
Noninvasive follicular neoplasm with papillary-like nuclear features (NIFTP): a new entity
Introduction
As also recommended by the National Cancer Institute in 2012 (1), a review of thyroid follicular neoplasms characterized by low clinical risk was performed. As a result, a nomenclature revision was proposed in an effort to reduce the psychological and clinical consequences associated with a diagnosis of cancer; moreover, the intent was to reduce the well-known overdiagnosis and overtreatment of thyroid cancers (1). In particular follicular variant of papillary thyroid carcinoma (FVPTC) represents 30% of papillary carcinomas and include encapsulated, encapsulated invasive, and non-encapsulated forms (2). An extensive reevaluation of encapsulated noninvasive and encapsulated invasive FVPTC by a panel of international experts ended in 2016 with the publication that proposed the introduction of noninvasive follicular neoplasm with papillary-like nuclear features (NIFTP) (3). The results of the study showed that encapsulated noninvasive FVPTC with specific histo-pathological characteristics had an extremely indolent behavior, with no adverse events in 109 patients during follow-up (13 years median), thus authors defined precise histological criteria to define these lesions.
The consequences of this reclassification affected all the aspects of thyroid pathology and clinics, with impact on the psychological sphere of patients. Indeed, the term neoplasia has replaced that of carcinoma: this change mirrors the indolent clinical behavior of NIFTP and should affect also clinicians’ choices in down-scaling treatment approaches (3). NIFTP are expected to have an excellent prognosis regardless of size (4), and could be managed with thyroid lobectomy similarly to patients with benign thyroid neoplasms as already advocated by clinical guidelines (5).
Histo-pathological diagnostic criteria
According to strict inclusion and exclusion histological criteria (3), NIFTP is an encapsulated or clearly demarcated papillary thyroid cancer with predominant follicles, and nuclear features of papillary thyroid carcinoma (PTC) Figure 1. The first essential criterion is then represented by the demonstration of the complete encapsulation of the lesion. As a direct consequence, the diagnosis of NIFTP can be rendered only after its complete resection and examination. The presence of papillary-like nuclear alterations has to be scored based on size and shape (nuclear enlargement, overlapping and/or elongation), nuclear membrane irregularities (irregular contours grooves, and/or pseudo-inclusions) and chromatin characteristics (chromatin clearing, margination of chromatin to membrane, and/or glassy nuclei). For each class of nuclear features a score of zero or one is assigned, yielding a range of scores from zero to three: for the diagnosis of NIFTP, a nuclear score between two and three has to be present.
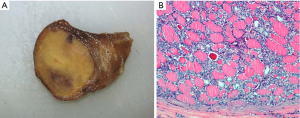
In addition, according to the initially proposed diagnostic criteria (3), the diagnosis of NIFTP could not be rendered if any of the following exclusion features are present: vascular or capsular invasion, more than 1% papillae, presence of psammoma bodies, more than 30% solid-trabecular architecture, high mitotic activity and presence of tumor necrosis. NIFTP, with all these diagnostic criteria, has been included in the last edition of the World Health Organization classification of endocrine tumors (6)
However, a refinement of diagnostic criteria has been proposed in 2018 (7): the papillae cut-off of 1% was modified, and in the presence of true well-formed papillae the lesion cannot be considered a NIFTP. Indeed, it has been shown that the presence of papillae (even if in less than 1% of tumor areas) is associated with higher frequency of BRAFV600E mutation and the occurrence of lymph node metastases (8, 9), compared with NIFTP with total absence of papillae. Moreover, several authors reported lymph node metastasis and distant metastasis in NIFTP patients (10-12).
Another important change in the revised diagnostic criteria is that in case of nuclear score of three, that indicates pronounced expression of PTC nuclear features, a careful revision of the entire tumor is recommended in order to exclude the presence of papillae (7).
