
Application of a modified lateral thoracic artery perforator flap in partial breast defects
Highlight box
Key findings
• Lateral thoracic artery perforator (LTAP) flap is a reliable method for local breast defect repair and can be considered in the case of breast-conserving surgery.
What is known and what is new?
• Breast-conserving surgery has become a common surgical treatment for breast cancer.
• There are relatively few reports on the use of LTAP as breast-conserving surgery.
What is the implication, and what should change now?
• This surgical approach can achieve wide resection of the primary breast tumor and ensure the safety of the resection margin. At the same time, the modified lateral thoracic artery perforator flap can also be used to repair the local breast defects with satisfactory aesthetic results.
IntroductionOther Section
Breast cancer has become the most common malignant tumor in the world (1). Breast-conserving surgery has been carried out since the late 1970s, and it has been accepted by an increasing number of patients with early breast cancer (2). According to studies, there is no statistically significant difference in the tumor recurrence rate or overall survival rate between breast-conserving surgery with radiotherapy and modified radical mastectomy (3-6). However, some patients are not satisfied with the breast shape after breast-conserving surgery. According to the literature, 20%-30% patients have breast shape changes after breast-conserving surgery (7). Compared with Western women, the breast volume of Asian women is generally smaller, and therefore more difficult to use residual glands to reshape the breast, necessitating autologous tissue flaps to fill the defect in the resection area (8).
In the past, breast surgeons often used autologous tissue, such as myocutaneous flaps or free flaps, to repair local breast defects. However, due to the long operation times, donor site morbidity and many complications of myocutaneous flaps or free flaps, many patients have difficulty accepting this repair method (9). For breast surgeons, the challenge lies in finding a minimally invasive and more suitable breast-conserving technique for local breast defects.
The pedicled perforator flap was first reported by Koshima in 1989 (10). This flap can make more efficient use of the tissue around the breast and is also an upgraded version of the traditional myocutaneous flap technique. The pedicled perforator flap of the lateral chest wall is considered to be a better method for repairing local breast defects, especially those in the lateral breast region. In 2014, Kim (11) took the lead in applying pedicled lateral thoracic artery perforator (LTAP) flaps to repair head, neck and limb wounds. In 2015, McCulley (12) applied an LTAP flap to partial breast reconstruction for the first time. At present, there are few studies on the application of LTAP flaps in breast repair. However, the incision designs reported in relevant papers (13-15) are all transverse fusiform flaps with lateral thoracic vessels as the axis, and the incision crosses between the breast and the lateral chest wall, leaving scars on the breast skin and affecting the postoperative breast aesthetics.
In view of these findings, our team modified the flap by designing a longitudinal fusiform flap at the lateral chest wall with the lateral thoracic perforator vessels as the axis, and the incision was concealed at the anterior axillary line. The modified flap can not only achieve radical cure of the tumor but also satisfy considerations regarding the aesthetics of the breast and achieve good clinical results. The aim of this study is to review a series of surgical procedures for local breast defects using a modified LTAP flap. Data on demographic characteristics, tumor size and location, type of axillary lymph node surgery, adjuvant chemotherapy and radiotherapy, and postoperative complications were retrospectively collected and analyzed. The surgical planning, indications, advantages and limitations were further discussed. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-529/rc).
MethodsOther Section
From January 2020 to June 2021, the medical records of patients who underwent partial breast defect repair with pedicled LTAPs in the Department of Breast Surgery, Affiliated Hospital of Guangdong Medical University, were retrospectively analyzed. Inclusion criteria: (I) age ≤80 years, (II) tumor size ≤5 cm, (III) breast defects ranged from 20% to 50%, (IV) accept radiation therapy. Exclusion criteria: reject radiation therapy. Data were collected on the demographic characteristics of these patients, tumor size and location, type of axillary lymph node surgery, adjuvant chemotherapy and radiotherapy, and postoperative complications. All patients were closely followed up by the breast surgeon during the postoperative period, and appropriate surveillance methods were proposed.
Generally, patients underwent physical examination, breast ultrasound, and mammography 3 to 6 months after surgery. In cases in which ultrasonography and mammography could not distinguish postoperative changes from local recurrence, magnetic resonance imaging was indicated.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Guangdong Medical University (No. KT2022-064-01), and all patients gave informed consent.
