
Risk factors and nomogram to predict skip metastasis in papillary thyroid carcinoma
Highlight box
Key findings
• This study found that the risk factors of skip metastasis in papillary thyroid carcinoma (PTC) were the maximum diameter (D) ≤10 mm, location in the upper portion and capsule invasion. The area under the curve of nomogram was 0.877, the accuracy was 85.32%, the sensitivity was 60.98%, and the specificity was 90.96%.
What is known and what is new?
• Skip metastasis in PTC is easily misdiagnosed before surgery, and it could lead to re-operation and affect the prognosis. The preoperative prediction of skip metastasis is very important for clinical management.
• Although there are a few studies about nomograms for predicting central lymph node metastases or lateral lymph node metastases of PTCs, there are few studies about nomograms for skip metastases. In this study, a nomogram was established to predict the risk of skip metastasis in PTC according to the clinical and ultrasonographic characteristics.
What is the implication, and what should change now?
• When PTC is located at the upper portion of thyroid, D ≤10 mm with capsule invasion, clinicians should be vigilant about the possibility of skip metastasis of cervical lymph nodes. The nomogram may be helpful to provide reference to clinical treatment decision-making.
Introduction
The incidence of thyroid carcinoma has been increasing in these years. Papillary thyroid carcinoma (PTC) is the most common pathological type while the rate of cervical lymph node metastasis is 12–81% (1,2). The metastatic pathway of PTC is generally continuous: it firstly invades the central lymph node (CLN), then the ipsilateral lateral lymph node (LLN), and finally involves the contralateral LLN and mediastinal lymph node (3,4). Skip metastases are defined as metastases in the LLN without CLN and the incidence of skip metastases is 6.5–27.5% (5). The 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer recommended therapeutic lateral lymph node dissection (LLND) only for patients with biopsy-proven lateral cervical lymph node metastases (6). When intraoperative pathology indicates negative of CLN, further LLND will not be performed unless preoperative ultrasound and fine needle aspiration biopsy (FNAB) indicate lateral lymph node metastases (LLNM). However, preoperative assessment of LLNM has a certain false negative rate, and its accuracy largely depends on the experience of pathologists and ultrasound doctors (7). Misdiagnosis of skip metastasis of PTCs before surgery will mean insufficient lymph node dissection during surgery, which could lead to re-operation and affect the prognosis. Therefore, the preoperative prediction of skip metastasis is very important for clinical management.
The nomogram models using regression analysis are frequently used in the study of tumor prognosis (8,9). These models which are based on multivariable analysis and integrate the results of logistic or Cox regression could predict the probability of clinical events by providing graphical presentations (8). Compared with traditional evaluation methods, nomogram model could produce more accurate and intuitive predictions. Although there are a few studies about nomograms for predicting CLN metastases (CLNM) or LLNM of PTCs (10,11), there are few studies about nomograms for skip metastases. The aim of our study is to establish a nomogram model according to the clinical and ultrasonographic characteristics of skip metastases in PTCs and evaluate its predictive value. We present this article in accordance with the TRIPOD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-376/rc).
Methods
Patients
This retrospective study was approved by the Ethics Committee of Ruijin Hospital Luwan Branch, Shanghai Jiao Tong University School of Medicine (Approval code: 2022-006), individual consent for this retrospective analysis was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The clinical and ultrasonographic characteristics of 311 patients with PTCs in Ruijin Hospital Luwan Branch from January 2017 to December 2021 were retrospectively analyzed.
Patients who met all of the following inclusion criteria were enrolled: (I) patients who underwent ultrasonography within 2 weeks before the first thyroid surgery; (II) PTC with LLNM was confirmed by postoperative pathology; (III) patients who did not receive radiotherapy and chemotherapy before ultrasound examination; (IV) thyroid function was tested in the patients. The exclusion criteria were as follows: (I) PTC with CLNM only; (II) patients who had the history of thyroid surgery; (III) the quality of image was poor or the clinical data were incomplete; (IV) patient with distant metastasis or other primary malignant tumor. After screening, a total of 218 eligible patients were enrolled.