The possible diagnosis of NIFTP when oncocytic component is present is still not clear. Some authors suggest that encapsulated FVPTC with oncocytic features can be classified as NIFTP if all the diagnostic criteria are met. In the multi-institutional cohort studied by Xu and colleagues, 61 noninvasive encapsulated FVPTC with oncocytic features were evaluated (13). No lymph node metastases nor structural recurrence were observed; in a subgroup of patients with 10.2 years median follow-up, none developed disease recurrence. In the series of Rosario and Mourão, none of the ten patients with “oncocytic NIFTP” developed structural disease or biochemical recurrence during follow-up (median 72 months) (14). Established that stringent diagnostic criteria for NIFTP must be adopted, these findings seem indicate that oncocytic appearance should not affect the indolent nature of this tumors.
The size of tumor should not impact on the possible diagnosis of NIFTP. However, the series of noninvasive FVPTC evaluated by the authors who proposed the nomenclature introduction, did not contain microPTCs (less than or equal to 1 cm). Shafique and colleagues tried to specifically address this aspect by evaluating eight patients with encapsulated noninvasive microFVPTC among a large series of microPTCs; none of the eight patients showed adverse events during follow-up (median 12.1 years).
NIFTP has been studied in cohorts of pediatrics patients (15,16); no lymph node metastases nor disease recurrence have been observed, suggesting that the same histological criteria can be applied also in pediatric age.
As already mentioned, according to the current guidelines, hemithyroidectomy is the most appropriate diagnostic and therapeutic management for NIFTP patients. However, the need for a total thyroidectomy is recommended in the presence of a significant contralateral nodule, lymph node metastases or extrathyroidal extension (17). Nevertheless, it seems that in many cases patients undergoing lobectomy will later require completion of thyroidectomy (18). Canberk and collaborators studied the NIFTP in the setting of multifocal and contralateral disease (19). The authors found that the frequency of co-occurring tumors in the contralateral lobe in NIFTP patients was not negligible (18% of cases); moreover, the majority of co-occurring lesions were malignant. This study showed that bilateral and multifocal disease are part of the spectrum of NIFTP neoplasms, and highlights the importance of a careful evaluation of both lobes even when NIFTP is preoperatively suspected.
Cytological features
Although the possibility to make a preoperative diagnosis of NIFTP has not been yet demonstrated, it is important to investigate the cytological characteristics of nodules that histologically prove NIFTP (17). Indeed, a preoperative identification of NIFTP, distinguishing it from its invasive or infiltrative counterpart, would be extremely helpful in reducing the surgical overtreatment of these lesions. However, the cytological features of NIFTP overlap with those of encapsulated and invasive FVPTC (2).
The feasibility of a preoperative diagnosis of NIFTP on cytological aspirates has been widely studied since their nomenclature revision. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) has been applied for the classification of thyroid nodules since 2009 (20). The system includes six diagnostic categories: (I) non-diagnostic; (II) benign; (III) atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS); (IV) suspicious for follicular neoplasm/follicular neoplasm (SFN/FN); (V) suspicious for malignancy (SM); (VI) malignant. Each class has a stratified risk of malignancy (ROM) and a specific management recommended.
It is well known that significant portions of NIFTPs and FVPTCs are classified as AUS/FLUS, SFN/FN and SM (17), but cytology alone is not sufficient to establish the degree of invasiveness of these lesions; indeed the differential diagnosis between NIFTP, encapsulated noninvasive or invasive FVPTC and infiltrative FVPTC essentially depends on the demonstration of invasion, either capsular or vascular. Despite this, some authors tried to find criteria able to provide a preoperative diagnosis, comparing the cyto-architectural features of follicular adenoma, NIFTP, invasive/infiltrative FVPTC and classical variant PTC (21-23). The results showed that NIFTP can be distinguished from classical PTC on the basis of the presence of multiple nuclear pseudoinclusions, papillae and nuclear clearing, but not from invasive FVPTC. Similarly, some authors have suggested that nuclear irregularities, grooves and pseudoinclusions could favor the diagnosis of invasive FVPTC (24). NIFTPs on fine-needle aspiration (FNA) cytology are mainly classified in one of the indeterminate categories of TBSRTC, similarly to the other thyroid neoplasms characterized by follicular architecture (17). While some have reported that a malignant preoperative cytological diagnosis is less likely in NIFTP (25), others have shown a high rate of preoperative malignant cytology in the TBSRTC category that corresponds to NIFTP (26). Hypothesizing that the proportion of NIFTP that are classified as malignant on cytology is small (17), the ROM observed in the indeterminate diagnostic categories of TBSRTC will be lower (27). In a review of the literature by Amendoeira and colleagues, the decrease of ROM is reported to be from 13.5% to 48% in SM, from 10% to 36% in SFN/FN and from 4.9% to 45% in AUS/FLUS nodules (28). Of note, the issue of the decreasing ROM in TBSRTC classes represents a controversial point, since NIFTP are non-malignant, but they cannot be considered benign entities (29).