Patient assessment and reconstruction procedures
All patients were examined by the same senior breast surgeon and individualized surgical treatment plan was made. According to the breast volume, the presence of ptosis, the size of the tumor, and the location of the tumor, the senior breast surgeon evaluated the suitability of the pedicled LTAP flap for local breast defect reconstruction.
Flap design
The pedicle vessels were evaluated by Doppler ultrasound. The lateral thoracic arteries and veins were first explored for their presence and accompanying each other. The lateral thoracic artery was then searched for between the third and fourth intercostal spaces in the anterolateral chest wall, and its location was marked. If there was no lateral thoracic artery on exploration, we looked for thoracodorsal vessel perforators or intercostal vessel perforators as the vascular pedicle of the flap. From the Doppler ultrasound, it can be seen that the lateral thoracic artery exits from the main trunk and runs toward the skin, tapering until it disappears.
Before surgery, the patient was placed in the supine position, and the extent of the longitudinal flap including the course of this perforator was marked with a black marker. The key point was to ensure adequate flap width and then adjust appropriately according to the actual weight of the excised tissue during the operation.
If the lateral thoracic vessels are injured or absent during the operation, the thoracodorsal vessel perforator, the intercostal vessel perforator, or the anterior branch of the thoracodorsal vessel were used as the alternative vessels for the flap (Figures 1-4).
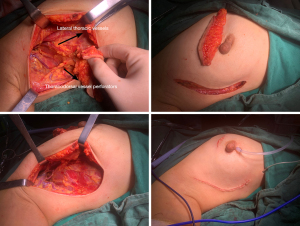

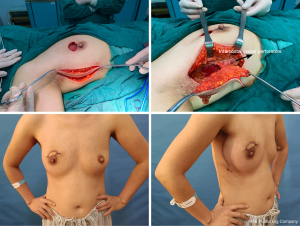

Postoperative complications
Hematoma, wound dehiscence, necrosis of breast skin and nipple-areola complex (NAC), partial and total flap necrosis, infection, and fat liquefaction were observed.
Evaluation of postoperative breast satisfaction
Postoperative breast satisfaction was investigated using an informal questionnaire, which was scored by the patients themselves. This questionnaire rated satisfaction as very satisfied, satisfied, disappointed, or regretful.
Statistical analysis
Statistical analyses were performed in SPSS Statistics 23.0 (IBM Corporation, Chicago, IL, USA). All values were expressed as percentages. The chi-square test and Fisher exact probability method were used to compare the characteristics of LTAP.
ResultsOther Section
From January 2020 to June 2021, a total of 126 patients were treated with LTAP flaps to repair local breast defects at the Affiliated Hospital of Guangdong Medical University. All flaps survived completely without donor site complications. None of the patients required revision surgery on the postoperative breast.
The median weight of the tumor specimen was 185 g (weight range, 170–320 g), and this glandular tissue accounted for 30% to 40% of the total breast volume. The average flap size was 10.5 cm ×2.5 cm (length range: 8–15 cm, width range, 2–4 cm). The minimum follow-up time was 6 months, with an average of 10 months (time range: 6–22 months). The mean operative time was 130 minutes (time range: 90–180 minutes), and the mean hospital stay was 3 days (time range, 2–5 days).
Characteristics of the tumor
All cases were unilateral breast cancer (68 right and 58 left). The locations of the cancer by number of cases were as follows: 30 cases (23.8%) were located in the upper outer quadrant, 32 cases (25.4%) in the lower outer quadrant, 40 cases (31.7%) in the upper inner quadrant, and 24 cases (19.0%) in the lower inner quadrant. Of the 126 patients, 84 (66.7%) had tumors less than or equal to 2 cm in diameter (T1), and 42 (33.3%) had tumors between 2 and 5 cm in diameter (T2). Ninety-seven patients (77.0%) had invasive breast cancer, 19 patients (15.08%) had both invasive carcinoma and ductal carcinoma in situ, and 10 patients (7.93%) had ductal carcinoma in situ (Table 1).