Instruments and methods
All ultrasound examinations were performed by a real-time ultrasound instrument (Aplio500, Toshiba), equipped with a 5–14 MHz transducer. The patients were asked to lie on the back with the neck fully exposed. The ultrasonographic characteristics were carefully evaluated, including Hashimoto’s thyroiditis (yes or no), multifocality (yes or no), tumor location (upper, middle, lower or isthmus), the maximum diameter (D) of tumor (D ≤10 or >10 mm), shape (irregular or regular), margin (ill-defined or well-defined), anteroposterior diameter/transverse diameter (A/T) ratio (A/T <1 or A/T ≥1), microcalcification (yes or no), intra-nodular vascularity (yes or no) and capsule invasion which meant that the shortest distance from the tumor boundary to the thyroid capsule or trachea was 0 mm (yes or no) (12).
The ultrasound images of each patient were retrospectively reviewed by two experienced radiologists with more than ten years of experience in thyroid ultrasound imaging who were blinded to the patients’ clinical histories and pathological diagnoses. If there were different opinions, the radiologists would consult the superior radiologist and reach an agreement.
Surgical techniques
All patients were operated for the first time. CLN + LLN dissection were performed according to the pathological results of FNAB. If LNM was confirmed by FNAB, the range of dissection was from the upper boundary to the sublingual nerve, from the lower boundary to the subclavian vein and from the lateral boundary to the anterior edge of the trapezius muscle.
According to the post-operative pathological results, the patients were divided into skip-positive group and skip-negative group for further analysis.
Statistical analysis
Statistical analyses were conducted by SPSS for Windows version 25.0 (SPSS Inc., Chicago, IL, USA). Chi-squared test or Fisher exact test was used for categorical variables, and binary logistic regression analysis was used for multivariate analysis. When P<0.05, the difference was statistically significant. The nomogram was established by using R Studio software (version 4.1.0). The ROC curve was used to evaluate its effectiveness. The calibration curve and the Hosmer-Lemeshow goodness of fit test (P>0.05) were used to analyze the consistency between the nomogram and the actual observation. The decision curve analysis (DCA) quantifying the standardized net benefit at different threshold probabilities was used to evaluate the clinical utility of the nomogram.
Results
Pathological results
There were 82 males and 136 females, with an average age of 42.38±13.04 years (range, 17–78 years). There were 41 cases in the skip-positive group, including 8 cases involving single region, 14 cases involving two regions, 18 cases involving three regions, and 1 case involving four regions, and there were 177 cases in the skip-negative group. The characteristics of our cohort are summarized in Table 1.
Table 1
| Variable | Value |
|---|---|
| Mean age, years | 42.38 |
| Gender | |
| Male | 82 (37.6) |
| Female | 136 (62.4) |
| Skip-positive group | 41 (18.8) |
| Single region (n=8) | |
| III | 5 |
| IV | 3 |
| Two regions (n=14) | |
| II + III | 5 |
| III + IV | 9 |
| Three regions (n=18) | |
| II + III + IV | 16 |
| III + IV + V | 1 |
| II + IV + V | 1 |
| Four regions (n=1) | |
| II + III + IV + V | 1 |
| Skip-negative group | 177 (81.2) |
Values are presented as n or n (%).
Univariate analysis of clinical and ultrasonographic characteristics between skip-positive group and skip-negative group
The univariate analysis results of clinical and ultrasonographic characteristics are listed in Table 2. There were statistical differences in tumor location, D and capsule invasion between skip-positive group and skip-negative group (P<0.05). No statistical differences were observed in sex, age, Hashimoto’s thyroiditis, multifocality, A/T ratio, shape, margin, microcalcification, intra-nodular vascularity and preoperative serum thyroglobulin (Tg) (P>0.05) (Table 2).
