
Novel technique for endoscopic-assisted nipple-sparing mastectomy and immediate breast reconstruction with endoscopic-assisted latissimus dorsi muscle flap harvest through a single axillary incision: a retrospective cohort study of comparing endoscopic and open surgery
Introduction
Immediate breast reconstruction (IBR) after nipple-sparing mastectomy (NSM) is a standard technique that provides satisfactory aesthetic results for early breast cancer patients (1). The latissimus dorsi muscle flap (LDMF) is a highly versatile and reliable transferred muscle flap and is thus commonly used for breast reconstruction (BR) (2). However, the traditional process of harvesting the LDMF results in a dorsal scar of 20–45 cm, which many patients find unacceptable (2,3). To minimize scarring, the endoscopic technique (ET) and robotic technique (RT) have been developed, but neither technique has been widely adopted (4). The ET has limitations in terms of negotiating the convex contour of the posterior chest wall and difficulty maintaining an optical window, which leads to operational difficulty, insufficient LDMF volume, and a prolonged operation time (3,5). The RT improves visualization and surgical dexterity but is still not as good as open surgery (6). Additionally, the RT has the disadvantages of high costs and a doubled docking time caused by changes in the patient’s position (3,7). More importantly, according to the reported literature, if the ET or RT is used to complete both the NSM and LDMF dissection in 1 operation, multiple incisions or ports are required, and the resulting scars are poorly concealed (3,8-15).
Due to the many defects of the ET and RT, the current surgical treatment standards have not changed, and open surgery is still the first choice of surgeons. However, with the increasing incidence and prolonged survival time of breast cancer patients worldwide, the demand for BR is increasing rapidly and patients’ aesthetic expectations have increased (16,17). After 2 years of investigation, our team created a novel endoscopic-assisted technique (NET) for IBR with LDMF after NSM that decreases the operation difficulty and shortens the operation time. The whole operation is completed by a single axillary incision hidden in the armpit and 2 cm × 0.5 cm incisions (with almost invisible scarring), the incisions are very difficult with the previously reported ET.
From the current experience of NET, this surgery is relatively safe and has achieved better aesthetic effects. However, there is no objective statistical analysis at present. Therefore, we conducted a retrospective study to assess the safety and efficacy, including aesthetic results, operation time, and postoperative outcomes of the NET compared to the traditional ET (TET) and open surgery for breast cancer patients. We also objectively evaluated patients’ satisfaction and quality of life (QoL) using the Breast-Q questionnaire. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-398/rc).
Methods
Patients
Patients’ breast surgery data of this retrospective study were consecutively retrieved by our team at the Breast Surgery Department, West China Hospital, Sichuan University, a prestigious and well-known medical center in China, from January 2013 to June 2021. The patients eligible for NSM and IBR were offered 3 options for their BR (i.e., implant only, implant, prosthetic mesh and LDMF, or LDMF and implant), all of which can be conducted by traditional open surgery or the ET. BR with LDMF is especially recommended for patients with ptosis breast and those who have high requirements for aesthetic outcomes. Before the NET was created in June 2020, patients were offered a choice of either the TET or open surgery. After June 2020, patients were offered the choice of the NET or open surgery. The decision was made by patients in consultation with doctors after the doctor described the operation, including the surgical trauma range, operation time, expense, postoperative complications, aesthetic results, and oncological safety. None of the patients required a skin paddle for the BR. We identified 45 breast cancer patients who underwent NSM and IBR with the LDMF or with implants and the LDMF using the NET, the TET, or open surgery. The number of cases in the hospital during the study period determined the sample size (18). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the biomedical ethics review committee of West China Hospital, Sichuan University (No. 488) and every patient signed a written consent before surgery.
Indications
Patients were eligible for IBR with LDMF after NSM if they met the following criteria: (I) had multicentric breast carcinoma, extensive intraductal neoplasia, and large unicentric carcinomas (<5 cm) that would not be suitable for breast-conserving surgery; (II) had large (>5 cm) unicentric carcinoma localized in mammary gland that had shrunk to <5 cm after neoadjuvant chemotherapy; (III) had no chest wall, skin, or nipple-areolar complex (NAC) tumor invasion (including Paget’s disease); and (IV) had contraindications to radiotherapy. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had inflammatory breast cancer; (II) had distant tumor metastasis; (III) were immunocompromised; and/or (IV) had severe comorbid conditions.
