
Seeding recurrence of follicular thyroid carcinoma after transoral endoscopic thyroidectomy vestibular approach: a case report
Highlight box
Key findings
• This study reports the first documented case of thyroid cancer recurrence along the surgical track following a transoral endoscopic thyroidectomy vestibular approach (TOETVA).
What is known and what is new?
• Track seeding recurrence after thyroidectomy, which involves the regrowth of thyroid tissue or cancer cells along the surgical dissection site has been previously documented for remote access approaches, but no such cases have been reported for TOETVA.
• Seeding recurrence of thyroid cancer along the surgical track after TOETVA can also occur.
What is the implication, and what should change now?
• This case report highlights the importance of preventing thyroid gland rupture during TOETVA. In the event of such rupture, vigilant postoperative monitoring is essential to promptly identify any clinical signs of thyroid cancer recurrence.
• Surgeons should be aware of this uncommon complication and provide informed guidance to patients, including recommendations for medical strategies to manage potential local seeding of thyroid tissue.
Introduction
Minimally invasive and remote access thyroid surgeries have become increasingly popular among thyroid cancer patients seeking improved cosmetic outcomes on top of oncologic outcomes (1). Remote access thyroidectomies can be performed via endoscopic or robotic approaches, including bilateral axillo breast, transaxillary, and transoral techniques (2-4). While these procedures offer several benefits over traditional open surgery, rare complications can still arise (5). One such complication is track seeding recurrence, which involves the regrowth of thyroid tissue or cancer cells along the surgical dissection site (6-8).
Seeding recurrence after thyroidectomy has been previously documented for remote access approaches, particularly in endoscopic and robotic transaxillary and bilateral axillo-breast approach surgeries (9-11). Reports included both malignant and benign implantations along the surgical track, and were treated with surgical re-exploration for excision of thyroid tissue and/or radioactive iodine (RAI) therapy (12,13).
To the best of our knowledge, there have been no previous reports of seeding recurrence in patients undergoing transoral endoscopic thyroidectomy vestibular approach (TOETVA). We share our experience with the first reported case of recurrence of thyroid cancer along the surgical track after TOETVA. We present this case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-480/rc).
Case presentation
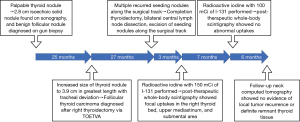
A 19-year-old female presented with a palpable lump on the right side of her neck, without any other significant symptoms. Medical, family, and psycho-social histories were insignificant. Thyroid function tests were normal, and the tests for thyroglobulin (Tg) and thyroperoxidase antibodies were negative. Ultrasonography (USG) revealed a Thyroid Imaging Reporting and Data System 3 isoechoic solid nodule without marked vascularity, measuring 2.8 cm in the right lower thyroid. In January 2018, fine needle aspiration (FNA) cytology of the lesion showed as atypia of undetermined significance, and in February 2018, repeat gun biopsy reported the lesion as a benign follicular nodule. During follow up in the outpatient clinic, the nodule increased in size to 3.9 cm in greatest length on USG and computed tomography (CT) in February 2020 (Figure 1). After inter-department consultation, lobectomy was recommended because of the prominence of the nodule and its potential for malignancy. In the same month, the patient underwent a TOETVA to perform a right thyroidectomy. During surgery, part of the thyroid capsule ruptured, but all visible thyroid tissue was meticulously suctioned without distilled water irrigation. The specimen was inserted into a plastic specimen bag and pulled out through the middle port incision of the lower vestibule. Histopathology of the right thyroid gland revealed one minimally invasive follicular thyroid carcinoma (FTC) measuring 3.0 cm in greatest diameter with minimal capsular invasion and minimal venous invasion, and one papillary microcarcinoma measuring 0.4 cm in greatest diameter. The postoperative course was uneventful with normal laboratory findings and vocal cord examination results.