The studies based on the cytological evaluation of NIFTP lesions confirm that thyroid nodules with microfollicular architecture and mild nuclear atypia on FNA, even performing a careful evaluation, cannot be further characterized on cytology and often require diagnostic surgery. For this reason, to improve the preoperative identification of NIFTPs, many authors investigated the usefulness of molecular testing.
Molecular characteristics of NIFTP
Molecular characteristics of NIFTP have been evaluated either on their preoperative FNAs or on tissue samples. Considering gene mutations and fusions, it has been widely demonstrated that NIFTPs are RAS-like tumors (30). They harbor mainly RAS mutations; BRAFK601E mutation can be present in some cases, while the BRAFV600E, more prevalent in classic PTC, should not be detected in NIFTP (7). In detail, in this review of the literature, we found 16 papers investigating RAS mutations in NIFTPs (3,8,10,31-43); the reported mutation frequency ranged from 20% to 100%, but 9 out of the 16 studies were centered in the range of 40–70%. Considering the BRAFK601E mutation, some studies reported the absence of this alteration in NIFTP, while at least eight papers detected this mutation in 2–11.5% of cases. In the majority of papers (5 out of 8), however, the frequency of BRAFK601E mutation in NIFTPs was below 5%. The presence of other RAS-like alterations such as EIF1AX mutations has been scarcely investigated in NIFTP. In the very first NIFTP cohort, RAS mutations coexisted with EIF1AX in two cases out of the 27 evaluated with molecular test (3). Similarly, the coexistence of EIF1AX mutation with a rare BRAF mutation in one out of 27 NIFTPs was reported (39).
In the same way, the main gene fusions found in NIFTP are those involving PPARG and THADA genes, typically detected in RAS-like thyroid neoplasms. PPARG rearrangements have been found in up to 22% (3,34,39,41) and THADA fusions in up to 40% of NIFTPs (3,36,39).
Besides BRAFV600E mutation, NIFTP should not have TP53 and TERT promoter mutations, commonly detected in high-risk cancer. However, these oncogenic events have been reported to be present in NIFTP: BRAFV600E mutation was detected in NIFTPs by some authors (8,31,32,37); TERT promoter mutations have been described so far in 1 out of 4 NIFTPs by Jiang an colleagues (10) and in 1 out of 15 NIFTPs by Song and colleagues (41). In the paper that recently proposed the refinement of criteria for NIFTP diagnosis, the absence of BRAFV600E-like and other high-risk mutations has been included as a secondary diagnostic criterion (7).