Table 1
| Variables | Number of cases | Proportion |
|---|---|---|
| Orientation | ||
| Right breast cancer | 68 | 53.9% |
| Left breast cancer | 58 | 46.1% |
| Locations | ||
| Upper outer quadrant | 30 | 23.8% |
| Lower outer quadrant | 32 | 25.4% |
| Lower inner quadrant | 40 | 31.7% |
| Upper inner quadrant | 24 | 19.0% |
| Diameter | ||
| <2 cm | 84 | 66.7% |
| 2–5 cm | 42 | 33.3% |
| Types of pathology | ||
| Invasive breast cancer | 97 | 77.0% |
| Ductal carcinoma in situ | 10 | 7.93% |
| Both of them | 19 | 15.08% |
Axillary lymph node dissection was performed
All patients underwent axillary dissection/sentinel lymph node biopsy through the medial incision of the flap. Among them, 85 patients (67.5%) had sentinel lymph node biopsy. Forty-one patients (32.5%) underwent complete axillary lymph node dissection (Level I, II, and III).
Adjuvant chemoradiotherapy
A total of 118 patients (93.7%) received some form of chemotherapy, and all patients received adjuvant radiotherapy. Radiotherapy was started after chemotherapy in all cases. Patients were followed up for a minimum of 6 months (time range, 6–22 months), local recurrence of the tumor was not identified.
Statistics of complications
In all 126 cases, the flaps survived completely without donor site complications. Edema occurred in 10 cases and disappeared after detumescence taking Aescuven Forte. Delayed venous injury occurred in 5 cases but resolved without intervention. The flaps survived completely without fat necrosis. Transient ischemia of the breast skin and nipple-breast complex occurred in 2 cases, and the symptoms were improved after wet compression with alcohol gauze. One patient had fat liquefaction and local redness and swelling in the breast, which disappeared after incision and drainage.
Patient satisfaction
Patients were followed up for a minimum of 6 months (time range, 6–22 months), and the cosmetic results of breasts were evaluated by independent personnel. 110 patients (87.3%) rated the nipple-areola composite cosmetic effect as good or very good, and 16 patients (12.7%) rated it as satisfactory. 116 (92.1%) patients rated the breast shape as good or very good, 8 patients (6.3%) rated it as satisfactory, and 2 patients (1.6%) rated it as unsatisfactory. 107 patients (84.9%) rated the breast symmetry as good or very good, 10 patients (7.9%) rated it as satisfactory, and 9 patients (7.1%) rated it as unsatisfactory. Some patients presented with breast asymmetry because the irradiated breast is more likely to retract after radiotherapy. Regarding the overall evaluation of the procedure, 121 patients (96.0%) were very satisfied or satisfied with their results. Five patients (4.0%) were unsatisfied with the procedure, but none regretted the procedure (Table 2).
Table 2
| Evaluation | Number of cases | Proportion |
|---|---|---|
| The nipple-areola composite cosmetic effect | ||
| Satisfactory | 16 | 12.7% |
| Good or very good | 110 | 87.3% |
| The breast shape | ||
| Satisfactory | 8 | 6.3% |
| Good or very good | 116 | 92.1% |
| Unsatisfactory | 2 | 1.6% |
| The breast symmetry | ||
| Satisfactory | 10 | 7.9% |
| Good or very good | 107 | 84.9% |
| Unsatisfactory | 9 | 7.1% |
| Overall effect | ||
| Satisfactory | 121 | 96.0% |
| Unsatisfactory | 5 | 4.0% |
DiscussionOther Section
In 1973, Veronesi proposed the concept of conservative breast reconstruction surgery for the first time and believed that when the diameter of breast cancer was less than 2 cm and there was no lymph node metastasis, partial mastectomy was safe (16). According to Fisher’s study (17), partial mastectomy combined with postoperative radiotherapy will not affect the survival rate of patients when the diameter of the breast cancer mass is less than 4 cm. After 30 years of development, a variety of breast-conserving surgery methods have appeared, but thus far, there is no consensus on how to repair partial breast defects during breast-conserving surgery.
Although myocutaneous flaps and free flaps have a stable blood supply and abundant tissue (18,19), they are difficult to popularize in primary hospitals because of the difficulty in learning, the difficulty in operation, and the great trauma to patients (20,21). With the successful application of perforator flaps in repairing head and neck and limb wounds (22,23), perforator flaps have gradually been introduced in the field of breast surgery.
A good perforator flap donor site should meet the following conditions:
- Predictable and constant perforator vessels;
- At least one perforator with a diameter greater than 0.5 mm. If the flap area is small, the perforator caliber can be smaller;
- A sufficiently long vascular pedicle can be separated
- The donor site should be sutured directly.