Evaluation of outcome measures
We retrieved data on age, body mass index (BMI), tumor site, breast cancer stage, mode of axillary surgery, mode of reconstruction, operation time, length of hospital stay, overall medical expenses, complications, outcomes from the BREAST-Q questionnaire, recurrence, and survival status at last follow-up. All the surgical procedures, including the TET, NET, and open surgery, were conducted by 2 surgeons during the study period. The operation time was measured as the interval between the first skin incision and the end of skin suturing, including axillary node surgery, NSM, LDMF harvesting, and BR. The overall medical expenses associated with the reported operations included both the surgical and non-surgical charges during hospitalization. We administered the BREAST-Q questionnaire (19) preoperatively and 1, 3, and 12 months postoperatively to all the patients to evaluate their QoL and patient-reported aesthetic results. As the follow-up time of patients receiving novel endoscopic surgery was <1 year, the 12-month follow-up score of this group was not determined, which could be a source of bias.
Surgical techniques
NET surgery
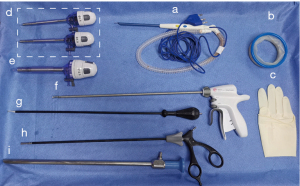
The NET procedure requires the following classic instruments, which are also used in endoscopic surgery and open breast cancer surgery (see Figure 1): (I) Peng’s multifunctional operative dissector (PMOD) (Shuyou Surgical, Hangzhou, China); (II) an 80-mm disposable wound protector (DWP) (Surkon Medical, Wuxi, China); (III) a sterile surgical glove (7#); (IV) 2 mm × 5.5 mm trocars (Aesculap Inc., Center Valley, USA); (V) a 12.5-mm trocar for endoscopy (Aesculap Inc.); (VI) an ultrasonic scalpel (Ethicon Inc., Somerville, USA); (VII) a coagulation hook (Aesculap Inc.); (VIII) forceps (Aesculap Inc.); and (IX) an endoscope (KARL-STORZ Inc., El Segundo, USA).
All the patients underwent dissection of the LDMF and NSM in the lateral decubitus position and then BR in the dorsal decubitus position (see Video 1). It is acceptable for LDMF harvesting or NSM to proceed first. In this study, we describe the procedures from LDMF harvesting to NSM. Both LDMF harvesting and NSM were operated from the deep to superficial surfaces. A 5- to 6-cm single axillary incision was made with cleavage lines. Sentinel lymph node biopsy or axillary lymph node dissection was first performed as needed under direct vision. Next, 5–10 cm towards the spine and 15 cm towards the iliac were separated in the deep surface of the latissimus dorsi (LD) under direct vision. The DWP was then inserted into the incision and wrapped by the opened end of 1 sterile surgical glove to seal the cavity (self-made access). Trocars were inserted through cuts in the fingertips of the glove, fixed with threads (see Figure 2A) and then connected to an insufflator to keep the pressure at 12 mmHg. The PMOD was inserted into the “HUAXI hole 2” (a manmade 0.5-cm incision located 18–20 cm below the armpit along the posterior axillary line) (see Figure 2B) and was used to dissociate the submuscular space with the assistance of grasping forceps under endoscopy. The subcutaneous layer was similarly dissected. The thoracodorsal artery and nerve were carefully preserved to prevent LDMF necrosis and atrophy and the thinning of the LDMF over time. Next, NSM was performed in the order of the subpectoral space (if an implant was placed in the subpectoral position, by a coagulation hook), retromammary space (by a coagulation hook with the assistance of forceps), and the subcutaneous layer of breast, which was dissected using the PMOD to retro-areolar tissue under direct vision, and the rest of the layer was then performed using the PMOD inserted through the “HUAXI hole 1” (located next to the areola in the upper-outer quadrant) (see Figure 2C).
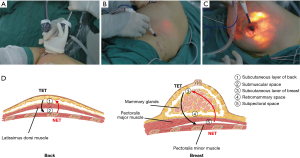
The surgical technique for NSM and the concept of the “HUAXI hole 1” has been described previously (20,21). The retro-areolar tissue should be carefully dissected by surgical scissors to protect the blood supply and prevent nipple ischemia. During the whole operation procedure, penetrating vessels were coagulated and cut with a PMOD and coagulation hook to ensure a clear visual field and maintain hemostasis. In areas with abundant blood vessels, an ultrasonic scalpel was used. Next, the LDMF was transposed to the front subcutaneous pocket. The patient’s position was changed from the lateral position to the supine position after the 2 drains were inserted. The implant (when necessary) was placed in the subpectoral position with the LDMF combined with the pectoralis major for patients with thin subcutaneous fat or in the prepectoral position with LDMF coverage for others. Typically, in the case of shrinkage of the LDMF, the size of the reconstructed breast was 20–30% bigger than the non-surgical side.
