
Changing trends in the management of ductal carcinoma in situ in Republic of Korea: a comprehensive analysis using Health Insurance Review and Assessment data [2009–2020]
Highlight box
Key findings
• We observed a significant increase in ductal carcinoma in situ (DCIS) breast surgeries, particularly breast-conserving surgery.
• Utilization of implant-based reconstruction and hypofractionated radiation therapy (RT) showed increasing trends.
What is known and what is new?
• The rise in DCIS diagnoses due to mammographic screening has been reported.
• This study adds insights into the rise of conservative surgical approaches, increased implant-based reconstructions, and preference for hypofractionated RT.
What is the implication, and what should change now?
• The evolving treatment landscape necessitates updated guidelines and extensive targeted research to optimize DCIS management.
• Monitoring surgical changes and discussing RT omission and adoption of hypofractionated RT are imperative for further advancements in DCIS management.
Introduction
Ductal carcinoma in situ (DCIS) is a neoplastic process confined to the ductal system of the breast that lacks histological evidence of invasion. These cells neither disrupt the basement membrane nor are involved in the surrounding breast stroma. Prior to the advent of mammography, pure DCIS was rarely diagnosed. However, owing to the widespread use of mammography, the diagnosis of DCIS has progressively increased over time, now comprising approximately 20–25% of screening-detected breast cancer cases in the United States (1). In Korea, the National Cancer Screening Program plays a crucial role in breast cancer screenings, advocating mammograms every 2 years for women aged 40 or older. The breast cancer screening rate exhibited a notable increase from 33% to 72% between 2004 and 2012 (2). The Korean Breast Cancer Society reported that DCIS accounted for 17.9% of all newly diagnosed breast lesions in 2017 (3), indicating a notable increase from 6.1% in 2000 and underscoring the impact of the national mammographic screening strategy and advancements in diagnostic methods.
The optimal treatment for DCIS remains controversial because there is no consensus regarding the risk of progression to invasive breast cancer or the impact of various therapeutic modalities on survival outcomes (4). The standard of care for DCIS is breast-conserving surgery (BCS) followed by whole-breast radiation therapy (RT), which reduces the risk of local recurrence by approximately 50% compared with BCS alone (5). Despite the effectiveness of BCS with RT, there is an ongoing debate regarding the necessity of adjuvant RT for all DCIS cases (6). This is particularly relevant in the context of the coronavirus disease 2019 (COVID-19) pandemic, which has given rise to unique challenges in the delivery of healthcare services, including RT. The pandemic has prompted a re-evaluation of treatment approaches, with an emphasis on minimizing hospital visits and treatment duration while ensuring optimal patient outcomes (7). In recent years, there has been growing interest in hypofractionated whole-breast irradiation (HF-WBI) as an alternative to conventional fractionation WBI (CF-WBI) in the management of DCIS. HF-WBI delivers high doses of radiation per fraction over a few treatment sessions, potentially offering increased convenience and reduced treatment burden for patients. This approach has shown promising results in invasive breast cancer (8,9), leading to investigations into its applicability and efficacy in DCIS treatment.
In light of these considerations, this study aims to analyze the adoption of hypofractionated RT for DCIS in the Korean population. By examining treatment patterns, we sought to provide valuable insights into the evolving landscape of DCIS management, particularly in the context of changing treatment paradigms influenced by the global pandemic and advancements in surgery and RT techniques. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-433/rc).
Methods
Data source
South Korea has a universal health coverage system—the National Health Insurance Service (NHIS)—that covers approximately 98% of the country’s population. The Health Insurance Review and Assessment Service (HIRA) reviews and evaluates the appropriateness of medical expenses claimed by medical institutions. The HIRA provides a Big Data Hub that allows researchers to analyze claims data and conduct population-based studies (10). The HIRA-National Patient Sample (HIRA-NPS) includes a stratified random sample of 3% of the total patients during each annual period from 2009 to 2018 and 5% during 2019 and 2020. The HIRA provides data on four types of patient samples: national, inpatient, pediatric, and aged population samples. We also collected the 1-year history of medical service use, demographics, and clinical details of the anonymized patients. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2022-128), and the requirement for individual consent for this retrospective analysis was waived.
Patient selection
The diagnostic code for DCIS was D05. Individuals diagnosed with invasive breast carcinoma (C50), whether before or after the diagnosis of DCIS, were excluded. The codes for breast surgery were as follows: N7131, N7132, N7138, and N7139 for total mastectomy (TM); N7133, N7134, N7136, and N7137 for BCS; and N7130 and N7135 for radical surgeries that include TM and BCS. The codes for RT were HD05 and HD06 for the three-dimensional conformal plan and HZ271 for intensity-modulated RT (IMRT). Patients with RT codes in the same year of surgery were assigned to the BCS group. Patients who did not undergo RT within the same year were assigned to the TM group. Subsequently, axillary and breast reconstruction surgeries were performed. The surgical and corresponding codes are listed in Table 1. For the analysis of radiation fractionation, the data of patients who received their first radiation treatment between February and November, annually, were collected separately.