Table 2
| Clinical and ultrasonographic characteristics | Skip-positive group | Skip-negative group | P value | χ2 |
|---|---|---|---|---|
| Sex | 0.611 | 0.259 | ||
| Male | 14 | 68 | ||
| Female | 27 | 109 | ||
| Age (years) | 0.615 | 0.974 | ||
| <45 | 23 | 109 | ||
| 45–55 | 12 | 39 | ||
| >55 | 6 | 29 | ||
| Hashimoto’s thyroiditis | 1.000 | 0.000 | ||
| Yes | 4 | 16 | ||
| No | 37 | 161 | ||
| Multifocality | 0.407 | 0.687 | ||
| Yes | 21 | 78 | ||
| No | 20 | 99 | ||
| Location | <0.001 | 41.137 | ||
| Isthmus | 3 | 8 | ||
| Upper | 30 | 39 | ||
| Middle | 6 | 95 | ||
| Lower | 2 | 35 | ||
| A/T ratio | 0.215 | 1.538 | ||
| <1 | 31 | 116 | ||
| ≥1 | 10 | 61 | ||
| Shape | 0.591 | 0.289 | ||
| Irregular | 26 | 120 | ||
| Regular | 15 | 57 | ||
| Margin | 0.677 | 0.173 | ||
| Ill-defined | 26 | 106 | ||
| Well-defined | 15 | 71 | ||
| Microcalcification | 0.31 | 1.031 | ||
| Yes | 31 | 146 | ||
| No | 10 | 31 | ||
| D (mm) | <0.001 | 16.43 | ||
| ≤10 | 20 | 33 | ||
| >10 | 21 | 144 | ||
| Capsule invasion | <0.001 | 21.899 | ||
| Yes | 34 | 75 | ||
| No | 7 | 102 | ||
| Intra-nodular vascularity | 0.375 | 0.786 | ||
| Yes | 9 | 51 | ||
| No | 32 | 126 | ||
| Tg (ng/mL) | 0.135 | – | ||
| ≤77 | 41 | 164 | ||
| >77 | 0 | 13 |
A/T, anteroposterior diameter/transverse diameter; D, the maximum diameter; Tg, thyroglobulin.
Multivariate analysis between skip-positive group and skip-negative group
The multivariate analysis results are listed in Table 3. The upper portion [odd ratio (OR) =3.113, 95% confidence interval (CI): 0.672–14.417], D ≤10 mm (OR =3.84, 95% CI: 1.572–9.378) and capsule invasion (OR =8.07, 95% CI: 2.983–21.835) were independent risk factors for skip metastases (P<0.05) (Figure 1).
Table 3
| Variable | P value | OR | 95% CI (lower) | 95% CI (upper) |
|---|---|---|---|---|
| Location | <0.001 | |||
| Isthmus as reference | ||||
| Upper | 0.146 | 3.113 | 0.672 | 14.417 |
| Middle | 0.115 | 0.263 | 0.05 | 1.383 |
| Lower | 0.127 | 0.204 | 0.027 | 1.571 |
| Capsule invasion | <0.001 | 8.07 | 2.983 | 21.835 |
| D | 0.003 | 3.84 | 1.572 | 9.378 |
OR, odds ratio; CI, confidence interval; D, the maximum diameter.
Nomogram for predicting skip metastases of cervical lymph nodes in PTCs
The nomogram was established according to the results of multivariate logistic regression analysis (Figure 2). Each risk factor could be scored. When PTC was located in the upper portion, it was 100 points, 9 points in the middle portion, 0 points in the lower portion and 58 points in the isthmus; 49 points when D ≤10 mm and 0 point when D >10 mm; 77 points for capsule invasion and 0 point for PTC without capsule invasion. The total points (line 5) could be obtained by summing the points of risk factors. The total points of each patient could generate the risk probability of skip metastasis on the corresponding risk axis (line 6) (Tables 4,5).