TET and open surgery
Under the TET, we used the same axillary incision as the NET, and the operation was similar to that reported in the literature (9,22,23). As retractors rather than air pressure were needed to make space to insert the endoscopic device, provide an optical cavity, and stretch the skin or muscle for the TET, self-made access and trocars were not used, and an airtight cavity was not formed. The procedure was performed from the superficial to deep surface for both the NSM and LDMF harvesting, and the subcutaneous dissection was carried out first, which is consistent with the traditional open surgery approach and opposite to the NET approach (see Figure 2D). A PMOD was also used for the initial dissection for each plane in the TET, but without a “HUAXI hole 1” and “HUAXI hole 2”, which were used for the PMOD insertion in the NET. More distant areas, which the PMOD cannot reach through an axillary incision, was dissected by a coagulation hook.
The traditional open technique has been described previously (24). It leaves a notable scar on the breast and back. Methods for preventing the postoperative complications described in the NET are also suitable for the TET and open technique.
Statistical analysis
The continuous variables are summarized as means and standard deviations, and the Student’s t-test or Wilcoxon signed rank test was used for comparisons. The categorical variables are presented as frequencies and proportions, and the chi-square test or Fisher’s exact test was used for comparisons. The BREAST-Q results were transformed to total scale scores ranging from 0 to 100 for each subtheme with higher scores representing greater satisfaction/better QoL (19). All the p values were 2-tailed, and a P value <0.05 was considered statistically significant. All the statistical analyses were performed using SPSS 25 for Windows (SPSS Inc., Chicago, IL, USA). Figures for the operation times and BREAST-Q scores were generated by R programming language (R version 3.3.1).
Results
Clinical and pathological characteristics
A total of 45 patients who underwent NSM and BR with LDMF or LDMF and implants were included in this study. A total of 17 patients underwent surgery with the ET, including 10 with the NET, and 7 with the TET. The remaining 28 patients underwent open surgery. The ET and open surgery (OPEN) groups were comparable in terms of age, BMI, tumor location, cup size, and disease stage (tumor and node stage) (see Table 1).
Table 1
| Characteristics | OPEN (n=28) | ET | P* | ||
|---|---|---|---|---|---|
| All (n=17) | TET (n=7) | NET (n=10) | |||
| Age (years), mean ± SD | 39.1±7.7 | 35.9±6.4 | 32.9±6.1 | 39.5±4.8 | 0.38 |
| BMI (kg/m2), mean ± SD | 22.3±4.6 | 21.3±1.3 | 21.1±0.9 | 21.8±1.5 | 0.34 |
| Location, n (%) | 0.58 | ||||
| Left breast | 16 (57.1) | 10 (58.8) | 2 (28.6) | 8 (80.0) | |
| Right breast | 12 (42.9) | 7 (41.2) | 5 (71.4) | 2 (20.0) | |
| Cup size, n (%) | >0.99 | ||||
| A–B | 18 (64.3) | 11 (64.7) | 6 (85.7) | 5 (50.0) | |
| C | 7 (25.0) | 5 (29.4) | 1 (14.3) | 4 (40.0) | |
| > C | 3 (10.7) | 1 (5.9) | 0 | 1 (10.0) | |
| T, n (%) | 0.24 | ||||
| Tis | 2 (7.1) | 3 (17.6) | 2 (28.6) | 1 (10.0) | |
| T1 | 12 (42.9) | 9 (52.9) | 4 (57.1) | 5 (50.0) | |
| T2 | 12 (42.9) | 4 (23.5) | 1 (14.3) | 3 (30.0) | |
| T3# | 2 (7.1) | 0 | 0 | 0 | |
| Tx | 0 | 1 (5.9) | 0 | 1 (10.0) | |
| N, n (%) | 0.43 | ||||
| N0 | 13 (46.4) | 12 (70.6) | 4 (57.1) | 8 (80.0) | |
| N1 | 10 (35.7) | 4 (23.5) | 3 (42.9) | 1 (10.0) | |
| N2 | 2 (7.1) | 0 | 0 | 0 | |
| N3 | 3 (10.7) | 1 (5.9) | 0 | 1 (10.0) | |
| NAC | 10 (35.7) | 1 (5.9) | 0 | 1 (10.0) | 0.02 |
*, P values represent inter-group comparison between OPEN and ET (including TET and NET). #, The 2 large unicentric carcinomas shrank to <5 cm after neoadjuvant chemotherapy. OPEN, open surgery; ET, endoscopic technique; TET, traditional endoscopic technique; NET, novel endoscopic technique; BMI, body mass index; NAC, neoadjuvant chemotherapy.