Table 1
| Code | Description | Category |
|---|---|---|
| N7130 | Radical mastectomy, including modified radical mastectomy and radical breast-conserving operations | BCS (with HD05, HD06, HZ271) |
| TM (without HD05, HD06, HZ271) | ||
| N7131 | Simple mastectomy | TM |
| N7132 | Subcutaneous mastectomy | TM |
| N7133 | Partial mastectomy | BCS |
| N7134 | Excision of accessory breast | BCS |
| N7135 | Radical mastectomy, including modified radical mastectomy and radical breast-conserving operations | BCS (with HD05, HD06, HZ271) |
| TM (without HD05, HD06, HZ271) | ||
| N7136 | Partial mastectomy with axillary lymphadenectomy | BCS |
| N7137 | Partial mastectomy | BCS |
| N7138 | Radical mastectomy including axillary lymph nodes | TM |
| N7139 | Radical mastectomy | TM |
| N7140 | Reconstruction of breast using myocutaneous flap of latissimus dorsi muscle | Breast reconstruction—autologous |
| N7141 | Reconstruction of breast using myocutaneous flap of latissimus dorsi muscle | Breast reconstruction—autologous |
| N7142 | Reconstruction of breast using myocutaneous flap of latissimus dorsi muscle | Breast reconstruction—autologous |
| N7143 | Reconstruction of breast using pedicled transverse rectus abdominis myocutaneous flap | Breast reconstruction—autologous |
| N7144 | Breast reconstruction, bilateral, with bilateral pedicle transverse rectus abdominis myocutaneous flaps | Breast reconstruction—autologous |
| N7145 | Reconstruction of breast using free transverse rectus abdominis myocutaneous flap | Breast reconstruction—autologous |
| N7146 | Reconstruction of breast using free transverse rectus abdominis myocutaneous flap | Breast reconstruction—autologous |
| N7147 | Breast reconstruction with deep inferior epigastric perforator skin flap | Breast reconstruction—autologous |
| N7148 | Reconstruction of breast with expander or prosthesis | Breast reconstruction—implant based |
| N7149 | Immediate insertion of breast prosthesis | Breast reconstruction—implant based |
| N7150 | Insertion of prosthesis for breast | Breast reconstruction—implant based |
| N7151 | Periprosthetic capsulectomy of breast | Breast reconstruction—implant based |
| N7152 | Nipple reconstruction | Breast reconstruction—nipple-areolar reconstruction |
| N7153 | Reconstruction of areola | Breast reconstruction—nipple-areolar reconstruction |
| P2121 | Excision of axillary lymph node | Excision |
| P2122 | Complete axillary lymphadenectomy | Axillary dissection |
| P2123 | Excision of sentinel lymph node | Sentinel lymph node biopsy |
| P2124 | Radionuclide scan of sentinel lymph node of breast | Sentinel lymph node biopsy |
BCS, breast-conserving surgery; TM, total mastectomy.
Statistical analysis
The baseline characteristics of the patients who received or did not receive hypofractionated RT were compared using the chi-square test for categorical variables. The parameters considered for evaluation included the year of diagnosis (2009–2014 and 2015–2020), patient age (<70 and ≥70 years), and axillary evaluation (performed or not performed). The hospitals of surgery were classified into the following regions: region I (Seoul vs. non-Seoul), region II [capital area (Seoul + Gyeonggi) vs. non-capital area], and region III [metropolitan area (capital area + metropolitan city) vs. non-metropolitan area]. The size of the hospital of surgery was determined according to the number of beds (<800 vs. ≥800). Based on the number of days for which HD05, HD06, or HZ271 was prescribed, patients were grouped into no-RT, hypofractionated RT (15–20 fractions), and conventional fractionation RT (21 fractions or more). The regions and sizes of the RT hospitals were also determined.
Results