The expression analysis of mRNA and miRNA panels in NIFTP has been reported by several groups. First of all, considering the Afirma test [Gene Expression Classifier, (GEC), now available as Genomic Sequencing Classifier (GSC)], it seems that the majority of NIFTPs are called as “suspicious” (10,44-46). There is still no consensus regarding how a NIFTP should be labeled by GEC. NIFTPs require surgery to be diagnosed, and the adequate treatment—if one could know that a nodule is a NIFTP before surgery—should be lobectomy. However, clinicians could be prompted toward total thyroidectomy in the light of a “suspicious” test result (47). In any case, not all NIFTPs have preoperative GEC “suspicious” result, and also GEC “benign” NIFTPs have been reported (44,48); since the majority of GEC “benign” nodules do not undergo surgery, it is reasonable hypothesizing that the rate of GEC “benign” nodules that proves NIFTP on histology could be underestimated. This hypothesis is supported by gene expression studies that demonstrated the heterogeneous nature of NIFTPs. In detail, Giannini and collaborators and Borrelli and collaborators, analyzing a mRNA panel and a miRNA panel respectively, reported that NIFTPs could be further divided in two different subgroups; comparing NIFTP expression profile to other thyroid lesions, they found that part of NIFTPs resemble to infiltrative FVPTCs and part to follicular adenomas (33,37). Pool and colleagues recently reported that, based on gene expression data, NIFTPs could be subdivided into three molecular groups, in particular a RAF-like, a RAS-like and a THADA-like, enriched in lesions with BRAF, RAS mutations and THADA fusions respectively (49). These findings demonstrate that NIFTPs are not a homogeneous group of lesions when their phenotype is evaluated; this is consistent with the hypothesis that NIFTP represents a pre-malignant lesion that could be observed in different moments of its morphological—and molecular—evolution: closer to a benign-like lesion it derives from, or closer to its invasive form.
Conclusions
Since 2016, the scientific community made great efforts in studying, describing and characterizing the new histological entity of NIFTP. Clinical and pathological guidelines endorsed the new terminology as well as the diagnostic criteria. NIFTPs have been characterized on histology, cytology and at molecular level. In all these fields, the common issue remains the difficult preoperative identification of this clinically indolent lesion, whose early diagnosis would allow a tailored surgical and clinical treatment. Finally, the last unmet point is to confirm the low-risk nature of NIFTPs in long-term follow-up series of patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 2014;15:e234-42. [Crossref] [PubMed]
- Baloch ZW, Shafique K, Flanagan M, et al. Encapsulated classic and follicular variants of papillary thyroid carcinoma: comparative clinicopathologic study. Endocr Pract 2010;16:952-9. [Crossref] [PubMed]
- Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol 2016;2:1023-9. [Crossref] [PubMed]
- Xu B, Tallini G, Scognamiglio T, et al. Outcome of Large Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2017;27:512-7. [Crossref] [PubMed]
- Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2017;27:481-3. [Crossref] [PubMed]
- International Agency for Research on Cancer, Lloyd RV, Osamura RY, et al. WHO classification of tumours of endocrine organs. 4th edition. Lyon: World Health Organization, 2017:355.
- Nikiforov YE, Baloch ZW, Hodak SP, et al. Change in Diagnostic Criteria for Noninvasive Follicular Thyroid Neoplasm With Papillarylike Nuclear Features. JAMA Oncol 2018;4:1125-6. [Crossref] [PubMed]
- Cho U, Mete O, Kim MH, et al. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol 2017;30:810-25. [Crossref] [PubMed]
- Kim TH, Lee M, Kwon AY, et al. Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology 2018;72:648-61. [Crossref] [PubMed]
- Jiang XS, Harrison GP, Datto MB. Young Investigator Challenge: Molecular testing in noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Cancer Cytopathol 2016;124:893-900. [Crossref] [PubMed]