There is no doubt that the LTAP flap satisfies the criteria for the perforator flap. The LTAP flap is similar to the lateral intercostal artery perforator flap, but the position is relatively higher, which is especially suitable for the repair of local breast defects. At the same time, it can also be combined with the lateral intercostal artery perforator to form a larger tissue flap to realize the repair of the whole breast defect.
The blood supply of the lateral thoracic artery can come from the thoracoacromial artery, axillary artery, thoracodorsal artery, subscapular artery or multiple arteries in the lateral thoracic region (24,25). Its anatomical position is relatively fixed, and it is generally located 2 cm outside the lateral margin of the breast between the third and fourth ribs (12). The size of the skin island is determined by the perforator location, the required flap size, and the skin laxity (26). The length of the skin island was generally strictly controlled within 8–15 cm. Levine (27) dissected the flap from the outside to the inside, from the pedicle to the skin island, which is suitable for delayed breast reconstruction. McCulley (12) dissected the lateral thoracic perforator flap from the inside to the outside, which can be suitable for immediate breast reconstruction or delayed breast reconstruction. Care should be taken to avoid injury to the pedicle vessels when dissecting the cephalic margin of the flap. When dissecting the LTAP during the operation, the perforators of the external branch of the intercostal artery may appear at the same time. At this time, it can be included in the design of the pedicle of the flap to fully ensure the blood supply of the flap. Therefore, the operation of the pedicle of the flap is relatively flexible, which can be separated to obtain a long vascular pedicle, or incorporate more perforating vessels in the pedicle (even combined with lateral intercostal perforating vessels) to form a tissue flap of sufficient size to meet the needs of reconstruction of large defects.
Before the operation, color Doppler ultrasound can be used to mark the location of the LTAP on the body surface and determine the caliber of the perforator. On the other hand, color Doppler ultrasound can also be used to observe the blood flow velocity and resistance of the perforator, judge the quality of the artery and vein, and determine whether there are vessel wall lesions, stenosis and other conditions. According to a study, the positive detection rate of color Doppler ultrasound for perforator vessels with a diameter greater than 0.7 mm is more than 90% (28). However, the diagnostic results of color Doppler ultrasound are greatly affected by human factors, and the results are closely related to the proficiency of the operator. The operator should have a certain knowledge of flap surgery, know the position and level of the target vessel, and fix the position for a long time during the detection. Due to the probing area limitation of the probe, the vascular information provided is segmental. If the patient’s economic conditions permit, magnetic resonance angiography (MRA) detection is feasible, which can improve the quality of perforator imaging, and the whole process only takes 10–20 minutes (29).
At present, there are few studies on implementation of the LTAP flap in the repair of local breast defects (12,30). All of the studies involve transverse fusiform flaps with the lateral thoracic vessels as the axis, and the incision runs between the breast and the lateral chest wall, which will leave scars on the breast skin and affect the aesthetics of the breast after the operation (31,32). We modified the design of the LTAP flap. After the location of the lateral thoracic vessels was marked, a longitudinal fusiform flap was designed at the lateral chest wall with the lateral thoracic perforator vessels as the axis, and the incision was concealed at the anterior axillary line. The upper boundary of the flap was 1 cm below the axilla, the lower boundary was at the inferior crease wall of the breast, and the boundary on both sides was determined according to the laxity of the skin of the patient. During the operation, the lateral thoracic vessels can be dissected first, and then the position of the flap can be adjusted accordingly. The area of the skin island can be appropriately reduced by carrying more fascial tissue around the perforator. To ensure the blood supply of the perforator flap, the fat tissue around the perforator pedicle is preserved as much as possible to avoid sacrificing too many branches of the vascular pedicle.
During flap dissection, intercostal artery perforators or thoracodorsal artery perforators may appear. If the mobility of the pedicle is not affected, the intercostal artery perforator or thoracodorsal artery perforator should be preserved as much as possible to increase the blood supply of the perforator flap. If the mobility of the pedicle of the flap is affected, the intercostal artery perforator or thoracodorsal artery perforator can be ligated.
For the defect in the medial breast, a tunnel was made in the base of the gland tissue in the lateral breast, and the LTAP flap was transferred through the tunnel to the defect in the medial breast. If the pedicle of the perforator flap is not long enough to reach the inner breast quadrant, the gland tissue of the outer quadrant can be freed to form a gland flap, and the defect in the medial quadrant can be repaired first. Then, the LTAP flap can be used to repair the defect in the lateral quadrant.