Between 2009 and 2020, the HIRA-NPS database included 304,100,188 claims (1,481,921 patients). Of these, 7,623 patients had the D05 diagnostic code. Among them, 1,248 (16.4%) patients underwent breast surgery and fulfilled the inclusion criteria (Figure 1). The sample was weighted to represent the Korean population, resulting in a sample size of 43,780 cases. The average number of breast surgeries for DCIS per year was 4,092, with a 10% annual increase from 2,211 in 2009 to 6,500 in 2020 (Figure 2). The number of BCS cases increased from 1,254 (56.7%) in 2009 to 4,400 (72.1%) in 2019 but decreased to 4,250 (65.4%) in 2020. The number of TM cases increased from 957 (43.3%) in 2009 to 2,250 (34.6%) in 2020, with a marked increase in both numbers and rates from 2014 to 2016. Since April 1, 2015, breast reconstruction surgery has been covered by the NHIS in South Korea, allowing the HIRA to collect data on the number of breast reconstruction cases. Between 2015 and 2020, 6,580 patients underwent reconstruction for DCIS: 5,123 (77.9%) underwent implant reconstruction and 1,374 (20.9%) underwent autologous reconstruction. In 2015, 462 (63.6%) of reconstructions were implant and 264 (36.4%) were autologous, while in 2020, 1,600 (88.9%) were implant and 200 (11.1%) were autologous (Figure 3). The annual rate of increase in implant reconstructions was 28%. Table 2 presents the annual trends in patient age, type of breast surgery, axillary surgery, reconstruction, adjuvant radiation, hospital region, and hospital size.

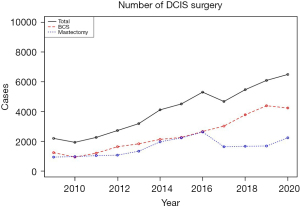
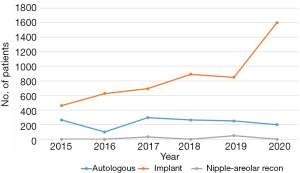
Table 2
| Variables | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||||||
| 20–29 | 33 (1.5) | 66 (3.4) | 66 (2.9) | 0 (0.0) | 99 (3.1) | 0 (0.0) | 99 (2.2) | 0 (0.0) | 99 (2.1) | 0 (0.0) | 50 (0.8) | 250 (3.9) |
| 30–39 | 363 (16.4) | 165 (8.5) | 429 (18.8) | 462 (16.9) | 363 (11.3) | 594 (14.4) | 594 (13.1) | 594 (11.2) | 660 (14.1) | 462 (8.4) | 650 (10.7) | 600 (9.3) |
| 40–49 | 1,056 (47.8) | 891 (45.8) | 627 (27.5) | 1,089 (39.8) | 1,518 (47.4) | 1,518 (36.8) | 1,782 (39.4) | 2,178 (41.0) | 1,551 (33.1) | 1,980 (36.1) | 2,050 (33.6) | 2,700 (41.9) |
| 50–59 | 528 (23.9) | 495 (25.4) | 660 (29.0) | 759 (27.7) | 825 (25.8) | 1,221 (29.6) | 1,221 (27.0) | 1,287 (24.2) | 1,551 (33.1) | 1,584 (28.9) | 1,650 (27.0) | 1,800 (27.9) |
| 60–69 | 198 (9.0) | 231 (11.9) | 396 (17.4) | 264 (9.6) | 132 (4.1) | 528 (12.8) | 627 (13.9) | 891 (16.8) | 594 (12.7) | 1,056 (19.3) | 1,500 (24.6) | 900 (14.0) |
| ≥70 | 33 (1.5) | 99 (5.1) | 99 (4.3) | 165 (6.0) | 264 (8.2) | 264 (6.4) | 198 (4.4) | 363 (6.8) | 231 (4.9) | 396 (7.2) | 200 (3.3) | 200 (3.1) |
| Combined age (years) | ||||||||||||
| <70 | 2,178 (98.5) | 1,848 (94.9) | 2,178 (95.7) | 2,574 (94.0) | 2,937 (91.8) | 3,861 (93.6) | 4,323 (95.6) | 4,950 (93.2) | 4,455 (95.1) | 5,082 (92.8) | 5,900 (96.7) | 6,250 (96.9) |
| ≥70 | 33 (1.5) | 99 (5.1) | 99 (4.3) | 165 (6.0) | 264 (8.2) | 264 (6.4) | 198 (4.4) | 363 (6.8) | 231 (4.9) | 396 (7.2) | 200 (3.3) | 200 (3.1) |
| Surgery type | ||||||||||||
| BCS | 1,254 (56.7) | 957 (49.2) | 1,221 (53.6) | 1,650 (60.2) | 1,848 (57.7) | 2,145 (52.0) | 2,277 (50.4) | 2,673 (50.3) | 3,036 (64.8) | 3,795 (69.3) | 4,400 (72.1) | 4,250 (65.4) |
| TM | 957 (43.3) | 990 (50.8) | 1,056 (46.4) | 1,089 (39.8) | 1,353 (42.3) | 1,980 (48.0) | 2,244 (49.6) | 2,640 (49.7) | 1,650 (35.2) | 1,683 (30.7) | 1,700 (27.9) | 2,250 (34.6) |
| Axillary surgery | ||||||||||||
| ALND | 0 (0.0) | 33 (6.2) | 0 (0.0) | 0 (0.0) | 33 (2.9) | 33 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Excision | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 33 (2.6) | 0 (0.0) | 0 (0.0) | 33 (0.9) | 0 (0.0) | 0 (0.0) |
| SNB | 561 (100.0) | 495 (93.8) | 627 (100.0) | 1,023 (100.0) | 1,122 (97.1) | 1,287 (97.5) | 1,221 (97.4) | 2,343 (100.0) | 2,772 (100.0) | 3,498 (99.1) | 3,950 (100.0) | 4,200 (100.0) |
| Reconstruction surgery | ||||||||||||
| Autologous | – | – | – | – | – | – | 264 (36.4) | 99 (13.6) | 297 (29.0) | 264 (22.9) | 250 (21.7) | 200 (11.1) |
| Implant | – | – | – | – | – | – | 462 (63.6) | 627 (86.4) | 693 (67.7) | 891 (77.1) | 850 (73.9) | 1,600 (88.9) |
| Nipple-areolar reconstruction | – | – | – | – | – | – | 0 (0.0) | 0 (0.0) | 33 (3.2) | 0 (0.0) | 50 (4.3) | 0 (0.0) |
| RT | ||||||||||||
| No | 1,551 (70.1) | 1,287 (66.1) | 1,584 (69.6) | 1,353 (49.4) | 1,914 (59.8) | 2,508 (60.8) | 3,003 (66.4) | 3,399 (64.0) | 2,937 (62.7) | 3,894 (71.1) | 3,700 (60.7) | 4,300 (66.2) |
| Yes | 660 (29.9) | 660 (33.9) | 693 (30.4) | 1,386 (50.6) | 1,287 (40.2) | 1,617 (39.2) | 1,518 (33.6) | 1,914 (36.0) | 1,749 (37.3) | 1,584 (28.9) | 2,400 (39.3) | 2,200 (33.8) |
| Surgery-RT | ||||||||||||
| BCS alone | 627 (28.4) | 330 (16.9) | 561 (24.6) | 330 (12.0) | 561 (17.5) | 594 (14.4) | 825 (18.2) | 792 (14.9) | 1,320 (28.2) | 2,244 (41.0) | 2,000 (32.8) | 2,100 (32.3) |
| BCS + RT | 627 (28.4) | 627 (32.2) | 660 (29.0) | 1,320 (48.2) | 1,287 (40.2) | 1,551 (37.6) | 1,452 (32.1) | 1,881 (35.4) | 1,716 (36.6) | 1,551 (28.3) | 2,400 (39.3) | 2,150 (33.1) |