- Hahn SY, Shin JH, Oh YL, et al. Role of Ultrasound in Predicting Tumor Invasiveness in Follicular Variant of Papillary Thyroid Carcinoma. Thyroid 2017;27:1177-84. [Crossref] [PubMed]
- Parente DN, Kluijfhout WP, Bongers PJ, et al. Clinical Safety of Renaming Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: Is NIFTP Truly Benign? World J Surg 2018;42:321-6. [Crossref] [PubMed]
- Xu B, Reznik E, Tuttle RM, et al. Outcome and molecular characteristics of non-invasive encapsulated follicular variant of papillary thyroid carcinoma with oncocytic features. Endocrine 2019;64:97-108. [Crossref] [PubMed]
- Rosario PW, Mourão GF. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a review for clinicians. Endocr Relat Cancer 2019;26:R259-66. [Crossref] [PubMed]
- Rossi ED, Mehrotra S, Kilic AI, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in the pediatric age group. Cancer Cytopathol 2018;126:27-35. [Crossref] [PubMed]
- Mariani RA, Kadakia R, Arva NC. Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: Should it also be reclassified in children? Pediatr Blood Cancer 2018;65:e26966. [Crossref] [PubMed]
- Ferris RL, Nikiforov Y, Terris D, et al. AHNS Series: Do you know your guidelines? AHNS Endocrine Section Consensus Statement: State-of-the-art thyroid surgical recommendations in the era of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Head Neck 2018;40:1881-8. [Crossref] [PubMed]
- Kluijfhout WP, Pasternak JD, Lim J, et al. Frequency of High-Risk Characteristics Requiring Total Thyroidectomy for 1–4 cm Well-Differentiated Thyroid Cancer. Thyroid 2016;26:820-4. [Crossref] [PubMed]
- Canberk S, Montezuma D, Taştekin E, et al. ‘The other side of the coin’: understanding noninvasive follicular tumor with papillary-like nuclear features in unifocal and multifocal settings. Hum Pathol 2019;86:136-42. [Crossref] [PubMed]
- Cibas ES, Ali SZ. NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol 2009;132:658-65. [Crossref] [PubMed]
- Maletta F, Massa F, Torregrossa L, et al. Cytological features of ‘noninvasive follicular thyroid neoplasm with papillary-like nuclear features’ and their correlation with tumor histology. Hum Pathol 2016;54:134-42. [Crossref] [PubMed]
- Bizzarro T, Martini M, Capodimonti S, et al. Young investigator challenge: The morphologic analysis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on liquid-based cytology: Some insights into their identification. Cancer Cytopathol 2016;124:699-710. [Crossref] [PubMed]
- Chandler JB, Colunga M, Prasad ML, et al. Identification of distinct cytomorphologic features in the diagnosis of NIFTP at the time of preoperative FNA: Implications for patient management. Cancer Cytopathol 2017;125:865-75. [Crossref] [PubMed]
- Ibrahim AA, Wu HH. Fine-Needle Aspiration Cytology of Noninvasive Follicular Variant of Papillary Thyroid Carcinoma Is Cytomorphologically Distinct From the Invasive Counterpart. Am J Clin Pathol 2016;146:373-7. [Crossref] [PubMed]
- Singh R, Avila J, Jo K, et al. Patients with Non-invasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features are Unlikely to have Malignant Preoperative Cytology. Ann Surg Oncol 2017;24:3300-5. [Crossref] [PubMed]
- Higuchi M, Hirokawa M, Kanematsu R, et al. Impact of the modification of the diagnostic criteria in the 2017 Bethesda System for Reporting Thyroid Cytopathology: a report of a single institution in Japan. Endocr J 2018;65:1193-8. [Crossref] [PubMed]
- Faquin WC, Wong LQ, Afrogheh AH, et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol 2016;124:181-7. [Crossref] [PubMed]
- Amendoeira I, Maia T, Sobrinho-Simões M. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): impact on the reclassification of thyroid nodules. Endocr Relat Cancer 2018;25:R247-58. [Crossref] [PubMed]
- Kakudo K. How to handle borderline/precursor thyroid tumors in management of patients with thyroid nodules. Gland Surg 2018;7:S8-18. [Crossref] [PubMed]
- Basolo F, Macerola E, Ugolini C, et al. The Molecular Landscape of Noninvasive Follicular Thyroid Neoplasm With Papillary-like Nuclear Features (NIFTP): A Literature Review. Adv Anat Pathol 2017;24:252-8. [Crossref] [PubMed]
