
The value of imaging combined with clinicopathological features in the diagnosis of high-risk breast lesions
Introduction
High-risk breast lesions (HRLs) are a group of morphologically and biologically heterogeneous diseases with an increased risk of breast cancer during follow-up after diagnosis (1-3). The high-risk lesions mainly include atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), flat epithelial atypia (FEA), papillary lesions, sclerosing adenosis (including radial scarring and complex sclerosing adenosis), and mucocele-like lesions (4-7). Currently, high-risk lesions are found mainly through image-guided biopsies, but because of sampling volume limitations or suboptimal targeting, there is a risk of upgrading to ductal carcinoma in situ (DCIS) or invasive malignancy at the time of surgical excision (8). As a result, surgical excision is often recommended (3). To some extent, this results in excessive treatment and unnecessary surgery.
The follow-up management of HRLs remains controversial, mainly because of the wide range of reported upgrade rates (9). If the upgrade rates of HRLs can be accurately predicted by combining various characteristics of lesions, we can guide clinical decision-making better and manage patients individually. At present, many scholars have discussed the factors affecting the upgrade rate of HRLs, including clinical, pathological, imaging, and other aspects. Previous studies have found that some characteristics are closely associated with upgrade, including age, menopausal status, lesion size, mode of biopsy, and imaging characteristics (10-14). Despite these efforts, there are still no definite characteristics that can reliably distinguish lesions requiring surgical excision from those that can be monitored. Current studies have some limitations. For example, the imaging characteristics used in the studies were limited to a single mode, and only the tumor’s imaging features were analyzed, whereas the background parenchyma features were ignored. Our study included clinicopathological features and imaging features of three modes [breast ultrasound, mammography, and magnetic resonance imaging (MRI)], comparing the value of different features in predicting the upgrade of HRLs to better stratify the risk of upgrade and assist in clinical decisions. Furthermore, we analyzed the background parenchymal enhancement (BPE) of MRI to study the effect of breast parenchymal features on the upgrade. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-155/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Fudan University Shanghai Cancer Center, Shanghai, China (No. 1507149-8), and individual consent for this retrospective analysis was waived. We continuously retrospectively analyzed patients who met the following inclusion criteria in the Fudan University Cancer Hospital from January 2017 to March 2018: (I) the pathology of the biopsies (including hollow-core needle biopsy, vacuum-assisted biopsy, and open biopsy) showed HRLs, including ADH, ALH, sclerosing adenosis (including radial scarring and complex sclerosing adenosis), intraductal papilloma, mucocele-like lesions, and FEA; (II) complete imaging data of the three modes—breast ultrasound, mammography, and MRI—are available; (III) all imaging examinations were performed before biopsy; and (IV) short-term follow-up was performed for 6 months to 1 year. The exclusion criteria included (I) poor image quality, and (II) lack of follow-up. Information about the patients’ age, maximum diameter of lesion, menopausal status, history of benign and malignant breast lesions, family history of malignant tumors, and clinical manifestations was collected from the clinical case system, and the image information before biopsy was obtained from the picture archiving and communication system.
Histopathologic analysis
Breast specimens were analyzed by an associate chief physician specializing in breast diagnosis in the Department of Pathology. The histologic analysis was based on the microscopic analysis of tissue sections stained with hematoxylin-eosin (H&E). Histological types were defined according to the fifth edition of the World Health Organization’s (WHO’s) pathological classification of breast tumors (15). Immunohistochemical methods were used to determine the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2), and the Ki-67 antigen. The pathologist was blinded to the results of the imaging examination.
High-risk lesion upgrade was defined as the presence of DCIS or invasive ductal carcinoma components at subsequent surgery based on histopathology (16). Based on their risk of upgrade reported in the literature (17-19), the lesions were categorized in order of severity as high-risk I (HR-I) (ADH, ALH, atypical papilloma, and sclerosing adenosis with atypical hyperplasia) and high-risk II (HR-II) (sclerosing adenosis, benign papilloma, mucocele-like lesions, and FEA). Although FEA is accompanied by atypical hyperplasia (AH), as the literature reports (3), the upgrade rate of FEA is much lower than that of other AH, so FEA was classified as HR-II.