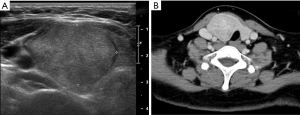
The patient was followed up in the outpatient clinic with USG every 6 months. In June 2022, which was 27 months after surgery, USG and CT showed multiple nodules in various areas including the postsurgical thyroid bed, and subcutaneous layers of the right lower lip, submental area, and mid to right upper neck levels I, IIA, and VI, and a 0.4 cm hypoechoic nodule in the left thyroid gland (Figure 2). FNA cytology of a soft tissue lesion at neck level IIA showed many bland-looking follicular cells. Re-review of the gun biopsy of the initial lesion was conducted by an experienced endocrine pathologist (Y.A.K.), and the gun biopsy revealed an indeterminate microfollicular proliferative lesion lacking a fibrous capsule or the adjacent non-lesional tissue in the specimen.

In June 2022, completion thyroidectomy of the left thyroid, bilateral central lymph node dissection and excision of multiple nodules, as well as parts of the adjacent strap muscles, were performed through a new low-collar incision on the neck and a small incision in the lower lip. Histopathology confirmed metastatic FTC in the soft tissues in the lower lip, strap muscles, and neck levels I, IIA, and VI, with a combined measurement of all tumor foci up to 4.1 cm in greatest diameter. Papillary microcarcinoma measuring 0.6 cm in greatest diameter was also found on the left thyroid gland. No tumor involvement was observed in the 25 lymph nodes that were resected. Due to the challenges encountered in accessing the upper neck and chin recurrences through the incisions, a neck CT scan was conducted on the second postoperative day. The scan aimed to assess the extent of remaining nodules, revealing their presence in the subcutaneous tissue of the upper neck and chin, prompting consideration for a second-look operation. A neck re-exploration through a submental incision was performed two weeks later to remove the nodules. On biopsy, the soft tissue lesions at neck levels I and VI were confirmed to be metastatic FTC, with the largest nodule measuring 1.5 cm in greatest length. The postoperative course was uneventful, and the patient was prescribed a daily dose of 100 mcg of levothyroxine.
In September 2022, the patient received RAI with 150 mCi of I-131 following the withdrawal of thyroxine. The patient’s thyrotropin (TSH) level was 105.4 mIU/L, Tg level was 59.04 ng/mL, and Tg antibody (Tg-Ab) level was 493 u/mL. Post-therapeutic whole-body scintigraphy (WBS) revealed focal uptakes in the right thyroid bed, upper mediastinum, and submental area. In April 2023, the patient received a second RAI with 100 mCi of I-131. The TSH level was 154.5 uIU/L, Tg level was <0.2 ng/mL, and Tg-Ab level was 91 U/mL. Post-therapeutic WBS showed no abnormal uptake (Figure 3). In October 2023, a follow-up neck CT scan showed no evidence of local tumor recurrence or definite remnant thyroid tissue (Figure 4). Figure 5 shows a brief timeline of key events in chronological order.


All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Discussion
TOETVA has emerged as a promising technique for thyroid surgery due to its minimal scarring and comparable postoperative outcomes to open thyroidectomy (14). However, as with any surgical procedure, there are unique complications associated with TOETVA that should be carefully considered, such as mental nerve injury (15). This case report highlights another rare but significant complication of TOETVA—seeding recurrence. Seeding recurrence after TOETVA has not been previously reported in literature, likely due to the relative novelty of the procedure and the limited number of centers that perform large number of TOETVA procedures (16). While TOETVA has shown promise as a minimally invasive alternative to conventional open thyroidectomy, studies on the long-term outcomes and complications are still limited (17). Recurrence of thyroid cancer is also possible even many years after surgery, underscoring the need for careful long-term monitoring of patients who undergo TOETVA or any thyroid surgery (18). Seeding recurrence after TOETVA can be challenging because the surgical incision is located in the vestibule of the oral cavity and the surgical track down passed the submandibular area, which can make it difficult to detect recurrence using follow-up ultrasound.
