
The novel use and feasibility of hemostatic oxidized regenerated cellulose agent (SurgiGuard®): multicenter retrospective study
Highlight box
Key findings
• SurgiGuard® showed stable feasibility and effectiveness with no direct adverse effect.
What is known and what is new?
• Oxidized regenerated cellulose is effective for hemostasis with various surgeries
• Among oxidized regenerated cellulose products, SurgiGuard® is effective and feasible.
What is the implication, and what should change now?
• Prospective comparative studies are needed in the future with this product.
Introduction
Perioperative bleeding is a major concern for surgeons, and efforts have been made by numerous surgeons and researchers to prevent perioperative bleeding (1). The reported prevalence of postoperative bleeding is 0.9–10% in various major surgeries, such as hepatectomy (2), pancreatic surgery (3), gastrointestinal tract surgery (4,5), cardiovascular surgery (6,7), nephrectomy (8), and liver transplantation (9). On a closer look, various recent studies have reported that the prevalence of capillary, venous and small artery bleeding is in the range of 3.3–30% (10-12). The evolution of hemostasis during the last few centuries of surgical history has resulted from the development of hemostatic agents and devices, as well as surgical skills and principles (1). Moreover, different types of bleeding occur, and appropriate methods should be applied in each situation. Several materials have been devised to control bleeding by understanding the mechanisms of the hemostatic process (13,14).
Several commercial hemostatic products assist with the hemostatic process. Polysaccharides are biologically derived polymers composed of sugar building blocks. Polysaccharide-based materials can be prepared and modified by chemical or physical methods (15). Since Frantz reported the potential for clinical application of oxidized cellulose as a topical hemostatic agent in 1943 (16), oxidized regenerated cellulose (ORC) has been developed and achieved notable clinical results (17).
SurgiGuard® (Samyang Biopharmaceuticals Corp., Seoul, Korea) is an absorbent hemostatic agent based on ORC. It is a hemostatic supplement used when other methods, such as the ligation of capillaries, veins, and arterial bleeding, are ineffective during surgery (18). The carboxyl group of oxidized cellulose has a low pH (acidity) through an oxidation reaction to promote hemostatic action and inhibit bacterial growth. In the case of in vitro, the product is suspended in water, and no appreciable solvation occurs; however, a drop in pH lower than 2.5 is observed. In in vivo studies (including previous papers reported by our group), after 24 h post-implantation, it has been noted that the product becomes completely gelatinous, and within 48 h, only small fragments remain. A rapid decrease in the pH (~2.5) of the fluid surrounding the site of implantation of the ORC has also been observed (19-22). The effectiveness of SurgiGuard® has been demonstrated to be equivalent to existing hemostatic agents in several animal studies, and the safety of the product has been demonstrated through biocompatibility tests and antimicrobial tests by NAMSA (Medical Research Organization, Toledo, OH, USA) (23).
To confirm that sustained use of this hemostatic material is feasible, it is important to clinically determine that the use of SurgiGuard® is effective compared to combination use with other hemostatic methods. Therefore, this study retrospectively reviewed data collected from patients who used SurgiGuard® to assess its effectiveness, safety and feasibility. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-675/rc).
Methods
Study population and study design
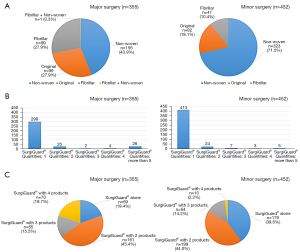
We collected the clinical data of patients who underwent surgery at seven different tertiary medical centers between January 2018 and December 2018. A total of 22 surgeons from 12 different departments participated in this study. To eliminate bias, all types of surgeries using a full anticoagulation agent or medication during surgery, such as cardiopulmonary surgery, were excluded from the study. Patients who underwent minor vascular surgery and kidney/liver transplantation with limited-dose heparin were included. We retrospectively investigated sex, diagnosis, surgical department, co-morbidities, medications, and perioperative findings (surgery, estimated blood loss, transfusion, serum hemoglobin level, time to hemostasis, drain usage). “Hemostasis” was defined as any type of oozing or pulsatile bleeding that was not observed at the bleeding site after application of SurgiGuard®. In cases of rebleeding even after the application of SurgiGuard®, the time until rebleeding occurred was also recorded. As the total patient cohort was heterogeneous, we divided it into two groups to assess the SurgiGuard® product: group A, who used SurgiGuard® alone (n=248), and group B, who used SurgiGuard® with other hemostatic products (n=559). All surgery types were categorized as major (total operative time ≥4 hours) or minor surgery (total operative time <4 hours; Figure 1).