Image interpretation
Because of the significant difference in the upgrade rates between HR-I and HR-II lesions, we researched the relationship between the imaging features and the upgrade rates of all HRLs, HR-I, and HR-II lesions, respectively. Two breast radiologists (with 6 and 15 years of experience in breast imaging diagnosis, respectively) reviewed these cases independently and reached a consensus after consultation. The analysis and evaluation of imaging manifestations of breast lesions were based on standards from the Breast Imaging Reporting and Data System (BI-RADS) proposed by the American College of Radiology (20). Mammographic features of lesions included mass, calcification, architectural distortion, and asymmetry; MRI features included mass, non-mass enhancement (NME), and BPE (minimal to mild or moderate to marked); and ultrasound features were divided into mass and other manifestations (such as heterogeneity, ductal dilation, and calcification). According to the BI-RADS lexicon (20) and clinical application, MRI BI-RADS 4 was still diagnosed as 4 A–C, and pathological results were taken as the gold standard to compare the efficacy of different imaging modes in diagnosing the upgrade of HRLs.
Statistical analysis
Independent sample t-tests and chi-square tests were used to compare the clinical features and imaging signs between the upgraded and non-upgraded group of HRLs. According to BI-RADS classification, seven points (1, 2, 3, 4A, 4B, 4C, and 5) were assigned as ordered classification variables. Using the pathological results as the gold standard, the diagnostic efficacy of mammography, ultrasound, and MRI for the upgrade rate of HRLs was compared by the receiver operating characteristic (ROC) curve. Binary logistic regression analysis was used to compare the relationship between the upgrade rate of HRLs and the clinical and imaging signs. P<0.05 indicated a significant difference. Statistical analyses were conducted using SPSS (version 26) and MedCalc software (version 20.0.3).
Results
Clinicopathological features
During the study period, 274 HRLs in 274 patients were diagnosed by biopsy. Of these lesions, 10 were excluded for poor image quality, and 34 were excluded for lack of follow-up. Therefore, 230 HRLs in 230 women were included in the study. The mean patient age was 48.3±10.6 years, and the mean maximum diameter of the lesions was 15.6±8.9 mm. According to the main pathological features, there were 43 cases of ADH, 6 cases of ALH, 89 cases of sclerosing adenosis, 87 cases of intraductal papilloma, 4 cases of mucocele-like lesions, and 1 case of FEA. One hundred twenty patients underwent surgery after biopsy, and the interval between biopsy and surgery was less than seven days. One hundred ten patients were confirmed complete resection of the lesion at biopsy, and they did not undergo surgery. Of 230 lesions, a total of 47 (20.4%) cases upgraded to cancer during follow-up surgery: 22 (9.5%) DCIS and 25 (10.9%) invasive. Table 1 shows the upgrade situation of different pathological types. There were statistical differences in age, maximum diameter of lesions, and menopausal status of HRLs between the upgraded group and the non-upgraded group (Table 2).