Asian women have smaller breasts and are more likely to have breast cancer at an early stage (33,34). The modified LTAP flap is a new option for breast reconstruction. It can be used to repair any quadrant defect of the breast, and the wound can be concealed at the anterior axillary line to ensure the aesthetic appearance of the breast. It is suitable for the reconstruction of local breast defects in most Asian women. From this study, we can see that its advantages are its simple technique, strong popularization, small trauma and short recovery period. Moreover, LTAP flaps have good aesthetic effects and a low incidence of complications in repairing local breast defects. In addition, the LTAP flap does not require complicated procedures such as vascular anastomosis and can also be used as a rescue measure after the failure of free flaps.
The drawback in using the LTAP flap is that sometimes the lateral thoracic artery is absent, so it needs to be explored by Doppler ultrasound before the operation. The 5 patients in our study who were not satisfied with the operation had poor cosmetic results due to either lean body mass or tumor location in the lower inner quadrant.
ConclusionsOther Section
The results of this study show that the modified LTAP flap is a reliable method for local breast defect repair and can be considered in the case of breast-conserving surgery. In selected patients, this approach allows as wide a resection of the primary breast tumor as possible with safe margins. At the same time, the modified LTAP flap can be used to repair local breast defects with satisfactory aesthetic results. However, due to the retrospective, single-center study, lack of a control group, and short follow-up time, some data may be biased. Next, we will continue to expand the sample size, increase the control group and extend the follow-up time to continue the study.
Breast-conserving surgery is a kind of surgery that is influenced by strong individual needs, and it is not possible that all the problems of breast-conserving surgery will be solved by one or two mainstream technologies. Understanding and mastering these new techniques of pedicled perforator flaps can undoubtedly expand the thinking of breast surgeons, increase the technical means of doctors, and serve patients better.
AcknowledgmentsOther Section
Funding: This work was funded by
FootnoteOther Section
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-529/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-529/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-529/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-529/coif). S.H. reports funding supports from Guangdong Medical Research Foundation (No. B2020036) and Affiliated Hospital of Guangdong Medical University Clinical Research Program (No. LCYJ2021B003). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Guangdong Medical University (No. KT2022-064-01), and all patients gave informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022;66:15-23. [Crossref] [PubMed]
- Catsman CJLM, Beek MA, Voogd AC, et al. The COSMAM TRIAL a prospective cohort study of quality of life and cosmetic outcome in patients undergoing breast conserving surgery. BMC Cancer 2018;18:456. [Crossref] [PubMed]
- Kelsall JE, McCulley SJ, Brock L, et al. Comparing oncoplastic breast conserving surgery with mastectomy and immediate breast reconstruction: Case-matched patient reported outcomes. J Plast Reconstr Aesthet Surg 2017;70:1377-85. [Crossref] [PubMed]
- Sun Y, Kim SW, Heo CY, et al. Comparison of quality of life based on surgical technique in patients with breast cancer. Jpn J Clin Oncol 2014;44:22-7. [Crossref] [PubMed]
- Bertozzi N, Pesce M, Santi PL, et al. Oncoplastic breast surgery: comprehensive review. Eur Rev Med Pharmacol Sci 2017;21:2572-85.