Operative data
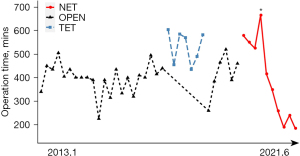
All the patients in the ET group successfully completed the surgery with no cases of intraoperative conversion to open surgery. Table 2 shows the operative data. The operation times in the OPEN and TET groups were relatively stable, with mean times of 400.9 min (median: 401.0 min) and 531.6 min (median: 570.0 min), respectively. The NET group (395.8 min) had a mean duration similar to that of the OPEN group. The operation time of the NET showed a consistent decrease (first operation: 579 min; last operation: 185 min) and gradually became stable (see Figure 3). The peak line shows a significantly longer duration due to the shooting of video footage of the surgery.
Table 2
| Operative data | OPEN (n=28) | ET (n=17) | P* | ||
|---|---|---|---|---|---|
| All (n=17) | TET (n=7) | NET (n=10) | |||
| Axillary surgery, n (%) | 0.10 | ||||
| SLNB | 13 (46.4) | 12 (70.6) | 4 (57.1) | 8 (80.0) | |
| ALND | 15 (53.6) | 5 (29.4) | 3 (42.9) | 2 (20.0) | |
| Reconstruction surgery, n (%) | |||||
| LDMF and implant | 6 (21.4) | 7 (41.2) | 1 (14.3) | 6 (60.0) | 0.14 |
| LDMF | 22 (78.6) | 10 (58.8) | 6 (85.7) | 4 (40.0) | |
| Implant volume (cc) | 150–355 | 150–395 | 270 | 150–395 | – |
| LDMF, mean ± SD | |||||
| Length | 21.3±5.2 | 20.7±6.2 | 15.3±3.1 | 24.1±5.2 | 0.64 |
| Width | 9.7±1.4 | 9.6±1.8 | 9.2±1.9 | 9.9±1.8 | 0.85 |
| Operation time (min) | |||||
| Median | 401.0 | 490.0 | 570.0 | 382.5 | 0.06 |
| Range | 226–520 | 185–665 | 435–604 | 185–665 | |
| Mean ± SD | 400.9±67.3 | 451.7±154.9 | 531.6±69.6 | 395.8±176.0 | |
| Perioperative parameters | |||||
| Complications#, n (%) | 17 (60.7) | 6 (35.3) | 3 (42.9) | 3 (30.0) | 0.09 |
| Chest hematoma/hemorrhage | 0 | 0 | 0 | 0 | |
| Donor-site hematoma/hemorrhage | 0 | 0 | 0 | 0 | |
| Breast skin flap necrosis | 3 (10.7) | 0 | 0 | 0 | |
| Donor-site skin necrosis | 1 (3.6) | 0 | 0 | 0 | |
| LDMF necrosis | 1 (3.6) | 0 | 0 | 0 | |
| Implant loss | 0 | 0 | 0 | 0 | |
| Infection | 2 (7.1) | 0 | 0 | 0 | |
| Chest seroma | 3 (10.7) | 0 | 0 | 0 | |
| Donor-site seroma | 9 (32.1) | 4 (23.5) | 2 (28.6) | 2 (20.0) | |
| Hypopigmentation of nipple areola | 4 (14.3) | 3 (17.6) | 2 (28.6) | 1 (10.0) | |
| Hospital stay (days), mean ± SD | 11.0±3.5 | 10.2±3.1 | 10.0±4.3 | 9.4±2.2 | 0.09 |
| Overall medical expenses (USD), mean ± SD | |||||
| Overall | 4,462±1,248 | 6,298±1,473 | <0.001 | ||
| LDMF and implant | 6,157±899 | 7,477±1,131 | 0.045 | ||
| LDMF | 4,000±875 | 5,472±1,075 | 0.002 | ||
*, P values represent inter-group comparison between OPEN and ET (including TET and NET); #, The number of patients was not the sum of each of the complications, as some of the patients suffered >1 complication. OPEN, open surgery; ET, endoscopic technique; TET, traditional endoscopic technique; NET, novel endoscopic technique; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; LDMF, latissimus dorsi muscle flap; USD, United States dollar.