Table 1
| Histopathologic type | Number of cases | Number of upgrades | Upgrade rate, % |
|---|---|---|---|
| Atypical ductal hyperplasia | 43 | 13 | 30.2 |
| Atypical lobular hyperplasia | 6 | 3 | 50.0 |
| Papillary lesion | 87 | 19 | 21.8 |
| Sclerosing adenosis | 89 | 11 | 12.4 |
| Mucocele-like lesions | 4 | 1 | 25.0 |
| Flat epithelial atypia | 1 | 0 | 0.0 |
| Total | 230 | 47 | 20.4 |
Table 2
| Features | All HRLs (n=230) | HR-I (n=109) | HR-II (n=121) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-upgraded (n=183) | Upgraded (n=47) | P | Non-upgraded (n=67) | Upgraded (n=42) | P | Non-upgraded (n=116) | Upgraded (n=5) | P | |||
| Age, years | 47.2±10.5 | 52.3±10.3 | 0.003* | 46.6±10.7 | 52.8±10.6 | 0.004* | 47.6±10.3 | 48.0±7.55 | 0.926 | ||
| Maximum diameter, mm | 14.96±8.95 | 17.89±8.58 | 0.044* | 13.96±7.15 | 18.19±8.69 | 0.007* | 15.54±9.82 | 15.40±7.92 | 0.974 | ||
| Side | 0.240 | 0.820 | 0.262 | ||||||||
| Left | 107 | 23 | 32 | 21 | 75 | 2 | |||||
| Right | 76 | 24 | 35 | 21 | 41 | 3 | |||||
| Menopausal status | 0.020* | 0.015* | 0.526 | ||||||||
| Post- | 60 | 24 | 21 | 23 | 39 | 1 | |||||
| Pre- | 123 | 23 | 46 | 19 | 77 | 4 | |||||
| Benign breast history | 0.954 | 0.656 | 0.493 | ||||||||
| Yes | 20 | 5 | 10 | 5 | 10 | 0 | |||||
| No | 163 | 42 | 57 | 37 | 106 | 5 | |||||
| Malignant breast history | 0.791 | 0.677 | 0.742 | ||||||||
| Yes | 32 | 9 | 15 | 8 | 17 | 1 | |||||
| No | 151 | 38 | 52 | 34 | 99 | 4 | |||||
| Family history of cancer | 0.208 | 0.145 | 0.031* | ||||||||
| Yes | 13 | 6 | 2 | 4 | 11 | 2 | |||||
| No | 170 | 41 | 65 | 38 | 105 | 3 | |||||
| Clinical symptoms | 0.165 | 0.374 | 0.371 | ||||||||
| Discharge | 10 | 4 | 5 | 4 | 5 | 0 | |||||
| Mass | 122 | 36 | 43 | 31 | 79 | 5 | |||||
| Negative | 51 | 7 | 19 | 7 | 32 | 0 | |||||
Numerical data are presented as the mean ± SD. Nonnumerical data are presented as the number of patients. *, P<0.05. HRLs, high-risk breast lesions.
HR-I patients (N=42/109, 38.5%) were significantly more likely to upgrade compared to HR-II (N=5/121, 4.1%, P<0.01). There were no significant differences in mean age (49.0±11.0 vs. 47.5±10.2 years old, P=0.3) and mean maximum diameter (15.59±8.01 vs. 15.53±9.72 mm, P=0.9) between HR-I and HR-II patients. In HR-I, there were also statistical differences in age, maximum diameter of lesions, and menopausal status between the upgraded group and the non-upgraded group (Table 2).
It has been reported that ER expression in breast epithelium is associated with AH, and ER percent staining and intensity are significantly increased in AH (21). Immunohistochemical examinations were performed in 126 of all 230 lesions. ER status analysis showed that of 79 cases associated with AH, ER+ accounted for 81.0% (64/79), and ER expression >70% accounted for 65.8% (52/79). Of 47 cases without AH, ER+ accounted for 95.7% (45/47), and ER expression >70% accounted for 55.3% (26/47). Among these 47 cases without AH, there were 34 benign intraductal papillomas in ER+ and 17 benign intraductal papillomas in ER >70%.
Imaging features
The correlation of imaging features in relation to the rate of upgrade among all HRLs, HR-I, and HR-II lesions is shown in Table 3. In all HRLs and HR-I lesions, MRI BPE (P<0.001; P<0.001), MRI manifestations (P=0.016; P=0.041) and ultrasound manifestations (P=0.015; P=0.024) were predictive of the upgrade. We further analyzed the specific manifestations of MRI (mass, NME, or negative) in the upgraded group and the non-upgraded group and found that none of the 26 MRI negative lesions had upgraded; the difference was statistically significant (χ2=7.53, P=0.006). We further analyzed the specific manifestations of ultrasound (mass, other manifestations, or negative) in the two groups compared and found that the upgrade rate of the 49 cases with the negative manifestation was significantly lower than those with non-negative manifestations; the difference was statistically significant (χ2=7.85, P=0.005). In the HR-II lesions, only MRI BPE was significantly different between the upgraded and non-upgraded groups (P=0.001). There was no statistical difference in mammographic signs between the upgraded and non-upgraded groups. MRI BPE was effective in predicting the upgrade of HRLs in all three subgroups and had a greater diagnostic value than ultrasound and mammography.