Thus, preoperative diagnosis of thyroid nodules is crucial in determining the appropriate surgical approach for their treatment (19). However, in the case of FTC, preoperative diagnosis can be particularly challenging because it shares many characteristics with benign thyroid nodules (20). Although a preoperative FNAC indicates a benign result, there is still a possibility of malignancy, including FTC or follicular variant papillary thyroid carcinoma (21). The risk of malignancy for benign cytology results can range from 2% to 7%, particularly in cases where the nodule is larger in size (21,22). Likewise, the present case illustrates an example where a preoperative biopsy suggested the presence of a benign follicular nodule, but the final histopathology report confirmed the diagnosis of FTC. Seeding recurrence is possible not only in malignant nodules, but can also occur in benign disease or thyroidal tissue fragments, particularly if it ruptures during surgery (23,24). Therefore, it is important that the surgeon handle the thyroid gland with caution during endoscopic procedures to preserve the thyroid capsule and ensure complete and uniform removal of the specimen in all cases (25). If rupture occurs, all fragments should be harvested. Regardless of the postoperative pathology results, close monitoring is necessary, especially in the case of malignancy.
Currently, there is no established guideline specifying the upper limit of thyroid nodule size for safe consideration of TOETVA. Generally, TOETVA is indicated for benign diseases, papillary microcarcinoma, and thyroid nodules smaller than 6 cm (26) with some surgeons suggesting a threshold of less than 4 cm (4). In this particular case, despite the relatively large size of the nodule, almost 4 cm, we chose TOETVA based on the benign preoperative biopsy results, the patient’s age, and the patient’s preference for this procedure. Although a large nodule size may not be a contraindication for TOETVA, surgeon judgment and expertise are crucial when dealing with larger nodule sizes.
Laparoscopic surgeries for other common cancers such as colon, ovaries, adrenal glands, and prostate cancers have reported seeding recurrence, such as port-site seeding and recurrence (27,28). The chances of direct tumor spillage during endoscopic thyroid surgery are high because of the friability of the thyroid tissue and the small working space available during surgery (29). The exact way in which tissue or tumor spreads at the point of trocar insertion during laparoscopic surgery is not yet known, but it is believed to be influenced by several factors. These may include the level of manipulation of the tumor during the procedure, the use of tumor morcellation to assist with extraction, the technique used for specimen removal, the use of gas insufflation and the possibility of a “chimney effect” (9,30).
In this present case, despite meticulous dissection and efforts to avoid trauma, the thyroid capsule was partially ruptured. There is a risk of non-visible seeding leading to prominent recurrences, even if all visible thyroid tissue is removed during surgery. Using a specimen bag to retrieve the resected specimen is a common preventative measure to minimize iatrogenic implantation, regardless of the type of disease (9). However, multifocal implantation along the surgical track still occurred in this present case even after removing all visible tissue fragments and using a plastic specimen bag during specimen retrieval, possibly due to cell exfoliation caused by repeated minor trauma from the endoscopic instruments and contact with the surgical track after the capsule rupture. While rinsing the surgical field with distilled water has been suggested to allow exfoliated cells to swell and rupture in a hypo-osmolar environment before concluding the procedure (13), further research is necessary before it can be recommended as a standard practice. Moreover, surgeons should also consider converting to an open approach in situations involving tumor spillage or an inability to fully retrieve it, even when dealing with a benign preoperative diagnosis.
RAI therapy is a viable option for managing recurrent or residual disease in patients with differentiated thyroid cancer, as it specifically targets thyroid tissue and can help eliminate remnant thyroid tissue and identify recurrence (19). While the effectiveness of adjuvant RAI therapy after reoperation in patients with recurrent differentiated thyroid cancer remains a topic of debate, various organizations continue to recommend its use in certain patients (31), because RAI therapy has been known to be effective especially in well-differentiated thyroid cancers (32). In this present case, the patient received RAI therapy after reoperation and achieved excellent outcomes. This shows that RAI therapy can be a valuable option for managing seeding recurrence following TOETVA in well-differentiated thyroid cancer cases and may improve patient outcomes in certain patients.
