
Correlations between dynamic-enhanced magnetic resonance imaging quantitative parameters and postoperative recurrence or metastasis and clinicopathological features in breast cancer patients—a retrospective cohort study
Introduction
The Breast cancer is the most common malignancy in women, and its incidence has increased in recent years (1). For breast cancer patients diagnosed for the first time, the current international breast cancer treatment guidelines recommend a dynamic-enhanced magnetic resonance imaging (MRI) examination to determine whether there is lymph node metastasis, evaluate tumor staging (T staging), clarify the scope of tumor invasion, etc., to help clinicians develop individualized treatment plans. MRI has an irreplaceable role in diagnosing and guiding the chemotherapy of invasive breast cancer (2-4).
In addition to the many qualitative indicators, there are quantitative indicators of MRI, including the apparent diffusion coefficient (ADC), and peak time. These quantitative indicators also play an important role for breast cancer patients. When the cell density of malignant tumors in patients is high, the diffusion movement of water molecules is limited, and the value of the ADC decreases; thus, the ADC can be used to distinguish between breast cancer and benign tumors and has better sensitivity and specificity than an ultrasound (5). The peak time is related to the microvascular and extravascular space in breast cancer patients, and angiogenesis is the basis for the rapid growth, infiltration, and metastasis of malignant tumors (6,7).
In a study focused on prostate cancer, it was found that the ADC and peak time may be potential biomarkers of early radiation response (8). Thus, we speculated that the ADC and peak time may have a certain relationship with the prognosis and clinical pathological characteristics of breast cancer patients and may have certain value in predicting the prognosis of breast cancer patients. However, as to date, no relevant studies appeared to have been conducted, we designed this study. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-400/rc).
Methods
General information
From January 2016 to June 2017, 214 invasive breast cancer patients treated at the Affiliated Kunshan Hospital of Jiangsu University were retrospectively and continuously enrolled in this study. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have been diagnosed with invasive breast cancer; (II) have received this diagnosis of breast cancer for the first time; (III) be aged ≥18 years; and (IV) have complete information. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had carcinoma in situ or benign mass; (II) had received special treatment, such as radiotherapy or chemotherapy; (III) had not undergone a breast enhancement MRI examination at the Affiliated Kunshan Hospital of Jiangsu University; (IV) had inflammatory breast cancer or lactating breast cancer; (V) had stage IV breast cancer; and/or (VI) failed to follow up. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Affiliated Kunshan Hospital of Jiangsu University (No. 20210092). Individual consent for this retrospective analysis was waived.
Observation indicators
The observation indicators were as follows: (I) general information: age, onset site, family history, and menopause; (II) MRI characteristics: ADC and peak time; and (III) pathological characteristics: maximum tumor diameter, estrogen receptor (ER) expression, progesterone receptor (PR) expression, nuclear-associated antigen Ki-67 index (%), human epidermal growth factor receptor 2 (HER-2) expression, a positive rate of axillary lymph nodes, subclavian lymph node metastasis, T staging, pathological type, histological grade, vascular tumor thrombus, and the 5-year recurrence or metastasis rate.
Examination methods
All the patients underwent dynamic-enhanced MRI within 72 h of preoperative surgery using the Philips Lntera 1.5T Achieva Nova Dual MR (Philips, Holland) with gadopentetate dimeglumine as the contrast medium (intravenously administered, concentration: 0.2 mmol/kg, speed: 2.5 mL/s).
Detection methods
The following detection methods were used: (I) ER: an expression level ≥10% indicated high expression; (II) PR: an expression level ≥10% indicated high expression; (III) Ki-67: an index >14% indicated high expression; (IV) HER2: intraoperative breast cancer tissue was taken from patients, immunohistochemical staining was performed, and the HER2 protein expression level was defined as (–), (1+), (2+), or (3+) according to the staining ratio and color intensity of HER2 protein expression level on the cell membrane. If the HER2 protein expression was (3+) or (2+) and fluorescence in situ hybridization (FISH) tests showed that the HER2 gene was amplified, HER2 was positive, or otherwise defined as negative; (V) positive axillary lymph node metastasis or subclavian lymph node metastasis: the presence of axillary lymph node metastasis or subclavian lymph node metastasis was determined according to the postoperative pathological results; (VI) vascular tumor thrombus: a immunohistochemical method using a ×20 microscopic was employed to detect whether vascular tumor thrombus was formed or not; and (VII) recurrence or metastasis: patients were followed-up for 5 years after surgery through clinical visit and cellphone to determine if they suffered from recurrence or metastasis.