- Kunkler IH, Williams LJ, Jack WJL, et al. Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer. N Engl J Med 2023;388:585-94. [Crossref] [PubMed]
- Ren JH, Wang Y, Zhang X, et al. A Clinical Analysis of Prognosis and Patient-Reported Outcomes of Oncoplastic Breast-Conserving Surgery for Early Breast Cancer: A Retrospective Cohort Study. Aesthetic Plast Surg 2023; Epub ahead of print. [Crossref]
- Hong S, Wang S, Liu J, et al. Usefulness of Lateral Thoracic Adipofascial Flaps After Breast-conserving Surgery in Small-to Moderate-sized Breasts. Clin Breast Cancer 2019;19:370-6. [Crossref] [PubMed]
- Vincent A, Hohman MH. Latissimus Dorsi Myocutaneous Flap. 2023 Mar 1. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Kim JT, Kim SW. Another option of perforator flap in the lateral thoracic area: lateral thoracic perforator flap. J Reconstr Microsurg 2014;30:443-50. [Crossref] [PubMed]
- McCulley SJ, Schaverien MV, Tan VK, et al. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:686-91. [Crossref] [PubMed]
- Holmström H, Lossing C. The lateral thoracodorsal flap in breast reconstruction. Plast Reconstr Surg 1986;77:933-43. [Crossref] [PubMed]
- Woerdeman LA, van Schijndel AW, Hage JJ, et al. Verifying surgical results and risk factors of the lateral thoracodorsal flap. Plast Reconstr Surg 2004;113:196-203; discussion 204-5. [Crossref] [PubMed]
- Munhoz AM, Montag E, Arruda EG, et al. The role of the lateral thoracodorsal fasciocutaneous flap in immediate conservative breast surgery reconstruction. Plast Reconstr Surg 2006;117:1699-710. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312:665-73. [Crossref] [PubMed]
- Mortada H, AlNojaidi TF, AlRabah R, et al. Morbidity of the Donor Site and Complication Rates of Breast Reconstruction with Autologous Abdominal Flaps: A Systematic Review and Meta-Analysis. Breast J 2022;2022:7857158. [Crossref] [PubMed]
- Wu ZY, Han J, Kim HJ, et al. Breast cancer outcomes following immediate breast reconstruction with implants versus autologous flaps: a propensity score-matched study. Breast Cancer Res Treat 2022;191:365-73. [Crossref] [PubMed]
- Blondeel N, Vanderstraeten GG, Monstrey SJ, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg 1997;50:322-30. [Crossref] [PubMed]
- Tribondeau P, Soffray F. Breast reconstruction with pedicled TRAM flap (a retrospective study of 115 consecutive cases). Ann Chir Plast Esthet 2008;53:309-17. [Crossref] [PubMed]
- Cho A, Hall FT. Review of perforator flaps in head and neck cancer surgery. Curr Opin Otolaryngol Head Neck Surg 2016;24:440-6. [Crossref] [PubMed]
- Zheng Y, Wei Z, Li H, et al. Application of anteromedial thigh perforator flap in repair of soft tissue defects of lower limbs. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019;33:1552-5. [Crossref] [PubMed]
- Kim JT, Kim SW. Perforator Flap versus Conventional Flap. J Korean Med Sci 2015;30:514-22. [Crossref] [PubMed]
- Shi J, Xu B, Shen GF, et al. Application of lateral thoracic flap in maxillofacial defect reconstruction: experience with 28 cases. J Plast Reconstr Aesthet Surg 2013;66:1369-75. [Crossref] [PubMed]
- Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg 2003;30:331-42. v. [Crossref] [PubMed]
- Levine JL, Soueid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg 2005;116:762-7. [Crossref] [PubMed]
- Hallock GG. Doppler sonography and color duplex imaging for planning a perforator flap. Clin Plast Surg 2003;30:347-57. v-vi. [Crossref] [PubMed]
- Hijjawi JB, Blondeel PN. Advancing deep inferior epigastric artery perforator flap breast reconstruction through multidetector row computed tomography: an evolution in preoperative imaging. J Reconstr Microsurg 2010;26:11-20. [Crossref] [PubMed]
- Zhygulin A, Fedosov A, Palytsia V. Modifications of the LICAP/LTAP Flap Technique in Partial Breast Reconstruction for Difficult Tumor Locations. Plast Reconstr Surg 2022;150:1219-22. [Crossref] [PubMed]
- Mangialardi ML, Baldelli I, Salgarello M, et al. Breast Reconstruction Using the Lateral Thoracic, Thoracodorsal, and Intercostal Arteries Perforator Flaps. Plast Reconstr Surg Glob Open 2021;9:e3334. [Crossref] [PubMed]
- Meybodi F, Cocco AM, Messer D, et al. The Modified Lateral Intercostal Artery Perforator Flap. Plast Reconstr Surg Glob Open 2019;7:e2066. [Crossref] [PubMed]
- Ellison-Loschmann L, McKenzie F, Highnam R, et al. Age and ethnic differences in volumetric breast density in new zealand women: a cross-sectional study. PLoS One 2013;8:e70217. [Crossref] [PubMed]
- Lim LY, Ho PJ, Liu J, et al. Determinants of breast size in Asian women. Sci Rep 2018;8:1201. [Crossref] [PubMed]