Table 3
| Features | All HRLs (n=230) | HR-I (n=109) | HR-II (n=121) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-upgraded (n=183) | Upgraded (n=47) | P | Non-upgraded (n=67) | Upgraded (n=42) | P | Non-upgraded (n=116) | Upgraded (n=5) | P | |||
| Mammography | 0.362 | 0.670 | 0.843 | ||||||||
| Mass | 50 | 17 | 20 | 16 | 30 | 1 | |||||
| Calcification | 72 | 14 | 24 | 12 | 48 | 2 | |||||
| Architectural distortion | 21 | 4 | 7 | 3 | 14 | 1 | |||||
| Asymmetry | 18 | 8 | 7 | 7 | 11 | 1 | |||||
| Negative | 22 | 4 | 9 | 4 | 13 | 0 | |||||
| MRI | 0.016* | 0.041* | 0.549 | ||||||||
| Mass | 76 | 26 | 29 | 23 | 47 | 3 | |||||
| NME | 81 | 21 | 29 | 19 | 52 | 2 | |||||
| Negative | 26 | 0 | 9 | 0 | 17 | 0 | |||||
| MRI BPE | <0.001* | <0.001* | 0.001* | ||||||||
| Minimal to mild | 155 | 16 | 61 | 15 | 94 | 1 | |||||
| Moderate to marked | 28 | 31 | 6 | 27 | 22 | 4 | |||||
| Ultrasound | 0.015* | 0.024* | 0.190 | ||||||||
| Mass | 92 | 27 | 33 | 25 | 59 | 2 | |||||
| Other manifestations | 45 | 17 | 15 | 14 | 30 | 3 | |||||
| Negative | 46 | 3 | 19 | 3 | 27 | 0 | |||||
*, P<0.05. HRLs, high-risk breast lesions; MRI, magnetic resonance imaging; NME, non-mass enhancement; BPE, breast parenchymal enhancement.
Univariate and multivariate analysis of the upgrade of HRLs
Univariate analysis was conducted on the clinical and imaging features related to the upgrade. As a result, age, the maximum diameter of the lesion, and moderate to marked BPE were the positive correlation factors for predicting the upgrade of HRLs, while pre-menopause, negative MRI, or ultrasound diagnosis were the negative correlation factors for predicting the upgrade of HRLs. The factors associated with upgrade in univariate analysis were introduced into multivariate logistic regression analysis. Age and BPE of MRI were found to be independent factors in predicting the upgrade of HRLs. The rate of upgrade of HRLs increased with age and MRI BPE (Table 4; Figures 1,2).
Table 4
| Features | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age | 1.045 | 1.014–1.007 | <0.01* | 1.070 | 1.006–1.139 | 0.031* | |
| Maximum diameter of lesions | 1.034 | 1.000–1.069 | <0.05* | 1.017 | 0.973–1.062 | 0.459 | |
| Premenopausal | 0.467 | 0.244–0.895 | <0.05* | 0.381 | 0.084–1.720 | 0.209 | |
| MRI-negative | 0.858 | 0.809–0.910 | <0.01* | 0 | 0 | 0.998 | |
| MRI-moderate to marked BPE | 10.725 | 5.193–22.151 | <0.01* | 31.562 | 10.158–98.069 | <0.01* | |
| Ultrasound-negative | 0.210 | 0.064–0.728 | <0.05* | 0.376 | 0.094–1.501 | 0.166 | |
*, P<0.05. HRLs, high-risk breast lesions; MRI, magnetic resonance imaging; BPE, breast parenchymal enhancement; OR, odds ratio; CI, confidence interval.