Axillary surgery and reconstruction surgery did not significantly differ between the ET and OPEN groups (P=0.10 and P=0.14, respectively). The size of the implant used for the BR ranged from 150 to 355 cc in the OPEN group and 150 to 395 cc in the NET group. Only 1 patient in the TET group received reconstruction with the LDMF and implant (270 cc). The mean length of the created muscle flap was longer in the NET group (24.1±5.2 cm) than the other 2 groups (OPEN: 21.3±5.2 cm; TET: 15.3±3.1 cm).
Postoperative parameters
There was no statistically significant difference (P=0.09) in the overall postoperative complication rates among the ET (30.0%) and OPEN (60.7%) groups (see Table 2). Skin necrosis occurred in 4 patients (14.3%, 3 patients with breast skin flap necrosis, and 1 patient with donor-site skin necrosis) in the OPEN group but 0 patients in the ET group (see Table 2). Other complications occurred in the OPEN and ET groups, respectively, as follows: 1 and 0 patients had LD necrosis; 2 and 0 patients had infections; 3 and 0 patients had chest seromas; 9 and 4 patients had donor-site seromas; and 4 and 3 patients had hypopigmentation of the nipple areola, (see Table 2). No cases of serious complications were observed in association with the surgery.
The lengths of hospital stay was similar between the 2 groups (11.0 vs. 10.2 days, respectively, P=0.09) (see Table 2). The mean overall costs of the OPEN and ET groups were 4,462 USD and 6,298 USD (P<0.001) (exchange rate: 1 USD =6.4 RMB). The ET group spent approximately 1,300–1,500 USD more than the OPEN group for the same reconstruction surgery (see Table 2).
Aesthetic results
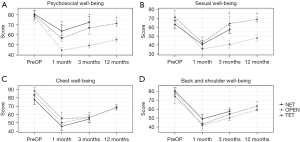
For patients who underwent the ET, there was no visible scar on or around the breast and back (NET: Figure 4A-4C; TET: Figure 4D-4F); thus, the ET surgery had better aesthetic results than the OPEN surgery (see Figure 4G-4I). Patients’ satisfaction with their breasts and back, according to BREAST-Q outcomes, were compared among the patients in the ET (including TET and NET) and OPEN groups to evaluate the aesthetic results. The 2 groups did not significantly differ in the preoperative BREAST-Q (baseline) scores (P=0.28 and 0.61, respectively). The ET group had significantly higher levels of satisfaction with breasts than the OPEN group at the 1-month (64.8 vs. 55.0, P=0.001), 3-month (66.9 vs. 58.7, P=0.01), and 12-month (65.5 vs. 59.3, P=0.02) follow-up time points, and patients’ levels of satisfaction with their backs were also higher in the ET group than the OPEN group (1-month: 61.0 vs. 46.0, P=0.01; 3-month: 64.1 vs. 47.8, P=0.01; 12-month: 72.8 vs. 47.7, P=0.01). Additionally, the BREAST-Q scores of the NET group were higher than or equivalent to those of the TET group after surgery. The results are set out in Figure 4J-4K.

QoL
The preoperative baseline was similar among the 2 groups (see Figure 5). Patients in the ET group had better psychosocial well-being after 1 month (60.2 vs. 44.6, P=0.002), 3 months (70.1 vs. 49.4, P<0.001), and 12 months (71.5 vs. 55.3, P=0.001) than those in the OPEN group (see Figure 5A). Patients in the ET group had a better sexual life after 3 months (60.7 vs. 40.8, P=0.001) and 12 months (68.8 vs. 47.8, P=0.003) than those in the OPEN group (see Figure 5B). Postoperative QoL scores after 1, 3, and 12 months did not differ significantly among the 2 groups in terms of chest well-being and back and shoulder well-being (see Figure 5C,5D). There was no obvious difference between the NET and TET groups at any follow-up time point for the 4 terms (shown in Figure 5).
Oncologic outcomes
The median follow-up time was 64, 45, and 8 months in the OPEN, TET and NET groups, respectively. In the OPEN group, 2 patients died, and 1 patient is currently receiving chemotherapy due to tumor metastasis. None of the patients presented with local or distant recurrence in the ET (NET and TET) groups.