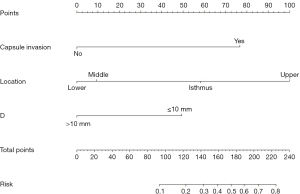
Table 4
| Risk factor | Classification | Points |
|---|---|---|
| Location | Upper | 100 |
| Middle | 9 | |
| Lower | 0 | |
| Isthmus | 58 | |
| D (mm) | ≤10 | 49 |
| >10 | 0 | |
| Capsule invasion | Yes | 77 |
| No | 0 |
D, the maximum diameter.
Table 5
| Total points | Risk |
|---|---|
| 93 | 0.1 |
| 123 | 0.2 |
| 143 | 0.3 |
| 159 | 0.4 |
| 174 | 0.5 |
| 189 | 0.6 |
| 205 | 0.7 |
| 225 | 0.8 |
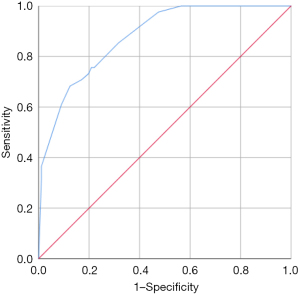
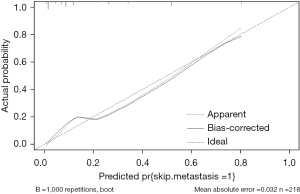
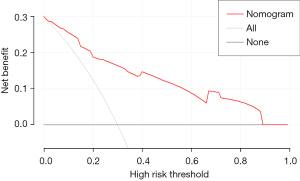
The area under curve (AUC) of the model was 0.877, and the accuracy, sensitivity and specificity were 85.32%, 60.98%, and 90.96%, respectively (Figure 3). The internal calibration plot showed that the predicted results of nomogram were basically consistent with the actual results (Figure 4). The Hosmer-Lemeshow goodness of fit test also showed the good consistency between the predicted and actual results (χ2=2.267, df=5, P=0.811). The DCA was used to assess the net benefit of nomogram-assisted decisions at different threshold probabilities. In the DCA, the nomogram achieved a greater net benefit than having all patients or none patients with a range of the threshold probability ranged from 0.02 to 0.88 (Figure 5). This result showed that the use of nomogram to predict skip metastasis in PTC patients would improve the detection rate of skip metastases and patients might benefit from this model.
Discussion
The 2015 American Thyroid Association (ATA) guidelines recommended therapeutic LLND only for patients with biopsy-proven LLNM, and the application of prophylactic central-compartment neck dissection (ipsilateral or bilateral) should be careful (6). However, the incidence of skip metastasis in PTC is about 6.5–27.5% (5). Skip metastasis is closely associated to local recurrence and prognosis (13). Re-operation may increase the risk of surgical complications, including recurrent laryngeal nerve injury. Therefore, even if no obvious metastatic lymph nodes are found in the central compartment, the LLN should be carefully evaluated to avoid misdiagnosis of skip metastasis. In order to screen high-risk patients who may have skip metastasis before surgery, we established a predictive model of skip metastasis. Nomogram can simply and intuitively reflect the relationship between variables in multivariate analysis, and predict the occurrence and prognosis of disease through scoring standards (14-16). This study analyzed the clinical and ultrasonic characteristics of PTCs with skip metastases, and the risk factors were screened out in order to establish the predictive nomogram model.
Univariate and multivariate logistic regression analyses between skip-positive group and skip-negative group showed that PTCs located in the upper portion, D ≤10 mm with capsule invasion were more prone to skip metastases (P<0.05). When the tumor is located in the upper portion of thyroid, there might be an independent lymphatic drainage pathway. The lymphatic drainage of the upper pole of the thyroid may follow the upper pole vessels, which directly drains to the lateral region and into the deep vein without passing through the central region (17,18). The study of lymphatic anatomy reported by Likhterov et al. (19) also showed the exclusive lymphatic pathway of the upper portion of the thyroid gland. Therefore, the LLN might be the first lymphatic drainage station for upper portion tumor, and it is more prone to skip metastasis. Understanding the pathway of disease spread can provide a better understanding of the reasons for skip metastasis.