| TM alone | 924 (41.8) | 957 (49.2) | 1,023 (44.9) | 1,023 (37.3) | 1,353 (42.3) | 1,914 (46.4) | 2,178 (48.2) | 2,607 (49.1) | 1,617 (34.5) | 1,650 (30.1) | 1,700 (27.9) | 2,200 (33.8) |
| TM + RT | 33 (1.5) | 33 (1.7) | 33 (1.4) | 66 (2.4) | 0 (0.0) | 66 (1.6) | 66 (1.5) | 33 (0.6) | 33 (0.7) | 33 (0.6) | 0 (0.0) | 50 (0.8) |
| Region of surgery I | ||||||||||||
| Non-Seoul | 1,155 (52.2) | 990 (50.8) | 990 (43.5) | 1,518 (55.4) | 1,782 (55.7) | 2,211 (53.6) | 2,310 (51.1) | 2,937 (55.3) | 2,640 (56.3) | 2,937 (53.6) | 3,050 (50.0) | 3,350 (51.5) |
| Seoul | 1,056 (47.8) | 957 (49.2) | 1,287 (56.5) | 1,221 (44.6) | 1,419 (44.3) | 1,914 (46.4) | 2,211 (48.9) | 2,376 (44.7) | 2,046 (43.7) | 2,541 (46.4) | 3,050 (50.0) | 3,150 (48.5) |
| Region of surgery II | ||||||||||||
| Non-capital | 858 (38.8) | 495 (25.4) | 528 (23.2) | 792 (28.9) | 957 (29.9) | 1,056 (25.6) | 1,320 (29.2) | 1,683 (31.7) | 1,848 (39.4) | 1,584 (28.9) | 1,950 (32.0) | 2,000 (30.8) |
| Capital area | 1,353 (61.2) | 1,452 (74.6) | 1,749 (76.8) | 1,947 (71.1) | 2,244 (70.1) | 3,069 (74.4) | 3,201 (70.8) | 3,630 (68.3) | 2,838 (60.6) | 3,894 (71.1) | 4,150 (68.0) | 4,500 (69.2) |
| Region of surgery III | ||||||||||||
| Non-metropolitan | 297 (13.4) | 231 (11.9) | 231 (10.1) | 198 (7.2) | 231 (7.2) | 429 (10.4) | 396 (8.8) | 759 (14.3) | 759 (16.2) | 693 (12.7) | 650 (10.7) | 500 (7.7) |
| Metropolitan | 1,914 (86.6) | 1,716 (88.1) | 2,046 (89.9) | 2,541 (92.8) | 2,970 (92.8) | 3,696 (89.6) | 4,125 (91.2) | 4,554 (85.7) | 3,927 (83.8) | 4,785 (87.3) | 5,450 (89.3) | 6,000 (92.3) |
| Size of hospital of surgery | ||||||||||||
| <800 beds | 891 (40.3) | 627 (32.2) | 990 (43.5) | 924 (33.7) | 1,551 (48.5) | 1,683 (40.8) | 1,353 (29.9) | 1,617 (30.4) | 1,749 (37.3) | 1,848 (33.7) | 2,350 (38.5) | 2,350 (36.2) |
| ≥800 beds | 1,320 (59.7) | 1,320 (67.8) | 1,287 (56.5) | 1,815 (66.3) | 1,650 (51.5) | 2,442 (59.2) | 3,168 (70.1) | 3,696 (69.6) | 2,937 (62.7) | 3,630 (66.3) | 3,750 (61.5) | 4,150 (63.8) |
Data are presented as number (%), unless otherwise indicated. BCS, breast-conserving surgery; TM, total mastectomy; ALND, axillary lymph node dissection; SNB, sentinel node biopsy; RT, radiation therapy.
The characteristics of the separate cohort for hypofractionated RT analysis are presented in Table 3. Figure 4 illustrates the trends in RT omission and the use of conventional fractionation RT and hypofractionated RT. Conventional fractionation RT was used in 429 (37.1%) patients in 2009, increasing to 1,287 (62.9%) in 2014, but decreasing to 700 (17.9%) by 2020 after 2014. In the later treatment period, the proportion of patients who received hypofractionated RT or omitted RT increased. Hypofractionated RT was used in 132 (6.5%) patients in 2014 and in 1,000 (25.6%) patients in 2020. The number of patients who did not receive RT was 594 (29.0%) in 2014 and 2,100 (53.8%) in 2020. The rate of RT omission was higher in the later treatment period [2015–2020], among patients over the age of 70 years, in metropolitan areas, and in small-volume hospitals. The rate of hypofractionated RT steadily increased during the later study period. In 2009, the rates of hypofractionated and conventional fractionation RT were 5.7% and 37.1%, respectively. In 2020, these rates were 25.6% and 17.9%, respectively. The rate of hypofractionated RT was significantly higher in the later period, after axillary surgery, among older patients, among those who received radiation in the capital area, and among those who received IMRT in large hospitals. The odds ratios are presented in Table 4.
Table 3
| Variables | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||||||
| 20–29 | 33 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 66 (3.8) | 0 (0.0) | 33 (1.7) | 0 (0.0) | 99 (3.6) | 0 (0.0) | 50 (1.2) | 100 (2.6) |
| 30–39 | 198 (17.1) | 66 (8.3) | 198 (17.6) | 231 (15.9) | 165 (9.4) | 330 (16.1) | 231 (11.7) | 165 (6.9) | 363 (13.3) | 396 (10.8) | 400 (9.5) | 250 (6.5) |
| 40–49 | 627 (54.3) | 396 (50.0) | 264 (23.5) | 693 (47.7) | 924 (52.8) | 792 (38.7) | 957 (48.3) | 990 (41.7) | 825 (30.1) | 1,287 (35.1) | 1,550 (36.9) | 1,600 (41.6) |
| 50–59 | 165 (14.3) | 264 (33.3) | 429 (38.2) | 297 (20.5) | 396 (22.6) | 561 (27.4) | 396 (20.0) | 693 (29.2) | 957 (34.9) | 1,254 (34.2) | 1,050 (25.0) | 1,150 (29.9) |