Discussion
In this study, we presented a novel endoscopic technique for NSM and LD harvesting through a single axillary incision [endoscopic NSM has been described previously (20,21)] and compared it to the TET and open surgery in terms of short-term surgical outcomes. As the ET (both the NET and TET) share some similarities in terms of manipulation, and the aesthetic effect and QoL of the breast cancer patients undergoing BR are greatly affected by the length and position of the incisions (the incision of the 2 endoscopic techniques are similar), we also compared all the outcome measures between the ET and the OPEN groups.
We found that the NET had a shorter operation time than the TET and had the potential of decreasing operation time than open surgery. Even with the unstable operation time, an appreciable difference (of 140.3 min) in the mean operation times was observed between the NET and TET groups. As the surgeons became proficient in the NET and the operation time stabilized, the duration of the last 5 NET procedures became relatively stable (at 240.0 min) and was much shorter than that of the open surgery (at 401 min); however, previous research has shown that open surgery has a shorter operation time than RT (3,8) (as far as we are aware, no report has compared operation time between open surgery and ET).
The NET has a number of innovations. First, the NSM and LDMF were dissected from the deep to the superficial planes, which allowed adequate air pressure to prop up the superficial tissue like a tent to provide good exposure to the optical space, and enabled us to avoid using various retractors (14,25) and prevented interference with the field of vision caused by the dissociated superficial tissue, which would have occurred if the subcutaneous plane had been dissected first, which is the approach that has been largely used in previous ET and RT procedures (9,22,23,26,27), including our TET. Second, due to the inflexibility of the endoscopic instruments, it is difficult to operate with the curvature of the back and breast. The insertion of a PMOD, which was able to operate in whatever direction to perform the surgery by endoscopy, through the “HUAXI hole 1” and “HUAXI hole 2” circumvented this problem. Additionally, the endoscope and forceps are more flexible and have more functional self-made access than the commercial gel-Port access. Third, a PMOD rather than a coagulation hook was used for the dissection of the relatively dense tissue, making the operational processes more fluent and convenient. All these innovations decreased the operation time.
In terms of the LD size, the length was determined by the breast size for the NET and OPEN groups, as both the techniques can harvest the entire LDMF. However, we were more inclined to recommend the TET than NET and OPEN surgery to patients with a relatively small breast size, as limitations related to having to negotiate the convex contour of the posterior chest wall and difficulties in maintaining an optical window result in a limited area of excisable LDMF and insufficient volume for BR (3,5). The problem was solved in the NET by the “HUAXI hole 2”, through which the PMOD can be inserted and which allows the distant muscle resection to be closer to the paravertebral origin or iliac bone. The volume of the obtained LDMF is sufficient to reconstruct a B cup breast, which meets the needs of most Asian women.
Safety was also evaluated in relation to the different surgical techniques. The ET group had a lower total complication rate than the OPEN group, but the difference was not statistically significant. The ET has the most potential advantages in terms of the ischemic flaps, as there is no incision on or around the breast and back, which has less effect on the blood supply of the flap (24,28,29). Additionally, image magnification by endoscope allows the intercostal perforators to be readily recognized and saved, and an accurate dissection of the skin flaps to be performed without compromising the vascularity, which contributes significantly to the overall circulation of both the NAC and the breast skin flap.
The overall medical expenses were compared between the ET and OPEN groups (The TET and NET groups had similar costs in terms of both the operation and non-operation costs during hospitalization). The difference between ET and OPEN groups amounted to about 1,300–1,500 USD, which mainly accounts for the use of the endoscopic instrument, and we think is acceptable compared to the 3,732 USD difference between the RT and ET for NSM (5).
The ET group was more satisfied with both their breasts and backs than the OPEN group. There was no obvious difference in the levels of satisfaction between the NET and TET groups, which is probably because the same concealed incision was used in both groups. In most previous reports in which the ET or RT has been used to complete the NSM and LDMF reconstruction, more than one incisions (3,8) or less concealed incision should be required (30), even for partial BR with LDMF (9,14). In 2018, Lai et al. reported inspiring results about the use of the RT for a single axillary incision-assisted NSM and IBR with LDMF, but the sample size was very small (27). However, our ET, whether TET or NET, can be used to perform the whole surgery at the same time with only 1 small and inconspicuous axillary wound. Additionally, the 0.5-cm scars resulting from the “HUAXI hole 1” and “HUAXI hole 2” (both for the NET) were almost invisible after the skin healed.
Additionally, patients in the ET group had better QoL in terms of their psychosocial and sexual well-being after the operation than those in the OPEN group, and no significant differences were found between the NET and TET groups. Due to the good aesthetic results and inconspicuous incisions, the appearances of the patients were similar to that of a normal person, which represents an encouraging result for both the patients and their spouses.