Previous studies suggested that skip metastasis was more common in small PTCs such as microcarcinomas, which were consistent with our findings (20,21). Our results showed that D ≤10 mm was a risk factor for skip metastases of PTCs. Zhang et al. reported that tumor size was significantly associated with lymph node metastasis (22), and the frequency of CLNM is known to increase with tumor size in PTC (23). Compared with small nodules (D ≤10 mm), large nodules are more invasive, and a wider range of lymph nodes may be involved, which could lead to a higher risk of concurrent CLNM and LLNM (24). Therefore, skip metastasis is less common in large PTC. For the small PTC, it is more likely that only LLN at the first lymphatic drainage station may be involved due to the independent lymphatic drainage pathway.
Capsule invasion is an important risk factor affecting cervical lymph node metastasis. There are abundant blood vessels and lymphatic vessels around the thyroid capsule and between the inner and outer capsule. When the tumor invades the capsule, the barrier effect of the capsule is damaged. The tumor invades the adjacent neck lymph nodes through blood vessels and lymphatic vessels, and even distant metastasis occurs (25). According to the previous studies (17,26), capsule invasion was also considered as a predictive factor of skip metastasis in PTC patients. In addition, the upper pole of thyroid tissue is relatively thin due to the butterfly shape of the thyroid gland. Therefore, even if the tumor is small, it can easily invade the capsule and even extend to the sternothyroid muscle or perithyroid soft tissue if it is located at the upper pole. Our findings showed that PTCs with capsule invasion in the upper pole might have a higher risk of skip metastasis.
Some studies (27,28) showed the important role of serum Tg in predicting node metastasis while the others (7,29) showed that there was weak correlation between Tg and metastatic disease. There is still controversy over whether preoperative serum Tg can predict lymph node metastasis in PTCs. In our study, there was no statistical difference in serum Tg between skip-positive group and skip-negative group.
Nomogram’s theory was put forward by French engineer Philbert Maurice d’Ocagne (1862–1938) in 1884 (30). It was first used in engineering. Nomogram is an effective model which transforms the complex regression equation into a visual graph. By integrating different prognosis and determinant variables, nomogram can generate the probability of clinical events and calculate the survival rate of tumor patients (30-32), so it has been widely applied in medical research. Compared with other prediction models, the prediction of nomograms is more accurate and straightforward. Previous studies have used nomogram model to predict CLNM or LLNM in PTCs and achieved good results (10,11). Wang et al. reported that the nomogram based on the five variables (age, gender, focal, BRAF and tumor size) could predict the CLNM in PTCs and the ROC curve showed high efficiency (11).In this study, we selected the risk factors for predicting skip metastases in PTCs patients by multivariate logistic regression analysis. Nomogram could predict skip metastases in PTCs by weighting the points of the risk factors. The larger points in the nomogram indicated higher risk of skip metastasis. We added the points to obtain the total points. Through the function conversion relationship between the total points and the risk of skip metastasis, the predicted value of skip metastasis in PTC patients can be calculated. For example, when PTC was located at the upper portion of thyroid, D ≤10 mm with capsule invasion, the corresponding points of risk factors were 100, 49 and 77, respectively. The total point of nomogram was 226, suggesting that the risk probability of skip metastasis of PTC cervical lymph nodes would exceed 80%. The AUC of the nomogram established in our study was 0.877. The accuracy, sensitivity and specificity were 85.32%, 60.98%, and 90.96%, respectively. The internal calibration plot and the Hosmer-Lemeshow goodness showed that the predicted results of nomogram were basically consistent with the actual results. Analysis of the decision curve showed that most PTC patients may benefit from the predictive nomogram model.This study had some limitations. First, because it was a retrospective single center study, there might be deviations in the selection of patients and the input of information. Second, the sample size was small, especially in the skip-positive group. All these need to be further studied by a large sample.
Conclusions
When PTC is located at the upper portion of thyroid, D ≤10 mm with capsule invasion, we should be vigilant about the possibility of skip metastasis of cervical lymph nodes. The nomogram may be helpful to provide reference to clinical treatment decision-making.