| 60–69 | 132 (11.4) | 66 (8.3) | 165 (14.7) | 132 (9.1) | 33 (1.9) | 231 (11.3) | 264 (13.3) | 396 (16.7) | 297 (10.8) | 429 (11.7) | 1,000 (23.8) | 550 (14.3) |
| ≥70 | 0 (0.0) | 0 (0.0) | 66 (5.9) | 99 (6.8) | 165 (9.4) | 132 (6.5) | 99 (5.0) | 132 (5.6) | 198 (7.2) | 297 (8.1) | 150 (3.6) | 200 (5.2) |
| Combined age (years) | ||||||||||||
| <70 | 1,155 (100.0) | 792 (100.0) | 1,056 (94.1) | 1,353 (93.2) | 1,584 (90.6) | 1,914 (93.5) | 1,881 (95.0) | 2,244 (94.4) | 2,541 (92.8) | 3,366 (91.9) | 4,050 (96.4) | 3,650 (94.8) |
| ≥70 | 0 (0.0) | 0 (0.0) | 66 (5.9) | 99 (6.8) | 165 (9.4) | 132 (6.5) | 99 (5.0) | 132 (5.6) | 198 (7.2) | 297 (8.1) | 150 (3.6) | 200 (5.2) |
| Axillary surgery | ||||||||||||
| ALND | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 33 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Excision | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 33 (4.8) | 0 (0.0) | 0 (0.0) | 33 (1.7) | 0 (0.0) | 0 (0.0) |
| SNB | 330 (100.0) | 165 (100.0) | 264 (100.0) | 627 (100.0) | 627 (95.0) | 594 (100.0) | 660 (95.2) | 825 (100.0) | 1,254 (100.0) | 1,881 (98.3) | 2,200 (100.0) | 2,250 (100.0) |
| RT | ||||||||||||
| No | 627 (54.3) | 330 (41.7) | 561 (50.0) | 330 (22.7) | 561 (32.1) | 594 (29.0) | 825 (41.7) | 792 (33.3) | 1,320 (48.2) | 2,244 (61.3) | 2,000 (47.6) | 2,100 (53.8) |
| Yes | 528 (45.7) | 462 (58.3) | 561 (50.0) | 1,122 (77.3) | 1,188 (67.9) | 1,452 (71.0) | 1,155 (58.3) | 1,584 (66.7) | 1,419 (51.8) | 1,419 (38.7) | 2,200 (52.4) | 1,800 (46.2) |
| Region of surgery I | ||||||||||||
| Non-Seoul | 264 (50.0) | 264 (57.1) | 330 (58.8) | 693 (61.8) | 627 (52.8) | 726 (50.0) | 759 (65.7) | 1,089 (68.8) | 990 (69.8) | 957 (67.4) | 1,200 (54.5) | 1,000 (55.6) |
| Seoul | 264 (50.0) | 198 (42.9) | 231 (41.2) | 429 (38.2) | 561 (47.2) | 726 (50.0) | 396 (34.3) | 495 (31.2) | 429 (30.2) | 462 (32.6) | 1,000 (45.5) | 800 (44.4) |
| Region of surgery II | ||||||||||||
| Non-capital | 363 (31.4) | 132 (16.7) | 231 (20.6) | 462 (31.8) | 561 (32.1) | 594 (29.0) | 726 (36.7) | 792 (33.3) | 1,089 (39.8) | 1,221 (33.3) | 1,450 (34.5) | 1,100 (28.2) |
| Capital area | 792 (68.6) | 660 (83.3) | 891 (79.4) | 990 (68.2) | 1,188 (67.9) | 1,452 (71.0) | 1,254 (63.3) | 1,584 (66.7) | 1,650 (60.2) | 2,442 (66.7) | 2,750 (65.5) | 2,800 (71.8) |
| Region of surgery III | ||||||||||||
| Non-metropolitan | 198 (17.1) | 33 (4.2) | 66 (5.9) | 99 (6.8) | 165 (9.4) | 198 (9.7) | 264 (13.3) | 231 (9.7) | 363 (13.3) | 561 (15.3) | 450 (10.7) | 300 (7.7) |
| Metropolitan | 957 (82.9) | 759 (95.8) | 1,056 (94.1) | 1,353 (93.2) | 1,584 (90.6) | 1,848 (90.3) | 1,716 (86.7) | 2,145 (90.3) | 2,376 (86.7) | 3,102 (84.7) | 3,750 (89.3) | 3,600 (92.3) |
| Size of hospital of surgery | ||||||||||||
| <800 beds | 396 (34.3) | 231 (29.2) | 462 (41.2) | 528 (36.4) | 891 (50.9) | 825 (40.3) | 660 (33.3) | 693 (29.2) | 1,254 (45.8) | 1,320 (36.0) | 1,750 (41.7) | 1,500 (38.5) |
| ≥800 beds | 759 (65.7) | 561 (70.8) | 660 (58.8) | 924 (63.6) | 858 (49.1) | 1,221 (59.7) | 1,320 (66.7) | 1,683 (70.8) | 1,485 (54.2) | 2,343 (64.0) | 2,450 (58.3) | 2,400 (61.5) |
| RT technique | ||||||||||||
| 3D | 528 (100.0) | 462 (100.0) | 561 (100.0) | 1,122 (100.0) | 1,188 (100.0) | 1,452 (100.0) | 1,155 (100.0) | 1,518 (95.8) | 1,287 (90.7) | 825 (58.1) | 1,700 (77.3) | 950 (52.8) |
| IMRT | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 66 (4.2) | 132 (9.3) | 594 (41.9) | 500 (22.7) | 850 (47.2) |
| RT fractionation | ||||||||||||
| No RT | 627 (54.3) | 330 (41.7) | 561 (50.0) | 330 (22.7) | 561 (32.1) | 594 (29.0) | 825 (41.7) | 792 (33.3) | 1,320 (48.2) | 2,244 (61.3) | 2,000 (47.6) | 2,100 (53.8) |
| 1–14 | 33 (2.9) | 0 (0.0) | 0 (0.0) | 66 (4.5) | 0 (0.0) | 33 (1.6) | 66 (3.3) | 33 (1.4) | 132 (4.8) | 66 (1.8) | 50 (1.2) | 100 (2.6) |
| 15–20 | 66 (5.7) | 33 (4.2) | 0 (0.0) | 66 (4.5) | 198 (11.3) | 132 (6.5) | 33 (1.7) | 330 (13.9) | 264 (9.6) | 792 (21.6) | 1,000 (23.8) | 1,000 (25.6) |
| ≥21 | 429 (37.1) | 429 (54.2) | 561 (50.0) | 990 (68.2) | 990 (56.6) | 1,287 (62.9) | 1,056 (53.3) | 1,221 (51.4) | 1,023 (37.3) | 561 (15.3) | 1,150 (27.4) | 700 (17.9) |
Data are presented as number (%), unless otherwise indicated. ALND, axillary lymph node dissection; SNB, sentinel node biopsy; RT, radiation therapy; 3D, three-dimensional; IMRT, intensity-modulated RT.