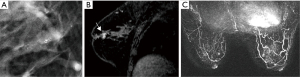
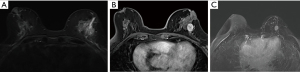
The efficacy of imaging diagnosis
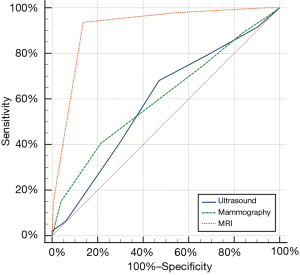
According to the Youden index, when BI-RADS 4A was used as the cut-off point, mammography, ultrasound, and MRI had the highest diagnostic efficacy in evaluating the upgrade of HRLs. Their sensitivities were 40.4%, 68.1%, and 93.6%; specificities were 78.69%, 53.0%, and 86.3%; and the areas under the ROC curve (AUCs) were 0.606, 0.590, and 0.913, respectively (Figure 3). The diagnostic value of MRI for HRLs was significantly higher than that of mammography and ultrasound (P<0.001; Figure 4).
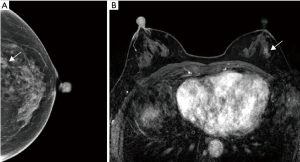
Discussion
In the diagnosis of HRLs, our study is unique because it combined clinical features, histological features, and multimodal imaging features, making it more comprehensive than previous studies. In terms of clinical features, we found that age, the maximum diameter of the lesions, and the menopausal status of the patients with HRLs were significantly correlated with upgrade, which was consistent with previous studies. Giuliani et al. found that the older the patients were, the higher the risk they had of the HRLs being upgraded (14), and Cheeney et al. found that the maximum diameter of the lesions was larger in upgraded HRLs (10).
In terms of histological features, we once again demonstrated that AH is an important factor associated with the upgrade of HRLs. Rakha et al. proposed to classify B3 lesions into B3a (without AH) and B3b (with AH) according to their AH components (17). Mayer et al. found that the upgrade rate of B3b lesions by biopsy was higher, and it was more meaningful to perform surgery (18). Our results are consistent with previous reports that the likelihood of upgrade in HR-II was significantly reduced (17-19), suggesting that the evaluation of AH components could be useful in guiding the follow-up management of HRLs. Because the upgrade rates of HR-I and HR-II are very different, it is important to select the correct follow-up management. We further investigated the factors influencing the upgrade in the HR-I and HR-II groups and found that only BPE of MRI had predictive value in both groups, which can guide the clinical selection of surgery or follow-up.
ER is reported to be positive in almost all breast AH (22), and ER expression is increased in AH (21). In this study, the positive rate of ER in HRLs with AH was 81.0%, and the ER expression >70% accounted for 65.8%, which was consistent with the report. The positive rate of ER in HRLs without AH was 95.7%, and ER >70% accounted for 55.3%. This may be due to the large number of benign intraductal papillomas in HRLs without AH. The pathogenesis of intraductal papillomas is related to the long-term proliferation of breast tissues under the dominant effect of estrogen, which promotes the proliferation of normal breast cells through ER (23). Therefore, there are more ER+ cases in intraductal papillomas, and the further increase of ER in AH may be one of the reasons why its hyperplasia degree is more active than that of normal hyperplasia.
In terms of imaging features, for mammography, Nguyen et al. linked calcification to the upgrade of HRLs (13). In our study, calcification was the most common X-ray sign, followed by mass, but the value of different X-ray signs for upgrading diagnosis was not found. For MRI, Preibsch et al. found the upgrade rates of NME and large lesions (>20 mm) were lower (11), whereas Okamoto et al. believed that the size and MRI features of lesions had no diagnostic value for the upgrade rate (24). Unlike previous research, the data in this study showed that the upgrade rate of HRLs with negative MRI findings was lower and the upgrade rate of HRLs with moderate and marked BPE was higher, but mass and NME had no diagnostic value for the upgrade of HRLs. For ultrasound, Giuliani et al. found that HRLs presenting as masses had a higher rate of upgrade (14). Our study found that HRLs with negative ultrasound manifestations had a lower rate of upgrade, while mass or other positive manifestations were not significantly associated with upgrade. In terms of diagnostic efficacy, we found that MRI was superior to mammography and ultrasound in diagnosing HRLs. MRI BPE was an independent factor in evaluating the upgrade of HRLs. A previous research has shown that BPE is influenced by hormone levels associated with age and the menstrual cycle and is a predictor of breast cancer risk (25). We found that BPE was a positive correlation indicator of the upgrade of HRLs, indicating that it could also indicate the correlation between HRLs and breast cancer risk, which could provide a reference for clinical decision-making in the follow-up treatment of HRLs.