Statistical analysis
The data analysis of this study was completed using SPSS 26.0, and a P value <0.05 (two-sided) indicated a statistically significant difference. The continuous measurement data of the 2 groups are expressed as the mean ± standard deviation, and the independent sample t-test was used to analyze differences between the 2 groups. The count data are represented as the n (%), and the chi-square test was used to analyze differences between the 2 groups. The diagnostic value of the ADC and peak time on postoperative recurrence or metastasis in breast cancer patients was analyzed using receiver operating characteristic (ROC) curves. The risk factors for postoperative recurrence or metastasis in breast cancer patients were explored by a multifactorial logistics regression analysis.
Results
Clinical pathological features of patients with postoperative recurrence or metastasis of breast cancer
Compared to patients without recurrence or metastasis, the ADC of patients with recurrence or metastasis was lower [(0.79±0.25)×10−3vs. (0.99±0.19)×10−3 mm2/s; P<0.001], the peak time was significantly reduced (156.92±25.08 vs. 178.56±31.10 s; P<0.001), and the maximum tumor diameter was significantly increased (2.79±1.94 vs. 1.91±0.88 cm; P<0.001). Compared to patients without recurrence or metastasis, the proportion of patients with a T staging ≥2, HER-2 positive, a high Ki-67 index, vascular tumor thrombus, and lymph node metastasis increased significantly, and the proportion of patients with high ER and PR expression was reduced in patients with recurrence or metastasis (P<0.05; see Table 1).
Table 1
| Variables | Recurrence or metastasis (n=39) | No recurrence or metastasis (n=175) | t/χ2 value | P value |
|---|---|---|---|---|
| Apparent diffusion coefficient (×10−3 mm2/s) | 0.79±0.25 | 0.99±0.19 | 5.625 | <0.001 |
| Peak time (s) | 156.92±25.08 | 178.56±31.10 | 4.058 | <0.001 |
| Age <40 years | 8 (20.51) | 29 (16.57) | 0.346 | 0.556 |
| Site of onset | 0.319 | 0.572 | ||
| Left | 19 (48.72) | 94 (53.71) | ||
| Right | 20 (51.28) | 81 (46.29) | ||
| Family history | 1 (2.56) | 3 (1.71) | 0.126 | 0.723 |
| Menopause | 15 (38.46) | 80 (45.71) | 0.680 | 0.410 |
| The largest diameter of the tumor (cm) | 2.79±1.94 | 1.91±0.88 | 4.347 | <0.001 |
| T staging | 19.964 | <0.001 | ||
| T1 | 12 (30.77) | 121 (69.14) | ||
| ≥T2 | 27 (69.23) | 54 (30.86) | ||
| Positive HER-2 | 19 (48.72) | 17 (9.71) | 34.674 | <0.001 |
| High Ki-67 | 31 (79.49) | 66 (37.71) | 22.457 | <0.001 |
| High ER | 9 (23.08) | 148 (84.57) | 61.718 | <0.001 |
| High PR | 13 (33.33) | 145 (82.86) | 40.485 | <0.001 |
| Vascular tumor thrombus | 9 (23.08) | 10 (5.71) | 11.884 | 0.001 |
| Breast cancer type | 1.774 | 0.183 | ||
| Non-special types | 37 (94.87) | 153 (87.43) | ||
| Special types | 2 (5.13) | 22 (12.57) | ||
| Lymph node metastasis | 55.499 | <0.001 | ||
| Yes | 33 (84.62) | 39 (22.29) | ||
| No | 6 (15.38) | 136 (77.71) | ||
| Subclavian lymph nodes metastasis | 0.681 | 0.409 | ||
| Yes | 1 (2.56) | 0 (0.00) | ||
| No | 38 (97.44) | 175 (100.00) | ||
| Histological grading | 1.760 | 0.185 | ||
| Grade II | 29 (74.36) | 146 (83.43) | ||
| Grade III | 10 (25.64) | 29 (16.57) |
Data are expressed as n (%) or mean ± standard deviation. HER-2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
Diagnostic value of ADC and peak time for postoperative recurrence or metastasis in breast cancer patients
The ADC and peak time had certain diagnostic value for postoperative recurrence or metastasis in breast cancer patients, and the areas under the curve were 0.821 [95% confidence interval (CI): 0.732–0.911; P<0.001] and 0.691 (95% CI: 0.609–0.774; P<0.001), respectively (see Figure 1 and Table 2).