Table 4
| Variables | RT omit vs. RT | Conv RT vs. hypofr RT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RT omit | RT | OR (95% CI) | P | Conv RT | Hypofr RT | OR (95% CI) | P | ||
| Treatment period | |||||||||
| 2009–2014 | 3,003 | 5,313 | 4,686 | 495 | |||||
| 2015–2020 | 9,281 | 9,577 | 1.15 (1.13–1.16) | <0.001 | 5,711 | 3,419 | 1.12 (1.1–1.13) | <0.001 | |
| Combined age (years) | |||||||||
| <70 | 11,357 | 14,229 | 10,067 | 3,716 | |||||
| ≥70 | 877 | 661 | 1.18 (1.15–1.22) | <0.001 | 330 | 198 | 1.1 (1.07–1.13) | <0.001 | |
| Axillary surgery | |||||||||
| No | 7,032 | 8,366 | 5,931 | 2,104 | |||||
| Yes | 5,252 | 6,524 | 1.04 (0.99–1.1) | – | 4,466 | 1,810 | 1.14 (1.06–1.23) | 0.001 | |
| Region of surgery† I | |||||||||
| Non-Seoul | 7,035 | 8,157 | 5,947 | 2,688 | |||||
| Seoul | 5,249 | 6,733 | – | – | 4,450 | 1,226 | 0.98 (0.97–1) | 0.008 | |
| Region of surgery† II | |||||||||
| Non-capital | 4,057 | 4,664 | 3,138 | 1,361 | |||||
| Capital area | 8,227 | 10,226 | 0.95 (0.93–0.96) | <0.001 | 7,259 | 2,553 | 1.04 (1.03–1.06) | <0.001 | |
| Region of surgery† III | |||||||||
| Non-metropolitan | 1,109 | 1,819 | 1,090 | 597 | |||||
| Metropolitan | 11,175 | 13,071 | 1.16 (1.14–1.19) | <0.001 | 9,307 | 3,317 | 0.98 (0.96–1) | 0.082 | |
| Size of hospital of surgery‡ | |||||||||
| <800 beds | 5,032 | 5,478 | 1.06 (1.04–1.07) | <0.001 | 4,003 | 1,243 | 0.99 (0.97–1) | 0.016 | |
| ≥800 beds | 7,252 | 9,412 | 6,394 | 2,671 | |||||
| RT technique | |||||||||
| 3D | – | – | 10,314 | 1,921 | |||||
| IMRT | – | – | – | – | 83 | 1,993 | 2.12 (2.08–2.15) | <0.001 | |
Data are presented as number, unless otherwise indicated. †, region of RT for hypofr RT analysis; ‡, size of hospital of RT for hypofr RT analysis. OR, odds ratio; RT, radiation therapy; conv, conventional fractionation; hypofr, hypofractionated; CI, confidence interval; 3D, three-dimensional; IMRT, intensity-modulated RT.
Discussion
Data from the HIRA-NPS database collected between 2009 and 2020 revealed an increasing trend in breast surgeries for DCIS, with the majority being BCS. After 2014, the uptake of implant-based breast reconstruction procedures significantly increased. The use of conventional fractionation RT decreased over time, whereas the use of hypofractionated RT and RT omission showed a steady increase, particularly in older patients.
The treatment pattern shift observed in South Korea for DCIS, with a gradual increase in lumpectomy with radiation (28.4–39.3%) and a decrease in mastectomy rates (43.3–27.9%), mirrors the trend in the United States. In the US, the adoption of lumpectomy with RT increased by almost 100% (24.2–46.8%), whereas that of unilateral mastectomy decreased by 60% (44.9–19.3%) (11). Currently, in both South Korea and the US, the most preferred treatment approach for DCIS is lumpectomy followed by WBI. These treatment trends are backed by the results of a representative meta-analysis conducted by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) in 2010, which included 3,700 locally excised DCIS cases from four randomized control trials (5). Adjuvant RT reduced the rate of ipsilateral breast events by approximately half compared to non-adjuvant RT (rate ratio, 0.46), including recurrent DCIS or invasive cancer, with an absolute 10-year risk reduction of 15.2%. Remarkably, RT was effective regardless of patient age, surgical extent, tamoxifen use, margins, or clinical presentation. Even in women with small, low-grade tumors and negative margins, a notable absolute reduction in the risk of ipsilateral breast events was observed. Long-term results from these trials reaffirmed the sustained effect of RT in reducing intramammary recurrence by half and maintaining an absolute risk reduction of 10–12%, although none of the studies showed a significant effect on breast cancer mortality (12-14). A recent study found that BCS + adjuvant RT had a low 6% overall recurrence rate at 85 months follow-up, with margin status, multifocality, hormone receptor status, and Her-2/Basal-like subtype identified as risk factors for local recurrence (15). Mastectomy, which is predominantly recommended when complete surgical removal through BCS is not feasible, has shown excellent outcomes. Studies on mastectomy have reported a high locoregional control rate of 96–100% and a low cancer-specific mortality rate of less than 4% (16,17). Given that the vast majority of the breast glandular tissue is removed during mastectomy, the incidence of invasive breast cancer recurrence is extremely rare. The reported ipsilateral breast recurrence rates are 0.8–1.9% after mastectomy, 4.1–8.8% after BCS + RT, and 7.2–15.4% after BCS alone (17,18). The move from mastectomy to lumpectomy with radiation is influenced by the increased diagnosis of small-sized DCIS, propelled by expanded screening programs (2). Increased patient preference for breast conservation and robust clinical evidence are also contributing to this shift.
The increase in TM rates from 2014 to 2016 can be attributed to the implementation of coverage by the NHIS in April 2015 (19). NHIS coverage significantly reduced the high cost of breast reconstruction, resulting in a notable decrease in the expenses faced by patients for autologous tissue- and prosthesis-based breast reconstruction, nipple-areolar complex reconstruction, and additional operations due to complications or deformities after reconstruction. According to a study, after NHIS coverage expansion, surgery-related costs, including anesthesia, inpatient care, and medication, decreased by half. Some patients strategically scheduled cancer operations post-April 2015 to lower expenses. A rise in post-2015 delayed breast reconstruction among breast cancer survivors with deformities may be attributed to reduced costs (20). Data on the type of breast reconstruction have become available since the implementation of NHIS coverage. In 2015, autologous and implant-based reconstruction accounted for 36.4% and 63.6% of all breast reconstruction surgeries, respectively. However, there was a rapid increase in the annual growth of 28% in the use of implant-based reconstruction, surpassing that of autologous tissue reconstruction. By 2020, the proportion of autologous reconstructions had decreased to 11.1%, while that of implant-based reconstructions had substantially increased to 88.9%. Consequently, the noteworthy rise in TM rates likely corresponds to an increase in subcutaneous mastectomies. The observed trend was similar to the surgical pattern observed among patients with invasive breast cancer. In comparison with the 2-year interval preceding April 2015, over the 2-year period following April 2015, there was a statistically significant surge in TM rates (41.9% vs. 36.6%) and a corresponding significant increase in the proportion of prosthesis-based reconstructions among those who underwent immediate breast reconstruction (67.5% vs. 44.6%) (20). Implant-based breast reconstruction has a disadvantage: it can result in a less natural breast shape than autologous tissue reconstruction and is susceptible to late capsular contracture related to radiation (21). Advancements in synthetic materials have significantly improved breast shape preservation, leading to increased acceptance and utilization of implant-based reconstruction (22). A common strategy involves inserting an expander during the initial surgery, followed by post-mastectomy RT and a two-stage implant exchange operation (23). Immediate implant reconstruction is a favorable option, especially in cases of DCIS, where adjuvant RT is rarely utilized. Implant-based reconstruction offers notable benefits, including short surgical and recovery durations (24); it also eliminates the need for additional hospital visits for RT. As we navigate the challenges posed by the pandemic, it is imperative to monitor and observe evolving trends in breast reconstruction treatments. This proactive approach will optimize patient care and achieve favorable outcomes.