In brief, our study has the following three advantages compared with other studies. First, we combined three imaging modes of mammography, ultrasound, and MRI for analysis, comparing the prediction efficiency of each mode. We found that MRI had the highest predictive efficacy, suggesting the importance of MRI follow-up for patients with HRLs. Second, we added features of breast parenchyma and found that BPE was an independent factor in predicting upgrade. Third, previous studies have proved that HRLs with AH are more likely to upgrade (17-19). To better determine the need for surgery, we further analyzed the factors that predicted the upgrade of HR-I and HR-II. We found that BPE was the only predictor of both groups and the odds ratio of BPE was highest. Based on this, we can infer that HR-I lesions with moderate to marked BPE are more suitable for surgery, and HR-II lesions with minimal to mild BPE can be considered for regular follow-up.
A limitation of our study is its retrospective design. We only included short-term follow-up data from 6 months to 1 year. Long-term follow-up monitoring of HRLs is still in progress. In addition, mammography in this study had no diagnostic value for the upgrade of HRLs. The reason may be that the analysis of image features is limited to the main signs, such as mass and calcification. The density, margin, morphology of the mass, and the distribution and characteristics of calcification were not targeted. In the future, more detailed research will focus on 1 or 2 features.
Conclusions
In conclusion, our study showed an increased rate of upgrade and ER expression in HRLs with AH. For different modes of imaging examination, MRI had better diagnostic efficacy than ultrasound and mammography for the upgrade of HRLs. Age and the BPE of the MRI can be used as independent factors to predict the upgrade of HRLs. The imaging features and diagnosis of HRLs still need further testing and will depend on clinical, pathological, and imaging multidisciplinary cooperation.
Acknowledgments
We would like to thank all the patients of this study for their participation. We would like to thank Yiji-to-Edit for their help in polishing our paper.
Funding: This project was supported by grants from Youth Medical Talents-Medical Imaging Practitioner Program [No. SHWRS (2020) 087], Cancer Research Program of National Cancer Center (No. NCC201909B06), and Shanghai Anticancer Association SOAR Project (No. SACA-AX202109).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-155/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-155/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-155/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-155/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Fudan University Shanghai Cancer Center, Shanghai, China (No. 1507149-8), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mooney KL, Bassett LW, Apple SK. Upgrade rates of high-risk breast lesions diagnosed on core needle biopsy: a single-institution experience and literature review. Mod Pathol 2016;29:1471-84. [Crossref] [PubMed]
- Dillon MF, McDermott EW, Hill AD, et al. Predictive value of breast lesions of "uncertain malignant potential" and "suspicious for malignancy" determined by needle core biopsy. Ann Surg Oncol 2007;14:704-11. [Crossref] [PubMed]
- Morrow M, Schnitt SJ, Norton L. Current management of lesions associated with an increased risk of breast cancer. Nat Rev Clin Oncol 2015;12:227-38. [Crossref] [PubMed]
- Chivukula M, Bhargava R, Tseng G, et al. Clinicopathologic implications of "flat epithelial atypia" in core needle biopsy specimens of the breast. Am J Clin Pathol 2009;131:802-8. [Crossref] [PubMed]