To assess the hemostatic effectiveness and user satisfaction of handling SurgiGuard® as a hemostasis supplement, we used a questionnaire containing a numeric 6-point scale. The hemostatic effectiveness score was given up to the first decimal place. From the surgeon’s point of view, 6 points indicated that the surgeon was very satisfied with the hemostatic effect, and 1 point indicated they were very dissatisfied. Regarding the satisfaction score, 6 points indicated that surgical handling was very satisfactory as a hemostatic supplement, and 1 point indicated it was very unsatisfactory (Appendix 1). In addition, we described adverse effects as more than grade 3 according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTC-AE). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Severance Hospital (Approval No. 4-2019-0576) and informed consent was taken from all the patients.
SurgiGuard®
SurgiGuard® is a type of ORC capable of assisting in managing small vessel bleeding. It is designed to achieve hemostasis when conventional surgical techniques are not available or are impractical. Indication for use is as follows: “During the operation, this product assists the hemostasis for capillary and venous, small artery bleeding”. Thus, this product is generally applied in a situation corresponding to the VIBe scale grade 1 or 2. Four types of SurgiGuard® products were used in this study (Figure 2). SurgiGuard Original® is the most common and has long and widely been used in a variety of surgeries. It offers good visibility of the surgical site due to the sheer knit structure. SurgiGuard Fabric® is denser than SurgiGuard Original® and made for heavier bleeding with faster hemostasis. In contrast, SurgiGuard Fibrillar® can be shaped or molded to various shapes for optimal adherence or used in multiple sites. Finally, SurgiGuard Non-woven® is an advanced product for maximized effect and superior handling. The non-woven structure increases surface contact with the bleeding site and can be applied not only in open surgery, but also minimally invasive surgery.
Statistical analysis
Continuous variables were analyzed using the independent samples t-test, and categorical variables were analyzed using Pearson’s chi-squared test or Fisher’s exact test. All results are expressed as mean and standard deviation or frequency and percentage. P<0.05 indicates significance. All statistical analyses were performed using SPSS® for Windows version 22.0 (IBM Corp., Armonk, NY, USA) and R version 3.3.1 (The R Project, Vienna, Austria).
Results
Clinical characteristics and use of products
A total of 807 patients were enrolled in this study, 29% of which underwent hepato-biliary-pancreas surgeries. The Obstetrics and Gynecology Department and Neurosurgery Department had 155 (19.2%) and 150 (18.6%) patients, respectively (Figure 3). The mean age was 53.0±18.5 years, and female patients were 54.5% of the cohort (n=440). A total of 360 (44.6%) patients who had no comorbidities, and 266 (33.0%) had hypertension, the most common comorbid disease. Regarding the type of surgery, 355 (44.0%) patients underwent major surgery and 452 (56.0%) patients underwent minor surgery (Table 1).
Table 1
| Variable | Patients (N=807) |
|---|---|
| Age, years, mean ± SD | 53.0±18.5 |
| Sex, n (%) | |
| Female | 440 (54.5) |
| Male | 367 (45.5) |
| Number of comorbidities, n (%) | |
| None | 360 (44.6) |
| 1 | 255 (31.6) |
| 2 | 135 (16.7) |
| 3 | 43 (5.3) |
| 4 | 14 (1.7) |
| Liver disease, n (%) | |
| No | 727 (90.1) |
| Yes | 80 (9.9) |
| Renal disease, n (%) | |
| No | 772 (95.7) |
| Yes | 35 (4.3) |
| Hypertension, n (%) | |
| No | 541 (67.0) |
| Yes | 266 (33.0) |
| Diabetes, n (%) | |
| No | 681 (84.4) |
| Yes | 126 (15.6) |
| Other disease, n (%) | |
| No | 604 (74.8) |
| Yes | 203 (25.2) |
| Operation type, n (%) | |
| Major (≥4 hours) | 355 (44.0) |
| Minor (<4 hours) | 452 (56.0) |
SD, standard deviation.