Conclusions
This case report highlights the importance of avoiding rupture of the thyroid gland during TOETVA. However, if the thyroid gland ruptures during the surgical procedure, close and careful monitoring after TOETVA is necessary to detect any clinical suspicion of thyroid cancer recurrence. Surgeons need to be conscious of this atypical complication and it should be addressed as a potential complication of TOETVA when obtaining informed consent before operation. Surgeons must be prepared to counsel patients appropriately, including the recommendation of medical strategies to manage any local seeding of thyroid tissue that may occur. Continued research is needed to better understand the risk factors for seeding recurrence after TOETVA and to develop strategies for preventing this rare but significant complication.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-480/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-480/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-480/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim DH, Kim SW, Kim GJ, et al. Efficacy and Safety of Minimally Invasive Thyroid Surgery: A Network Meta-Analysis. Laryngoscope 2023;133:2470-9. [Crossref] [PubMed]
- Lee KE, Choi JY, Youn YK. Bilateral axillo-breast approach robotic thyroidectomy. Surg Laparosc Endosc Percutan Tech 2011;21:230-6. [Crossref] [PubMed]
- Kim K, Kang SW, Kim JK, et al. Robotic Transaxillary Hemithyroidectomy Using the da Vinci SP Robotic System: Initial Experience With 10 Consecutive Cases. Surg Innov 2020;27:256-64. [Crossref] [PubMed]
- Chai YJ, Chae S, Oh MY, et al. Transoral Endoscopic Thyroidectomy Vestibular Approach (TOETVA): Surgical Outcomes and Learning Curve. J Clin Med 2021;10:863. [Crossref] [PubMed]
- de Vries LH, Aykan D, Lodewijk L, et al. Outcomes of Minimally Invasive Thyroid Surgery - A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2021;12:719397. [Crossref] [PubMed]
- Bakkar S, Frustaci G, Papini P, et al. Track Recurrence After Robotic Transaxillary Thyroidectomy: A Case Report Highlighting the Importance of Controlled Surgical Indications and Addressing Unprecedented Complications. Thyroid 2016;26:559-61. [Crossref] [PubMed]
- Tamiolakis D, Antoniou C, Venizelos J, et al. Papillary thyroid carcinoma metastasis most probably due to fine needle aspiration biopsy. A case report. Acta Dermatovenerol Alp Pannonica Adriat 2006;15:169-72.
- Uchida N, Suda T, Inoue T, et al. Needle track dissemination of follicular thyroid carcinoma following fine-needle aspiration biopsy: report of a case. Surg Today 2007;37:34-7. [Crossref] [PubMed]
- Beninato T, Kleiman DA, Scognamiglio T, et al. Tract recurrence of a follicular thyroid neoplasm following transaxillary endoscopic thyroidectomy. Thyroid 2012;22:214-7. [Crossref] [PubMed]
- Espiard S, Petyt G, Lion G, et al. Ectopic Subcutaneous Implantation of Thyroid Tissue After Gasless Transaxillary Robotic Thyroidectomy for Papillary Thyroid Cancer. Thyroid 2015;25:1381-2. [Crossref] [PubMed]
- Jung JS KS, Jung HY, Han SW, et al. Simultaneous Seeding of Follicular Thyroid Adenoma Both Around the Operative Bed and Along the Subcutaneous Tunnel of the Upper Chest Wall after Endoscopic Thyroidectomy. J Korean Soc Radiol 2017;76:138-41.
- Chabrillac E, Zerdoud S, Fontaine S, et al. Multifocal recurrence on the transaxillary robotic thyroidectomy incision. Eur Ann Otorhinolaryngol Head Neck Dis 2020;137:59-60. [Crossref] [PubMed]
- Fregoli L, Bakkar S, Papini P, et al. First report of benign track seeding after robot-assisted transaxillary thyroid surgery. Am J Otolaryngol 2021;42:102811. [Crossref] [PubMed]
- Lee MJ, Oh MY, Lee JM, et al. Comparative surgical outcomes of transoral endoscopic and robotic thyroidectomy for thyroid carcinoma: a propensity score-matched analysis. Surg Endosc 2023;37:1132-9. [Crossref] [PubMed]
- Zhang D, Famá F, Caruso E, et al. How to Avoid and Manage Mental Nerve Injury in Transoral Thyroidectomy. Surg Technol Int 2019;35:101-6.