Acknowledgments
Funding: This project was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-376/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-376/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-376/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-376/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Ethics Committee of Ruijin Hospital Luwan Branch, Shanghai Jiao Tong University School of Medicine (Approval code: 2022-006), individual consent for this retrospective analysis was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pavlidis ET, Pavlidis TE. Role of prophylactic central neck lymph node dissection for papillary thyroid carcinoma in the era of de-escalation. World J Clin Oncol 2023;14:247-58. [Crossref] [PubMed]
- Zhang K, Qian L, Chen J, et al. Preoperative Prediction of Central Cervical Lymph Node Metastasis in Fine-Needle Aspiration Reporting Suspicious Papillary Thyroid Cancer or Papillary Thyroid Cancer Without Lateral Neck Metastasis. Front Oncol 2022;12:712723. [Crossref] [PubMed]
- Guarnizo A, López Palacio R, Carrillo Bayona JA. Skip and Mediastinal Metastasis in Papillary Thyroid Cancer. Radiol Imaging Cancer 2023;5:e230018. [Crossref] [PubMed]
- Ryu YJ, Kwon SY, Lim SY, et al. Predictive Factors for Skip Lymph Node Metastasis and Their Implication on Recurrence in Papillary Thyroid Carcinoma. Biomedicines 2022;10:179. [Crossref] [PubMed]
- Jiwang L, Jinghui B, Fengqin F, et al. Comprehensive analysis of clinicopathologic and sonographic features in thyroid cancer with skip lymph node metastasis: establish and assessment of a prediction nomogram. Braz J Otorhinolaryngol 2023;89:101301. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Zhao M, Shi X, Zou Z, et al. Predicting skip metastasis in lateral lymph nodes of papillary thyroid carcinoma based on clinical and ultrasound features. Front Endocrinol (Lausanne) 2023;14:1151505. [Crossref] [PubMed]
- Valent D, Krismer F, Grossauer A, et al. Nomogram to Predict the Probability of Functional Dependence in Early Parkinson’s Disease. J Parkinsons Dis 2023;13:49-55. [Crossref] [PubMed]
- Zhang D, Hu J, Liu Z, et al. Prognostic nomogram in patients with epithelioid sarcoma: A SEER-based study. Cancer Med 2023;12:3079-88. [Crossref] [PubMed]
- Hu Q, Zhang WJ, Liang L, et al. Establishing a Predictive Nomogram for Cervical Lymph Node Metastasis in Patients With Papillary Thyroid Carcinoma. Front Oncol 2021;11:766650. [Crossref] [PubMed]
- Wang Z, Chang Q, Zhang H, et al. A Clinical Predictive Model of Central Lymph Node Metastases in Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne) 2022;13:856278. [Crossref] [PubMed]
- Seong CY, Chai YJ, Lee SM, et al. Significance of distance between tumor and thyroid capsule as an indicator for central lymph node metastasis in clinically node negative papillary thyroid carcinoma patients. PloS One 2018;13:e0200166. [Crossref] [PubMed]
- Jiang Q, Zhai M, Lin X, et al. Case Report: A papillary thyroid microcarcinoma patient with skip lymph node metastasis and multiple distant metastasis. Front Surg 2022;9:1019846. [Crossref] [PubMed]
- Li C, Chen Z, Zhao H, et al. A nomogram based on metabolic profiling to discriminate lung cancer among patients with lung nodules. J Int Med Res 2023;51:3000605231161204. [Crossref] [PubMed]
- Zou W, Wu D, Wu Y, et al. Nomogram predicts risk of perineural invasion based on serum biomarkers for pancreatic cancer. BMC Gastroenterol 2023;23:315. [Crossref] [PubMed]