SurgiGuard Non-woven® was the most commonly used product in both types of surgery. More than 70% of minor surgeries used the non-woven product, and solitary use occurred in more than 83% of minor surgeries. In contrast, in major surgery, 26 (7.3%) patients used more than five products (Figure 4).
Perioperative findings
Regarding the type of surgery, we found a significant difference between the two groups. Minor surgery was the most common in group A (72.2%) and major surgery was the most common in group B (51.2%; P<0.001).
We found no significant differences between groups A and B concerning preoperative and postoperative hemoglobin levels. In group B, 503 patients (90.0%) had an indwelling postoperative surgical drain, and the amount on postoperative day 1 was 198.7±299.2 mL. In contrast, in group A, 136 patients (54.8%) had an inserted drain, and the amount was 128.2±136.9 mL (P<0.001).
The estimated blood loss was significantly higher in group B than group A (mean 261.6 vs. 506.7 mL, P=0.001). Similarly, the postoperative transfusion rate was higher in group B than group A (7.7% vs. 23.8%, P<0.001). The mean time to hemostasis was 4.0±2.7 minutes in group A and 6.3±9.8 minutes in group B.
We found no significant differences regarding the mean time to hemostasis between the two groups (P=0.685). Intraoperative re-bleeding after SurgiGuard® application occurred in 7 patients (group B only), but we found no significant differences between the two groups (P=0.174). The mean time to re-bleeding was 6.3±11.6 min (Table 2).
Table 2
| Variable | Group A (N=248) | Group B (N=559) | P |
|---|---|---|---|
| Preoperative hemoglobin, g/dL, mean ± SD | 12.7±1.7 | 12.8±2.0 | 0.663 |
| Postoperative day 1 hemoglobin, g/dL, mean ± SD | 11.5±1.7 | 11.5±1.8 | 0.802 |
| Postoperative drain, n (%) | <0.001 | ||
| No | 112 (45.2) | 56 (10.0) | |
| Yes | 136 (54.8) | 503 (90.0) | |
| Postoperative day 1 drainage amount, mL, mean ± SD | 128.2±136.9 | 198.7±299.2 | <0.001 |
| Operation type, n (%) | <0.001 | ||
| Major | 69 (27.8) | 286 (51.2) | |
| Minor | 179 (72.2) | 273 (48.8) | |
| Estimated blood loss (mL), mean ± SD | 261.6±452.4 | 506.7±1,403.2 | 0.001 |
| Postoperative transfusion, n (%) | <0.001 | ||
| No | 229 (92.3) | 426 (76.2) | |
| Yes | 19 (7.7) | 133 (23.8) | |
| Time to hemostasis, min, mean ± SD | 0.685 | ||
| No postoperative bleeding | 4.0±2.7 | 6.3±9.8 | |
| Re-bleeding after SurgiGuard® | N/A | 6.3±11.6 | |
| Intra-operative re-bleeding, n (%) | 0.174 | ||
| No | 248 (100.0) | 552 (98.7) | |
| Yes | 0 (0.0) | 7 (1.3) | |
| Additional treatment, n (%) | N/A | ||
| SurgiGuard® + thrombin | 0 (0.0) | 1 (0.2) | |
| SurgiGuard® + Floseal | 0 (0.0) | 2 (0.4) | |
| Surgicel + TachoSil | 0 (0.0) | 1 (0.2) | |
| Surgicel + Tisseel + Fibrillar + Gelfoam | 0 (0.0) | 1 (0.2) | |
| Surgicel + Greenplast + Fibrillar | 0 (0.0) | 1 (0.2) | |
| Floseal | 0 (0.0) | 1 (0.2) |
Group A: used SurgiGuard® alone; Group B: used SurgiGuard® with other hemostatic products. SD, standard deviation; N/A, not available.