- Lee SE, Hwang TS, Choi YL, et al. Molecular Profiling of Papillary Thyroid Carcinoma in Korea with a High Prevalence of BRAFV600E Mutation. Thyroid 2017;27:802-10. [Crossref] [PubMed]
- Zhao L, Dias-Santagata D, Sadow PM, et al. Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer Cytopathol 2017;125:323-31. [Crossref] [PubMed]
- Borrelli N, Denaro M, Ugolini C, et al. miRNA expression profiling of ‘noninvasive follicular thyroid neoplasms with papillary-like nuclear features’ compared with adenomas and infiltrative follicular variants of papillary thyroid carcinomas. Mod Pathol 2017;30:39-51. [Crossref] [PubMed]
- Howitt BE, Chang S, Eszlinger M, et al. Fine-needle aspiration diagnoses of noninvasive follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2015;144:850-7. [Crossref] [PubMed]
- Ohori NP, Wolfe J, Carty SE, et al. The influence of the noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) resection diagnosis on the false-positive thyroid cytology rate relates to quality assurance thresholds and the application of NIFTP criteria. Cancer Cytopathol 2017;125:692-700. [Crossref] [PubMed]
- Valderrabano P, Khazai L, Leon ME, et al. Evaluation of ThyroSeq v2 performance in thyroid nodules with indeterminate cytology. Endocr Relat Cancer 2017;24:127-36. [Crossref] [PubMed]
- Giannini R, Ugolini C, Poma AM, et al. Identification of Two Distinct Molecular Subtypes of Non-Invasive Follicular Neoplasm with Papillary-Like Nuclear Features by Digital RNA Counting. Thyroid 2017;27:1267-76. [Crossref] [PubMed]
- Proietti A, Sartori C, Macerola E, et al. Low frequency of TERT promoter mutations in a series of well-differentiated follicular-patterned thyroid neoplasms. Virchows Arch 2017;471:769-73. [Crossref] [PubMed]
- Brandler TC, Liu CZ, Cho M, et al. Does Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features (NIFTP) Have a Unique Molecular Profile? Am J Clin Pathol 2018;150:451-60. [Crossref] [PubMed]
- Sohn SY, Lee JJ, Lee JH. Molecular Profile and Clinicopathologic Features of Follicular Variant Papillary Thyroid Carcinoma. Pathol Oncol Res 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Song YS, Won JK, Yoo SK, et al. Comprehensive Transcriptomic and Genomic Profiling of Subtypes of Follicular Variant of Papillary Thyroid Carcinoma. Thyroid 2018;28:1468-78. [Crossref] [PubMed]
- Johnson DN, Furtado LV, Long BC, et al. Noninvasive Follicular Thyroid Neoplasms With Papillary-like Nuclear Features Are Genetically and Biologically Similar to Adenomatous Nodules and Distinct From Papillary Thyroid Carcinomas With Extensive Follicular Growth. Arch Pathol Lab Med 2018;142:838-50. [Crossref] [PubMed]
- Reinke RH, Larsen SR, Mathiesen JS, et al. Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features is Rare: A Population Based Study of Incidence. Head Neck Pathol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Song SJ. Pre-operative features of non-invasive follicular thyroid neoplasms with papillary-like nuclear features: An analysis of their cytological, Gene Expression Classifier and sonographic findings. Cytopathology 2017;28:488-94. [Crossref] [PubMed]
- Walts AE, Sacks WL, Wu HH, et al. A retrospective analysis of the performance of the RosettaGX® RevealTM thyroid miRNA and the Afirma Gene Expression Classifiers in a cohort of cytologically indeterminate thyroid nodules. Diagn Cytopathol 2018;46:901-7. [Crossref] [PubMed]
- Samulski TD. Usage trends and performance characteristics of a ‘gene expression classifier’ in the management of thyroid nodules: An institutional experience. Diagn Cytopathol 2016;44:867-73. [Crossref] [PubMed]
- Hang JF, Westra WH, Cooper DS, et al. The impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on the performance of the Afirma gene expression classifier. Cancer Cytopathol 2017;125:683-91. [Crossref] [PubMed]
- Harrell RM, Eyerly-Webb SA, Golding AC, et al. Statistical comparison of Afirma GSC and Afirma GEC outcomes in a community endocrine surgical practice: early findings. Endocr Pract 2019;25:161-4. [Crossref] [PubMed]
- Pool C, Walter V, Bann D, et al. Molecular characterization of tumors meeting diagnostic criteria for the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Virchows Arch 2019;474:341-51. [Crossref] [PubMed]