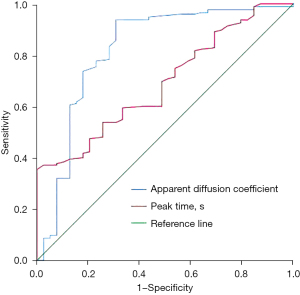
Table 2
| Variables | Area under the curve | P value | 95% confidence interval | Optimal diagnostic cut-offs | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Apparent diffusion coefficient value | 0.821 | <0.001 | 0.732 | 0.911 | 0.78 | 0.937 | 0.692 |
| Peak time(s) | 0.691 | <0.001 | 0.609 | 0.774 | 167.5 | 0.594 | 0.667 |
Risk factors for the recurrence or metastasis of breast cancer after surgery
An ADC <0.78×10−3 mm2/s, a peak time <167.50 s, T staging ≥2, vascular tumor thrombus and positive lymph nodes were risk factors for postoperative recurrence in breast cancer patients, and high ER expression was a protective factor for postoperative recurrence or metastasis in breast cancer patients (P<0.05; see Table 3).
Table 3
| Variables | B | S.E. | Wald | P value | Exp (B) | 95% confidence interval |
|---|---|---|---|---|---|---|
| Apparent diffusion coefficient <0.78 (×10−3 mm2/s) | 2.984 | 1.039 | 8.241 | 0.004 | 19.768 | 2.577–151.619 |
| Peak time <167.50 s | 1.742 | 0.846 | 4.242 | 0.039 | 5.708 | 1.088–29.947 |
| T staging ≥2 | 4.808 | 1.599 | 9.042 | 0.003 | 122.474 | 5.334–2,812.360 |
| High ER | –2.891 | 1.358 | 4.531 | 0.033 | 0.056 | 0.004–0.795 |
| High PR | –3.016 | 1.656 | 3.316 | 0.069 | 0.049 | 0.002–1.259 |
| Vascular tumor thrombus | 3.343 | 1.544 | 4.686 | 0.030 | 28.304 | 1.372–583.914 |
| Positive lymph nodes | 5.751 | 1.729 | 11.066 | 0.001 | 314.407 | 10.617–9,310.547 |
| Constant | –18.839 | 5.341 | 12.442 | <0.001 | – | – |
S.E., standard error; ER, estrogen receptor; PR, progesterone receptor.
Association of ADC with clinical pathological features of breast cancer patients
We found that ADC was associated with the site of onset, T staging, vascular tumor thrombus, and lymph node metastasis in breast cancer patients (P<0.05; see Table 4).