- Berg WA. Image-guided breast biopsy and management of high-risk lesions. Radiol Clin North Am 2004;42:935-46. vii. [Crossref] [PubMed]
- Georgian-Smith D, Lawton TJ. Controversies on the management of high-risk lesions at core biopsy from a radiology/pathology perspective. Radiol Clin North Am 2010;48:999-1012. [Crossref] [PubMed]
- Sewell CW. Pathology of high-risk breast lesions and ductal carcinoma in situ. Radiol Clin North Am 2004;42:821-30. v. [Crossref] [PubMed]
- Yu CC, Ueng SH, Cheung YC, et al. Predictors of underestimation of malignancy after image-guided core needle biopsy diagnosis of flat epithelial atypia or atypical ductal hyperplasia. Breast J 2015;21:224-32. [Crossref] [PubMed]
- Falomo E, Adejumo C, Carson KA, et al. Variability in the Management Recommendations Given for High-risk Breast Lesions Detected on Image-guided Core Needle Biopsy at U.S. Academic Institutions. Curr Probl Diagn Radiol 2019;48:462-6. [Crossref] [PubMed]
- Cheeney S, Rahbar H, Dontchos BN, et al. Apparent diffusion coefficient values may help predict which MRI-detected high-risk breast lesions will upgrade at surgical excision. J Magn Reson Imaging 2017;46:1028-36. [Crossref] [PubMed]
- Preibsch H, Wanner LK, Staebler A, et al. Malignancy rates of B3-lesions in breast magnetic resonance imaging - do all lesions have to be excised? BMC Med Imaging 2018;18:27. [Crossref] [PubMed]
- Bahl M, Barzilay R, Yedidia AB, et al. High-Risk Breast Lesions: A Machine Learning Model to Predict Pathologic Upgrade and Reduce Unnecessary Surgical Excision. Radiology 2018;286:810-8. [Crossref] [PubMed]
- Nguyen CV, Albarracin CT, Whitman GJ, et al. Atypical ductal hyperplasia in directional vacuum-assisted biopsy of breast microcalcifications: considerations for surgical excision. Ann Surg Oncol 2011;18:752-61. [Crossref] [PubMed]
- Giuliani M, Rinaldi P, Rella R, et al. A new risk stratification score for the management of ultrasound-detected B3 breast lesions. Breast J 2018;24:965-70. [Crossref] [PubMed]
- Yang WT, Bu H. Updates in the 5(th) edition of WHO classification of tumours of the breast. Zhonghua Bing Li Xue Za Zhi 2020;49:400-5. [PubMed]
- Burbank F. Stereotactic breast biopsy of atypical ductal hyperplasia and ductal carcinoma in situ lesions: improved accuracy with directional, vacuum-assisted biopsy. Radiology 1997;202:843-7. [Crossref] [PubMed]
- Rakha EA, Lee AH, Jenkins JA, et al. Characterization and outcome of breast needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Int J Cancer 2011;129:1417-24. [Crossref] [PubMed]
- Mayer S, Kayser G, Rücker G, et al. Absence of epithelial atypia in B3-lesions of the breast is associated with decreased risk for malignancy. Breast 2017;31:144-9. [Crossref] [PubMed]
- Huang YY, Park H, McLaren S, et al. B3 lesion upgrade rates in a tertiary Australian breast centre: a 8-year experience (2012-2019). ANZ J Surg 2020;90:2521-6. [Crossref] [PubMed]
- Mercado CL. BI-RADS update. Radiol Clin North Am 2014;52:481-7. [Crossref] [PubMed]
- Barr FE, Degnim AC, Hartmann LC, et al. Estrogen receptor expression in atypical hyperplasia: lack of association with breast cancer. Cancer Prev Res (Phila) 2011;4:435-44. [Crossref] [PubMed]
- Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer 2001;8:47-61. [Crossref] [PubMed]
- Allred DC, O'Connell P, Fuqua SA, et al. Immunohistochemical studies of early breast cancer evolution. Breast Cancer Res Treat 1994;32:13-8. [Crossref] [PubMed]
- Okamoto S, Chen ST, Covelli JD, et al. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: imaging characteristics, outcome of surgical excision or imaging follow-up. Breast Cancer 2020;27:405-14. [Crossref] [PubMed]
- King V, Brooks JD, Bernstein JL, et al. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260:50-60. [Crossref] [PubMed]


