudel-Fitzgerald C, Poole EM, Idahl A, et al. The Association of Work Characteristics With Ovarian Cancer Risk and Mortality. Psychosom Med 2017;79:1059-67. [Crossref] [PubMed]
- Rich NE, Parvathaneni A, Sen A, et al. High Neutrophil-Lymphocyte Ratio and Delta Neutrophil-Lymphocyte Ratio Are Associated with Increased Mortality in Patients with Hepatocellular Cancer. Dig Dis Sci 2022;67:2666-76. [Crossref] [PubMed]
- Sutandyo N, Jayusman AM, Widjaja L, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as mortality predictor of advanced stage non-small cell lung cancer (NSCLC) with COVID-19 in Indonesia. Eur Rev Med Pharmacol Sci 2021;25:3868-78. [PubMed]
- de la Cruz-Ku G, Chambergo-Michilot D, Torres-Roman JS, et al. Neutrophil-to-lymphocyte ratio predicts early mortality in females with metastatic triple-negative breast cancer. PLoS One 2020;15:e0243447. [Crossref] [PubMed]
- Kang M, Jeong CW, Kwak C, et al. Preoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget 2017;8:12891-901. [Crossref] [PubMed]
- Sanna E, Tanca L, Cherchi C, et al. Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer. Diagnostics (Basel) 2021;11:1298. [Crossref] [PubMed]
- Liontos M, Andrikopoulou A, Koutsoukos K, et al. Neutrophil-to-lymphocyte ratio and chemotherapy response score as prognostic markers in ovarian cancer patients treated with neoadjuvant chemotherapy. J Ovarian Res 2021;14:148. [Crossref] [PubMed]
- Zhang X, Jiang Y, Wang Y, et al. Prognostic role of neutrophil-lymphocyte ratio in esophageal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e13585. [Crossref] [PubMed]
- Mellor KL, Powell AGMT, Lewis WG. Systematic Review and Meta-Analysis of the Prognostic Significance of Neutrophil-Lymphocyte Ratio (NLR) After R0 Gastrectomy for Cancer. J Gastrointest Cancer 2018;49:237-44. [Crossref] [PubMed]
- Szor DJ, Dias AR, Pereira MA, et al. Prognostic Role of Neutrophil/Lymphocyte Ratio in Resected Gastric Cancer: A Systematic Review and Meta-analysis. Clinics (Sao Paulo) 2018;73:e360. [Crossref] [PubMed]
- Xu ZG, Ye CJ, Liu LX, et al. The pretransplant neutrophil-lymphocyte ratio as a new prognostic predictor after liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. Biomark Med 2018;12:189-99. [Crossref] [PubMed]
- Min GT, Li YM, Yao N, et al. The pretreatment neutrophil-lymphocyte ratio may predict prognosis of patients with liver cancer: A systematic review and meta-analysis. Clin Transplant 2018;32:e13151. [Crossref] [PubMed]
- Lu C, Zhou L, Ouyang J, et al. Prognostic value of lymphocyte-to-monocyte ratio in ovarian cancer: A meta-analysis. Medicine (Baltimore) 2019;98:e15876. [Crossref] [PubMed]
- Gong J, Jiang H, Shu C, et al. Prognostic value of lymphocyte-to-monocyte ratio in ovarian cancer: a meta-analysis. J Ovarian Res 2019;12:51. [Crossref] [PubMed]
- Pinto MP, Balmaceda C, Bravo ML, et al. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol Oncol 2018;151:10-7. [Crossref] [PubMed]
- Yin X, Wu L, Yang H, et al. Prognostic significance of neutrophil-lymphocyte ratio (NLR) in patients with ovarian cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e17475. [Crossref] [PubMed]
- Komura N, Mabuchi S, Yokoi E, et al. Comparison of clinical utility between neutrophil count and neutrophil-lymphocyte ratio in patients with ovarian cancer: a single institutional experience and a literature review. Int J Clin Oncol 2018;23:104-13. [Crossref] [PubMed]
- Zhang T, Liu Q, Zhu Y, et al. Lymphocyte and macrophage infiltration in omental metastases indicates poor prognosis in advance stage epithelial ovarian cancer. J Int Med Res 2021;49:3000605211066245. [Crossref] [PubMed]
- Soibi-Harry AP, Amaeshi LC, Garba SR, et al. The relationship between pre-operative lymphocyte to monocyte ratio and serum cancer antigen-125 among women with epithelial ovarian cancer in Lagos, Nigeria. Ecancermedicalscience 2021;15:1288. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