Table 4
| Variables | Apparent diffusion coefficient (×10−3 mm2/s) | t value | P value |
|---|---|---|---|
| Age (years) | 1.325 | 0.187 | |
| <40 (n=37) | 0.99±0.25 | ||
| ≥40 (n=177) | 0.94±0.21 | ||
| Site of onset | 2.014 | 0.045 | |
| Left side (n=113) | 0.98±0.21 | ||
| Right (n=101) | 0.92±0.22 | ||
| Family history | 0.965 | 0.336 | |
| Yes (n=4) | 0.85±0.12 | ||
| No (n=210) | 0.95±0.22 | ||
| Menopause | 1.367 | 0.173 | |
| Yes (n=95) | 0.93±0.23 | ||
| No (n=119) | 0.97±0.21 | ||
| T staging | 2.287 | 0.023 | |
| T1 (n=133) | 0.98±0.21 | ||
| ≥ T2 (n=81) | 0.91±0.21 | ||
| Positive HER-2 | 1.746 | 0.082 | |
| Yes (n=36) | 0.89±0.26 | ||
| No (n=178) | 0.96±0.21 | ||
| High Ki-67 | 1.887 | 0.061 | |
| Yes (n=97) | 0.92±0.22 | ||
| No (n=117) | 0.98±0.21 | ||
| High ER | 1.240 | 0.217 | |
| Yes (n=157) | 0.96±0.19 | ||
| No (n=57) | 0.92±0.27 | ||
| High PR | 1.195 | 0.233 | |
| Yes (n=158) | 0.96±0.21 | ||
| No (n=56) | 0.92±0.25 | ||
| Vascular tumor thrombus | 2.125 | 0.035 | |
| Yes (n=19) | 0.85±0.20 | ||
| No (n=195) | 0.96±0.22 | ||
| Breast cancer type | 0.287 | 0.774 | |
| Non-special types (n=190) | 0.95±0.21 | ||
| Special types (n=24) | 0.94±0.25 | ||
| Lymph node metastasis | 3.262 | 0.001 | |
| Yes (n=72) | 0.88±0.24 | ||
| No (n=142) | 0.98±0.20 | ||
| Histological grading | 0.004 | 0.997 | |
| Grade II (n=175) | 0.95±0.22 | ||
| Grade III (n=39) | 0.95±0.20 |
Data are expressed as mean ± standard deviation. HER-2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
Correlation between peak time and clinical pathological characteristics of breast cancer patients
Peak time was related to a high Ki-67 index, high ER expression, and high PR expression (P<0.05; see Table 5).
Table 5
| Variables | Peak time (s) | t value | P value |
|---|---|---|---|
| Age (years) | 1.109 | 0.269 | |
| <40 (n=37) | 179.78±27.16 | ||
| ≥40 (n=177) | 173.54±31.93 | ||
| Site of onset | 1.025 | 0.307 | |
| Left side (n=113) | 176.68±31.23 | ||
| Right (n=101) | 172.31±31.12 | ||
| Family history | 0.686 | 0.493 | |
| Yes (n=4) | 164.00±43.44 | ||
| No (n=210) | 174.82±31.01 | ||
| Menopause | 1.177 | 0.240 | |
| Yes (n=95) | 171.81±31.42 | ||
| No (n=119) | 176.86±30.95 | ||
| T staging | 1.099 | 0.273 | |
| T1 (n=133) | 176.44±30.55 | ||
| ≥ T2 (n=81) | 171.62±32.17 | ||
| Positive HER-2 | 0.663 | 0.508 | |
| Yes (n=36) | 171.47±30.64 | ||
| No (n=178) | 175.25±31.34 | ||
| High Ki-67 | 2.217 | 0.028 | |
| Yes (n=97) | 169.47±30.23 | ||
| No (n=117) | 178.88±31.45 | ||
| High ER | 2.854 | 0.005 | |
| Yes (n=157) | 178.22±31.25 | ||
| No (n=57) | 164.68±29.02 | ||
| High PR | 2.078 | 0.039 | |
| Yes (n=158) | 177.23±31.35 | ||
| No (n=56) | 167.23±29.76 | ||
| Vascular tumor thrombus | 1.186 | 0.237 | |
| Yes (n=19) | 166.53±26.57 | ||
| No (n=195) | 175.41±31.55 | ||
| Breast cancer type | 1.321 | 0.188 | |
| Non-special types (n=190) | 175.62±31.65 | ||
| Special types (n=24) | 166.71±26.52 | ||
| Lymph node metastasis | 0.613 | 0.540 | |
| Yes (n=72) | 172.78±31.10 | ||
| No (n=142) | 175.55±31.30 | ||
| Histological grading | 0.074 | 0.941 | |
| Grade II (n=175) | 174.69±32.10 | ||
| Grade III (n=39) | 174.28±27.06 |
Data are expressed as mean ± standard deviation. HER-2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
Discussion
Currently, dynamic-enhanced MRI is widely used in the diagnosis of breast cancer to develop individualized treatment plans (9-11). We found that the correlations between the ADC and peak time, and postoperative recurrence or metastasis and the clinical pathological features of breast cancer patients were examined, and it was found that the decreased ADC and peak time were risk factors for postoperative recurrence or metastasis in breast cancer patients and were related to the patients’ clinical pathological features.