In the later treatment periods, especially after 2018, there was a significant increase in the use of hypofractionated RT, accounting for over half of the patients receiving RT. Clinical evidence and the establishment of hypofractionated RT as a standard treatment for invasive carcinoma are considered the main factors contributing to the growing adoption of hypofractionated RT. In 2018, the American Society for Radiation Oncology (ASTRO) revised its guidelines to advocate for the use of HF-WBI as a viable treatment option for DCIS. Although comprehensive randomized controlled trials (RCTs) targeting pure DCIS cases are lacking, the evidence from RCTs on micro-invasive carcinoma, observational approaches, and population-based studies cumulatively provides robust support for the application of hypofractionation in DCIS treatment (8). Subsequently, a clinical trial conducted by the Trans-Tasman Radiation Oncology Group (TROG) in Australia and New Zealand compared HF-WBI and CF-WBI in DCIS. The trial revealed no statistically significant disparities in the 5-year rates of freedom from local recurrence between CF-WBI (94.9%) and HF-WBI (94.9%) groups (25). The Danish Breast Cancer Group (DBCG) HYPO trial randomized women with adenocarcinoma or DCIS to receive 50 Gy in 25 fractions or 40 Gy in 15 fractions. Among the 246 DCIS patients, locoregional recurrence was observed in 7.7% of cases, with no discernible distinction between the treatment groups [hazard ratio (HR), 1.40; 95% confidence interval (CI): 0.49–4.05; P=0.053] (26). In South Korea, the cost analysis indicates that hypofractionated RT (42.56 Gy/16 fractions) led to a significant saving of 675.64 US dollars (USD) (26.6% reduction) compared to conventional fractionation RT (50.4 Gy/28 fractions). The reduction in patient out-of-pocket costs is approximately 34.80 USD. Furthermore, the adoption of hypofractionated RT has the potential to further decrease indirect costs by shortening the treatment period to 2 weeks (27). In the later part of our study, an increasing trend was observed among Korean women with DCIS who underwent BCS and opted to omit RT. Notably, this trend was prominent among elderly individuals and those treated at metropolitan surgical centers. Unfortunately, factors crucial for the decision to omit RT, such as histological grade, size, and diagnostic approach, could not be analyzed from the available dataset. In the US, during the COVID-19 pandemic, omission of RT was recommended for patients with mammographically detected lesions <2.5 cm, low- or intermediate-grade tumors, adequate margins (≥2 mm), and those aged over 40 years (28). Notably, this recommendation is specific to unique circumstances. Recent prospective studies have suggested omission of RT for individuals classified in the “good risk group”; hence, caution is required when advising omission. The Radiation Therapy Oncology Group (RTOG) 9804 identified good-risk patients as those with mammographically detected low- or intermediate-grade DCIS measuring less than 2.5 cm, with margins ≥3 mm (29). The trial compared the outcomes of RT vs. observation after surgery. The 7-year results indicated a low ipsilateral local failure rate in both arms (0.9% in the RT arm and 6.7% in the observation arm); however, the RT arm had a significantly low failure rate (HR, 0.11; P<0.001). Notably, for patients with high nuclear grade and large tumor size and young patients, the addition of RT should be considered, as it can significantly affect overall survival (6). Currently, three clinical trials [COMET in the USA, LORIS in the UK, and LORD in The Netherlands/European Union (EU)] are actively recruiting patients to address this nuanced issue (30-32).
Our study has several limitations. First, the retrospective nature of our analysis of a national claims database may have led to potential bias and incomplete clinical information. An example is the possible misclassification of lumpectomy patients who underwent surgery in December using N7135 code and received RT in the subsequent year, potentially leading to misrepresentation as mastectomy cases. Second, certain key factors that influence the decision, such as histological grade, tumor size, and diagnostic approach, could not be assessed. The database lacks detailed information on patient preferences, socioeconomic statuses, and genetic profiles, which could affect treatment choices. Considering the generalizability of our findings in a clinical context, the lack of specific individualized treatment data emphasizes the need for further research to tailor therapeutic approaches to individual patients.
Conclusions
In conclusion, our study provides insights into the changing treatment landscape of DCIS in South Korea. We observed an increasing trend in breast surgeries for DCIS, particularly BCS, and increased utilization of implant-based breast reconstruction. The use of RT has also evolved, with a notable increase in hypofractionated RT and omission of RT. While recommendations for RT omission exist, caution is advised, and decisions should be tailored to individual patient factors and tumor characteristics. As we navigate the complexities of breast cancer treatment, continuous monitoring and analysis of evolving trends are crucial for optimizing patient care and outcomes. Efforts to further understand the impact of treatment decisions and to explore novel approaches through ongoing clinical trials will contribute to advancements in DCIS management.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-433/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-433/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-433/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-433/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). The study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2022-128), and the requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heller SL, Plaunova A, Gao Y. Ductal carcinoma in situ and progression to invasive cancer: a review of the evidence. J Breast Imaging 2021;3:135-43.