To date, none of the patients in the TET and NET groups have presented with recurrence. However, oncological safety needs to be examined more closely. Thus, studies with larger sample sizes and longer follow-up times need to be conducted. Additionally, the retrospective nature and small sample size of the present study may have led to possible selection and confounding biases and may have affected the comparability between the groups. Further, the NET is still in the exploratory stage, and only 10 procedures have been completed. The operation time for the last 5 procedures was relatively stable and significantly lower than that of the open surgery, but due to the small sample size, we cannot conclude that NET has certain advantages in this respect.
Conclusions
NSM and LDMF reconstruction conducted using the ET through a single axillary incision provided better aesthetic results, a better QoL, an equivalent hospital stay, and a lower complication rate than open surgery. Additionally, the NET appears to address the issue of the longer operation time and the limited volume of the obtained LDMF caused by the limitations of the TET. The NET may become the recommended method for NSM and LDMF reconstruction; however, first, its effectiveness and safety require further validation by future prospective studies.
Acknowledgments
Funding: This study was supported by grants from Key projects of Sichuan Provincial Health Commission (No. 21PJ042); Incubation project of West China Hospital, Sichuan University (No. 2022HXFH004); Natural Science Foundation of Sichuan Province (No. 2022NSFSC0744); Key research and development projects of Sichuan Provincial Department of science and technology (No. 2021YFS0104).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-398/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-398/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-398/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the biomedical ethics review committee of West China Hospital, Sichuan University (No. 488) and every patient signed a written consent before surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Missana MC, Pomel C. Endoscopic latissimus dorsi flap harvesting. Am J Surg 2007;194:164-9. [Crossref] [PubMed]
- Adams WP Jr, Lipschitz AH, Ansari M, et al. Functional donor site morbidity following latissimus dorsi muscle flap transfer. Ann Plast Surg 2004;53:6-11. [Crossref] [PubMed]
- Winocour S, Tarassoli S, Chu CK, et al. Comparing Outcomes of Robotically Assisted Latissimus Dorsi Harvest to the Traditional Open Approach in Breast Reconstruction. Plast Reconstr Surg 2020;146:1221-5. [Crossref] [PubMed]
- Kim DG, Kim JS, Lee JS, et al. The Usefulness of Endoscopic Harvesting of the Latissimus Dorsi Flap for Breast Reconstruction Using a Single-Port and CO2 Gas Insufflation Technique. Aesthetic Plast Surg 2021;45:2681-90. [Crossref] [PubMed]
- Lai HW, Chen ST, Tai CM, et al. Robotic- Versus Endoscopic-Assisted Nipple-Sparing Mastectomy with Immediate Prosthesis Breast Reconstruction in the Management of Breast Cancer: A Case-Control Comparison Study with Analysis of Clinical Outcomes, Learning Curve, Patient-Reported Aesthetic Results, and Medical Cost. Ann Surg Oncol 2020;27:2255-68. [Crossref] [PubMed]
- Park KU, Tozbikian GH, Ferry D, et al. Residual breast tissue after robot-assisted nipple sparing mastectomy. Breast 2021;55:25-9. [Crossref] [PubMed]
- Toesca A, Peradze N, Galimberti V, et al. Robotic Nipple-sparing Mastectomy and Immediate Breast Reconstruction With Implant: First Report of Surgical Technique. Ann Surg 2017;266:e28-30. [Crossref] [PubMed]
- Houvenaeghel G, El Hajj H, Schmitt A, et al. Robotic-assisted skin sparing mastectomy and immediate reconstruction using latissimus dorsi flap a new effective and safe technique: A comparative study. Surg Oncol 2020;35:406-11. [Crossref] [PubMed]
- Lee J, Jung JH, Kim WW, et al. Endoscopy-assisted muscle-sparing Latissimus Dorsi muscle flap harvesting for partial breast reconstruction. BMC Surg 2020;20:192. [Crossref] [PubMed]