- Liu L, Wang H, Zhao B, et al. Nomogram to predict the progression of patients with primary membranous nephropathy and nephrotic syndrome. Int Urol Nephrol 2022;54:331-41. [Crossref] [PubMed]
- Zhu S, Wang Q, Zheng D, et al. A Novel and Effective Model to Predict Skip Metastasis in Papillary Thyroid Carcinoma Based on a Support Vector Machine. Front Endocrinol (Lausanne) 2022;13:916121. [Crossref] [PubMed]
- Yang Z, Heng Y, Zhao Q, et al. The proposed modification of TNM staging and therapeutic strategy for skip metastasis in papillary thyroid carcinoma: A multicenter retrospective cohort study. Cancer Med 2023;12:13270-8. [Crossref] [PubMed]
- Likhterov I, Reis LL, Urken ML. Central compartment management in patients with papillary thyroid cancer presenting with metastatic disease to the lateral neck: Anatomic pathways of lymphatic spread. Head Neck 2017;39:853-9. [Crossref] [PubMed]
- Zhao L, Wu F, Zhou T, et al. Risk factors of skip lateral cervical lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Endocrine 2022;75:351-9. [Crossref] [PubMed]
- Zhang X, Chen Y, Chen W, et al. Combining Clinicopathologic and Ultrasonic Features for Predicting Skip Metastasis of Lateral Lymph Nodes in Papillary Thyroid Carcinoma. Cancer Manag Res 2023;15:1297-306. [Crossref] [PubMed]
- Zhang Z, Zhang X, Yin Y, et al. Integrating BRAF(V600E) mutation, ultrasonic and clinicopathologic characteristics for predicting the risk of cervical central lymph node metastasis in papillary thyroid carcinoma. BMC Cancer 2022;22:461. [Crossref] [PubMed]
- Zhan WW, Zhou P, Zhou JQ, et al. Differences in sonographic features of papillary thyroid carcinoma between neck lymph node metastatic and nonmetastatic groups. J Ultrasound Med 2012;31:915-20. [Crossref] [PubMed]
- Meng C, Wang W, Zhang Y, et al. The influence of nodule size on the aggressiveness of thyroid carcinoma varies with patient’s age. Gland Surg 2021;10:961-72. [Crossref] [PubMed]
- Zhang F, Zheng B, Yu X, et al. Risk Factors for Contralateral Occult Carcinoma in Patients With Unilateral Papillary Thyroid Carcinoma: A Retrospective Study and Meta-Analysis. Front Endocrinol (Lausanne) 2021;12:675643. [Crossref] [PubMed]
- Li F, Zhou FJ, Zhu TW, et al. Nomogram for predicting skip metastasis in cN0 papillary thyroid cancer patients at increased risk of lymph node metastasis. Adv Clin Exp Med 2023;32:753-61. [Crossref] [PubMed]
- Signore A, Lauri C, Di Paolo A, et al. Predictive Role of Serum Thyroglobulin after Surgery and before Radioactive Iodine Therapy in Patients with Thyroid Carcinoma. Cancers (Basel) 2023;15:2976. [Crossref] [PubMed]
- Huang Z, Song M, Wang S, et al. Preoperative serum thyroglobulin is a risk factor of skip metastasis in papillary thyroid carcinoma. Ann Transl Med 2020;8:389. [Crossref] [PubMed]
- Smulever A, Pitoia F. Thirty years of active surveillance for low-risk thyroid cancer, lessons learned and future directions. Rev Endocr Metab Disord 2024;25:65-78. [Crossref] [PubMed]
- Cai X, Aierken X, Ahmat A, et al. A Nomogram Model Based on Noninvasive Bioindicators to Predict 3-Year Risk of Nonalcoholic Fatty Liver in Nonobese Mainland Chinese: A Prospective Cohort Study. Biomed Res Int 2020;2020:8852198. [Crossref] [PubMed]
- Sun S, Yang W, Yang Y, et al. Nomogram for predicting survival after lymphatic metastasis in esophageal cancer: A SEER analysis. Medicine (Baltimore) 2023;102:e34189. [Crossref] [PubMed]
- Li Y, Bao Y, Zheng H, et al. A nomogram for predicting severe myelosuppression in small cell lung cancer patients following the first-line chemotherapy. Sci Rep 2023;13:17464. [Crossref] [PubMed]