The tissues of malignant tumors got a higher cell density; therefore, the tissue gaps are reduced, which in turn results in limitation of free movement of water molecules. So, the ADC decreases. Normal or benign tumor tissues have low cell density. Previous studies have confirmed that the ADC has a high sensitivity and specificity for the differential diagnosis of benign and malignant mammary tumors (12-15). In recent years, studies have also explored the relationship between ADC and the prognosis of breast cancer patients, and shown that ADCs are related to histological grading, the Ki-67 index, tumor size, molecular subtypes, and axillary lymph node metastasis; thus, the ADC appears to be related to the heterogeneity of breast cancer (16,17), which was supported by the findings of our study. Our study showed that the ADC was related to the site of onset, T staging, vascular tumor thrombus, and lymph node metastasis, and that tumor density tends to be higher in patients with high T staging, vascular tumor thrombus, and lymph node metastasis, which results in a reduced ADC. In addition, we found that the ADC had high value in predicting postoperative recurrence or metastasis in breast cancer patients, and a reduction in the ADC was a risk factor for postoperative recurrence or metastasis.
Peak time refers to the time required for the breast cancer tissue signal intensity to reach its peak; the shorter the time, the richer the neovascularization in the tumor body. Our study showed that a decrease in peak time was a risk factor for postoperative recurrence or metastasis in breast cancer, which indicated that patients with more angiogenesis in breast cancer tumors were more likely to suffer from recurrence or metastasis after surgery. Previous studies have also confirmed that angiogenesis is a risk factor for postoperative recurrence or metastasis in breast cancer patients (18-20). A further analysis showed that peak time was more lowly expressed in high Ki-67 patients and more highly expressed in high ER and PR patients. As a high Ki-67 index is a risk factor for a poor prognosis in patients (21), while high ER and PR expression levels are protective factors for a poor prognosis (22-24), we indirectly illustrated that peak time is associated with patient prognosis.
Limitations
This study had a number of limitations. First, it was a retrospective clinical study. Second, it failed to further measure cell density and angiogenesis in breast cancer tissue.
Conclusions
The peak time and even more notably, the ADC have good value in predicting the recurrence or metastasis of breast cancer after surgery, and are related to the clinical pathological characteristics of breast cancer patients.
Acknowledgments
Funding: The study was supported by the Suzhou Science and Technology Plan Project (Minsheng Technology) (No. SYSD2019022).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-400/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-400/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-400/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Affiliated Kunshan Hospital of Jiangsu University (No. 20210092). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Chen K, Liu JQ, Wu W, et al. Clinical practice guideline for breast-conserving surgery in patients with early-stage breast cancer: Chinese Society of Breast Surgery (CSBrS) practice guidelines 2021. Chin Med J (Engl) 2021;134:2143-6. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of anthracycline-based postoperative chemotherapy on blood glucose and lipid profiles in patients with invasive breast cancer. Ann Palliat Med 2021;10:5502-8. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Tao D, Wang Y, Zhang X, et al. Identification of Angiogenesis-Related Prognostic Biomarkers Associated With Immune Cell Infiltration in Breast Cancer. Front Cell Dev Biol 2022;10:853324. [Crossref] [PubMed]