- Elliott LF, Ghazi BH, Otterburn DM. The scarless latissimus dorsi flap for full muscle coverage in device-based immediate breast reconstruction: an autologous alternative to acellular dermal matrix. Plast Reconstr Surg 2011;128:71-9. [Crossref] [PubMed]
- Selber JC, Baumann DP, Holsinger FC. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg 2012;129:1305-12. [Crossref] [PubMed]
- Iglesias M, Gonzalez-Chapa DR. Endoscopic latissimus dorsi muscle flap for breast reconstruction after skin-sparing total mastectomy: report of 14 cases. Aesthetic Plast Surg 2013;37:719-27. [Crossref] [PubMed]
- Santanelli di Pompeo F, Laporta R, Sorotos M, et al. Latissimus dorsi flap for total autologous immediate breast reconstruction without implants. Plast Reconstr Surg 2014;134:871e-9e. [Crossref] [PubMed]
- Yang CE, Roh TS, Yun IS, et al. Immediate partial breast reconstruction with endoscopic latissimus dorsi muscle flap harvest. Arch Plast Surg 2014;41:513-9. [Crossref] [PubMed]
- Chung JH, You HJ, Kim HS, et al. A novel technique for robot assisted latissimus dorsi flap harvest. J Plast Reconstr Aesthet Surg 2015;68:966-72. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Houvenaeghel G, Bannier M, Rua S, et al. Robotic breast and reconstructive surgery: 100 procedures in 2-years for 80 patients. Surg Oncol 2019;31:38-45. [Crossref] [PubMed]
- Yadon ZE, Rodrigues LC, Davies CR, et al. Indoor and peridomestic transmission of American cutaneous leishmaniasis in northwestern Argentina: a retrospective case-control study. Am J Trop Med Hyg 2003;68:519-26. [Crossref] [PubMed]
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 2009;124:345-53. [Crossref] [PubMed]
- Zhang S, Xie Y, Liang F, et al. Video-assisted Transaxillary Nipple-sparing Mastectomy and Immediate Implant-based Breast Reconstruction: A Novel and Promising Method. Aesthetic Plast Surg 2022;46:91-8. [Crossref] [PubMed]
- Zhou J, Liu X, Feng Y, et al. Breakthrough in breast reconstruction in the context of COVID-19: safety and efficiency of endoscopic breast reconstruction at a day surgery center. Gland Surg 2021;10:2477-89. [Crossref] [PubMed]
- Liu C, Luan J, Ouyang Y, et al. Breast Reconstruction in Poland Syndrome Patients with Latissimus Dorsi Myo Flap and Implant: An Efficient Endoscopic Approach Using Single Transverse Axillary Incision. Aesthetic Plast Surg 2019;43:1186-94. [Crossref] [PubMed]
- Franceschini G, Visconti G, Garganese G, et al. Nipple-sparing mastectomy combined with endoscopic immediate reconstruction via axillary incision for breast cancer: A preliminary experience of an innovative technique. Breast J 2020;26:206-10. [Crossref] [PubMed]
- Du Z, Zhou Y, Chen J, et al. Retrospective observational study of breast reconstruction with extended latissimus dorsi flap following skin-sparing mastectomy. Medicine (Baltimore) 2018;97:e10936. [Crossref] [PubMed]
- Owaki T, Kijima Y, Yoshinaka H, et al. Present status of endoscopic mastectomy for breast cancer. World J Clin Oncol 2015;6:25-9. [Crossref] [PubMed]
- Lai HW, Chen ST, Lin SL, et al. Technique for single axillary incision robotic assisted quadrantectomy and immediate partial breast reconstruction with robotic latissimus dorsi flap harvest for breast cancer: A case report. Medicine (Baltimore) 2018;97:e11373. [Crossref] [PubMed]
- Lai HW, Lin SL, Chen ST, et al. Robotic nipple sparing mastectomy and immediate breast reconstruction with robotic latissimus dorsi flap harvest - Technique and preliminary results. J Plast Reconstr Aesthet Surg 2018;71:e59-61. [Crossref] [PubMed]
- Lai HW, Chen ST, Lin SL, et al. Robotic Nipple-Sparing Mastectomy and Immediate Breast Reconstruction with Gel Implant: Technique, Preliminary Results and Patient-Reported Cosmetic Outcome. Ann Surg Oncol 2019;26:42-52. [Crossref] [PubMed]
- Jeon DN, Kim J, Ko BS, et al. Robot-assisted breast reconstruction using the prepectoral anterior tenting method. J Plast Reconstr Aesthet Surg 2021;74:2906-15. [Crossref] [PubMed]
- Yuan H, Xie D, Xiao X, et al. The Clinical Application of Mastectomy With Single Incision Followed by Immediate Laparoscopic-Assisted Breast Reconstruction With Latissimus Dorsi Muscle Flap. Surg Innov 2017;24:349-52. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