- Hong S, Lee YY, Lee J, et al. Trends in Cancer Screening Rates among Korean Men and Women: Results of the Korean National Cancer Screening Survey, 2004-2018. Cancer Res Treat 2021;53:330-8. [Crossref] [PubMed]
- Kang SY, Kim YS, Kim Z, et al. Breast Cancer Statistics in Korea in 2017: Data from a Breast Cancer Registry. J Breast Cancer 2020;23:115-28. [Crossref] [PubMed]
- O'Keefe TJ, Harismendy O, Wallace AM. Histopathological growth distribution of ductal carcinoma in situ: tumor size is not "one size fits all". Gland Surg 2022;11:307-18. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr 2010;2010:162-77. [Crossref] [PubMed]
- Sagara Y, Freedman RA, Vaz-Luis I, et al. Patient Prognostic Score and Associations With Survival Improvement Offered by Radiotherapy After Breast-Conserving Surgery for Ductal Carcinoma In Situ: A Population-Based Longitudinal Cohort Study. J Clin Oncol 2016;34:1190-6. [Crossref] [PubMed]
- Wakefield DV, Eichler T, Wilson E, et al. Variable Effect of the COVID-19 Pandemic on Radiation Oncology Practices in the United States. Int J Radiat Oncol Biol Phys 2022;113:14-20. [Crossref] [PubMed]
- Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018;8:145-52. [Crossref] [PubMed]
- Meattini I, Becherini C, Boersma L, et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol 2022;23:e21-31. [Crossref] [PubMed]
- Service HIRA. HIRA Bigdata Open portal. 2023. Accessed 2023 Aug 11. Available online: https://opendata.hira.or.kr/home.do
- Worni M, Akushevich I, Greenup R, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst 2015;107:djv263. [Crossref] [PubMed]
- Donker M, Litière S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol 2013;31:4054-9. [Crossref] [PubMed]
- Wärnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol 2014;32:3613-8. [Crossref] [PubMed]
- Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 2011;12:21-9. [Crossref] [PubMed]
- Tomasicchio G, Picciariello A, Stucci LS, et al. Outcome and risk factors for local recurrence after breast conserving surgery in patients affected by ductal carcinoma in situ. Minerva Surg 2022;77:536-41. [Crossref] [PubMed]
- Bijker N, Donker M, Wesseling J, et al. Is DCIS breast cancer, and how do I treat it? Curr Treat Options Oncol 2013;14:75-87. [Crossref] [PubMed]
- Halperin EC, Wazer DE, Perez CA, et al. editors. Perez & Brady's Principles and Practice of Radiation Oncology. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2018.
- Thompson AM, Clements K, Cheung S, et al. Management and 5-year outcomes in 9938 women with screen-detected ductal carcinoma in situ: the UK Sloane Project. Eur J Cancer 2018;101:210-9. [Crossref] [PubMed]
- Welfare MoHa. Discussing Strategies for Expanded Selective Coverage and Enhanced Mid-term Sustainability of Breast Reconstruction Surgery. Sejong 2015. Available online: https://www.mohw.go.kr/eng/index.jsp
- Hong KY, Son Y, Chang H, et al. Trends in breast reconstruction: Implications for the National Health Insurance Service. Arch Plast Surg 2018;45:239-45. [Crossref] [PubMed]
- Jagsi R, Momoh AO, Qi J, et al. Impact of Radiotherapy on Complications and Patient-Reported Outcomes After Breast Reconstruction. J Natl Cancer Inst 2018;110:157-65. [Crossref] [PubMed]
- Govshievich A, Somogyi RB, Brown MH. Conservative mastectomies and immediate reconstruction with the use of ADMs. Gland Surg 2015;4:453-62. [Crossref] [PubMed]
- Ho AY, Hu ZI, Mehrara BJ, et al. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol 2017;18:e742-53. [Crossref] [PubMed]
- Ganesh Kumar N, Kung TA. Guidelines for breast reconstruction during the COVID-19 pandemic: Are we considering enough evidence? Breast J 2020;26:2108-9. [Crossref] [PubMed]
- Chua BH, Link EK, Kunkler IH, et al. Radiation doses and fractionation schedules in non-low-risk ductal carcinoma in situ in the breast (BIG 3-07/TROG 07.01): a randomised, factorial, multicentre, open-label, phase 3 study. Lancet 2022;400:431-40. [Crossref] [PubMed]
- Offersen BV, Alsner J, Nielsen HM, et al. Hypofractionated Versus Standard Fractionated Radiotherapy in Patients With Early Breast Cancer or Ductal Carcinoma In Situ in a Randomized Phase III Trial: The DBCG HYPO Trial. J Clin Oncol 2020;38:3615-25. [Crossref] [PubMed]
- Lee A, Kim HY, Kim TH, et al. Hypofractionated Radiotherapy for Early-Stage Breast Cancer: A Propensity Score Matched Analysis. J Korean Med Sci 2022;37:e64. [Crossref] [PubMed]
- Braunstein LZ, Gillespie EF, Hong L, et al. Breast Radiation Therapy Under COVID-19 Pandemic Resource Constraints-Approaches to Defer or Shorten Treatment From a Comprehensive Cancer Center in the United States. Adv Radiat Oncol 2020;5:582-8. [Crossref] [PubMed]
- McCormick B, Winter K, Hudis C, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol 2015;33:709-15. [Crossref] [PubMed]
- Hwang S. Comparing an Operation to Monitoring, With or Without Endocrine Therapy (COMET) Trial For Low Risk DCIS (COMET). 2017. Available online: https://clinicaltrials.gov/study/NCT02926911?term=Comparing%20an%20Operation%20to%20Monitoring,%20with%20or%20without%20Endocrine%20Therapy&rank=1
- Francis A, Bartlett JMS, Billingham LJ, et al. Abstract OT2-3-01: The LORIS trial: A multicentre, randomized phase III trial of standard surgery versus active monitoring in women with newly diagnosed low risk ductal carcinoma in situ. Cancer Res 2013;73:OT2-3-01.
- Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study. Eur J Cancer 2015;51:1497-510. [Crossref] [PubMed]