- Khodabandeh Z, Valilo M, Velaei K, et al. The potential role of nicotine in breast cancer initiation, development, angiogenesis, invasion, metastasis, and resistance to therapy. Breast Cancer 2022;29:778-89. [Crossref] [PubMed]
- Lee SL, Lee J, Craig T, et al. Changes in apparent diffusion coefficient radiomics features during dose-painted radiotherapy and high dose rate brachytherapy for prostate cancer. Phys Imaging Radiat Oncol 2018;9:1-6. [Crossref] [PubMed]
- Zhang H, Yin Y, Tao W, et al. Clinical Observation of MRI Scanning Combined with Clinical Nursing for Surgical Breast Cancer Patients. Int J Anal Chem 2022;2022:6863281. [Crossref] [PubMed]
- Taourel P. MRI to Detect Contralateral Breast Cancer in Patients with Newly Diagnosed Breast Cancer: An Increase in Overall Survival to Be Confirmed. Radiology 2022;304:308-9. [Crossref] [PubMed]
- Freitas V, Li X, Amitai Y, et al. Contralateral Breast Screening with Preoperative MRI: Long-Term Outcomes for Newly Diagnosed Breast Cancer. Radiology 2022;304:297-307. [Crossref] [PubMed]
- McDonald ES, Romanoff J, Rahbar H, et al. Mean Apparent Diffusion Coefficient Is a Sufficient Conventional Diffusion-weighted MRI Metric to Improve Breast MRI Diagnostic Performance: Results from the ECOG-ACRIN Cancer Research Group A6702 Diffusion Imaging Trial. Radiology 2021;298:60-70. [Crossref] [PubMed]
- Tuan Linh L, Minh Duc N, Minh Duc N, et al. Correlations between apparent diffusion coefficient values and histopathologic factors in breast cancer. Clin Ter 2021;172:218-24. [PubMed]
- Yin H, Jiang Y, Xu Z, et al. Apparent Diffusion Coefficient-Based Convolutional Neural Network Model Can Be Better Than Sole Diffusion-Weighted Magnetic Resonance Imaging to Improve the Differentiation of Invasive Breast Cancer From Breast Ductal Carcinoma In Situ. Front Oncol 2022;11:805911. [Crossref] [PubMed]
- Surov A, Meyer HJ, Wienke A. Can apparent diffusion coefficient (ADC) distinguish breast cancer from benign breast findings? A meta-analysis based on 13 847 lesions. BMC Cancer 2019;19:955. [Crossref] [PubMed]
- Choi BB. Associations Between Apparent Diffusion Coefficient Values and the Prognostic Factors of Breast Cancer. J Comput Assist Tomogr 2019;43:931-6. [Crossref] [PubMed]
- Ren C, Zou Y, Zhang X, et al. Diagnostic value of diffusion-weighted imaging-derived apparent diffusion coefficient and its association with histological prognostic factors in breast cancer. Oncol Lett 2019;18:3295-303. [Crossref] [PubMed]
- Shakouri A, Kahroba H, Hamishekar H, et al. Nanoencapsulation of Hirudo medicinalis proteins in liposomes as a nanocarrier for inhibiting angiogenesis through targeting VEGFA in the Breast cancer cell line (MCF-7). Bioimpacts 2022;12:115-26. [Crossref] [PubMed]
- Ayoub NM, Jaradat SK, Al-Shami KM, et al. Targeting Angiogenesis in Breast Cancer: Current Evidence and Future Perspectives of Novel Anti-Angiogenic Approaches. Front Pharmacol 2022;13:838133. [Crossref] [PubMed]
- Hussen BM, Salihi A, Abdullah ST, et al. Signaling pathways modulated by miRNAs in breast cancer angiogenesis and new therapeutics. Pathol Res Pract 2022;230:153764. [Crossref] [PubMed]
- Al-Keilani MS, Elstaty R, Alqudah MA. The Prognostic Potential of Neurokinin 1 Receptor in Breast Cancer and Its Relationship with Ki-67 Index. Int J Breast Cancer 2022;2022:4987912. [Crossref] [PubMed]
- Xie F, Liu L, Yang H, et al. The Impact of Reproductive Factors on the Risk of Breast Cancer by ER/PR and HER2: A Multicenter Case-Control Study in Northern and Eastern China. Oncologist 2022;27:e1-8. [Crossref] [PubMed]
- Weinfurtner RJ, Raghunand N, Stringfield O, et al. MRI Response to Pre-operative Stereotactic Ablative Body Radiotherapy (SABR) in Early Stage ER/PR+ HER2- Breast Cancer correlates with Surgical Pathology Tumor Bed Cellularity. Clin Breast Cancer 2022;22:e214-23. [Crossref] [PubMed]
- Wang X, He J, Jiang S, et al. Multi-ligand modified PC@DOX-PA/EGCG micelles effectively inhibit the growth of ER+, PR+ or HER2+ breast cancer. J Mater Chem B 2022;10:418-29. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