- Fernandez-Ranvier G, Meknat A, Guevara D, et al. Transoral Endoscopic Thyroidectomy Vestibular Approach: A Single-institution Experience of the First 50 Cases. Surg Innov 2020;27:439-44. [Crossref] [PubMed]
- Nguyen HX, Nguyen HX, Nguyen TTP, et al. Transoral endoscopic thyroidectomy by vestibular approach in Viet Nam: surgical outcomes and long-term follow-up. Surg Endosc 2022;36:4248-54. [Crossref] [PubMed]
- Hwangbo Y, Kim JM, Park YJ, et al. Long-Term Recurrence of Small Papillary Thyroid Cancer and Its Risk Factors in a Korean Multicenter Study. J Clin Endocrinol Metab 2017;102:625-33. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Lloret J, Ganado T, Subhi I, et al. An attempt to reduce unnecessary surgical procedures... Can ultrasound characteristics help in differentiating adenoma vs carcinoma in follicular thyroid neoplasms? Radiologia (Engl Ed) 2023;65:22-31. [Crossref] [PubMed]
- Chai YJ, Suh H, Yi JW, et al. Factors associated with the sensitivity of fine-needle aspiration cytology for the diagnosis of follicular variant papillary thyroid carcinoma. Head Neck 2016;38:E1467-71. [Crossref] [PubMed]
- Ali SZ, Baloch ZW, Cochand-Priollet B, et al. The 2023 Bethesda System for reporting thyroid cytopathology. J Am Soc Cytopathol 2023;12:319-25. [Crossref] [PubMed]
- Kim HS, Kim SH, Kim JH, et al. Multifocal hot spots demonstrated by whole-body 131I scintigraphy and SPECT/CT after transaxillary endoscopic thyroidectomy. Clin Nucl Med 2015;40:260-2. [Crossref] [PubMed]
- Huang EYF, Kao NH, Lin SY, et al. Concordance of the ACR TI-RADS Classification With Bethesda Scoring and Histopathology Risk Stratification of Thyroid Nodules. JAMA Netw Open 2023;6:e2331612. [Crossref] [PubMed]
- Anuwong A, Kim HY, Dionigi G. Transoral endoscopic thyroidectomy using vestibular approach: updates and evidences. Gland Surg 2017;6:277-84. [Crossref] [PubMed]
- Anuwong A, Sasanakietkul T, Jitpratoom P, et al. Transoral endoscopic thyroidectomy vestibular approach (TOETVA): indications, techniques and results. Surg Endosc 2018;32:456-65. [Crossref] [PubMed]
- Eng MK, Katz MH, Bernstein AJ, et al. Laparoscopic port-site metastasis in urologic surgery. J Endourol 2008;22:1581-5. [Crossref] [PubMed]
- Kuhry E, Schwenk WF, Gaupset R, et al. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev 2008;2008:CD003432. [Crossref] [PubMed]
- Kim MR, Jo S, Shim HK. Port-Site Implantation Diagnosed by Iodine-131 Post-Ablation Single-Photon Emission Tomography-Computed Tomography After Robotic Thyroidectomy: A Case Report. Am J Case Rep 2019;20:1695-8. [Crossref] [PubMed]
- Greco F, Wagner S, Reichelt O, et al. Huge isolated port-site recurrence after laparoscopic partial nephrectomy: a case report. Eur Urol 2009;56:737-9. [Crossref] [PubMed]
- Raghupathy J, Tan BKJ, Song HJJMD, et al. The efficacy of adjuvant radioactive iodine after reoperation in patients with persistent or recurrent differentiated thyroid cancer: a systematic review. Langenbecks Arch Surg 2023;408:21. [Crossref] [PubMed]
- Lin JD, Kuo SF, Huang BY, et al. The efficacy of radioactive iodine for the treatment of well-differentiated thyroid cancer with distant metastasis. Nucl Med Commun 2018;39:1091-6. [Crossref] [PubMed]