Effectiveness and satisfaction grade
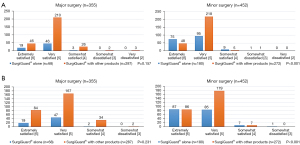
In major surgery, we found no significant differences in the hemostatic effectiveness grade or the handling satisfaction grade between the two groups. In minor surgery, the proportion of patients with the most satisfying scale (≥ grade 5) was small in group A compared to group B (hemostatic effectiveness grade ≥5: 94.4% vs. 97.8%, user handling satisfaction grade ≥5: 95.6% vs. 97.4%; Figure 5).
Effectiveness score and reasons for ineffective cases
In both major and minor surgery, the effectiveness score was higher with SurgiGuard® used alone (major surgery, 5.3±0.5 vs. 5.1±0.6, P=0.048; minor surgery, 5.4±0.6 vs. 5.2±0.4, P<0.001). We identified three cases of ‘very dissatisfied’ responses, the reasons for which were comorbidities, concomitant drug use, and vascular injury (Table 3).
Table 3
| SurgiGuard® use | Major | Minor | |||||
|---|---|---|---|---|---|---|---|
| Group A (N=68) | Group B (N=287) | P | Group A (N=180) | Group B (N=272) | P | ||
| Effectiveness score, mean ± SD | 5.3±0.5 | 5.1±0.6 | 0.048 | 5.4±0.6 | 5.2±0.4 | <0.001 | |
| Reason for not-effective, n (%) | N/A | N/A | |||||
| Comorbidities (liver, renal, sepsis, etc.) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | |||
| Concomitant drug (aspirin, warfarin, etc.) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | |||
| Vascular injury | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | |||
Group A: used SurgiGuard® alone; Group B: used SurgiGuard® with other hemostatic products. SD, standard deviation; N/A, not available.
Adverse effect
A total of nine postoperative adverse effects were reported: bleeding, postoperative intestinal obstruction, abscess, fever, leg swelling, and respiratory dysfunction. There were no general dysfunctions such as liver/renal dysfunction, postoperative fever or localized wound infection. The most common adverse effect was bleeding or leakage from the treated site (3 patients, 33.3%), and one patient required an intraoperative transfusion. Postoperative intestinal obstruction occurred in 2 patients (22.2%). However, these complications did not directly correlate with SurgiGuard® use (Table 4).
Table 4
| Variable | Group A (N=248) | Group B (N=559) | P |
|---|---|---|---|
| Adverse effect, n (%) | <0.001 | ||
| No | 248 (100.0) | 550 (98.4) | |
| Yes | 0 (0.0) | 9 (1.6) | |
| Specific adverse effect, n (%) | |||
| Bleeding/leakage from treated site | 0 (0.0) | 3 (33.3)† | |
| Postoperative intestinal obstruction | 0 (0.0) | 2 (22.2) | |
| Abscess | 0 (0.0) | 1 (11.1) | |
| Fever | 0 (0.0) | 1 (11.1) | |
| Leg swelling | 0 (0.0) | 1 (11.1) | |
| Respiratory dysfunction | 0 (0.0) | 1 (11.1) | |
| Correlation with SurgiGuard®, n (%) | |||
| No relation | 0 (0.0) | 9 (100.0) |
†, including one patient who required intraoperative transfusion. Group A: used SurgiGuard® alone; Group B: used SurgiGuard® with other hemostatic products.
Discussion
A topical hemostatic agent should not only have a certain ability to achieve hemostasis, but also minimum tissue reactivity. Given these characteristics, polysaccharide-based materials are a good source for topical hemostatic agents. Polysaccharides are naturally derived polymers with advantages such as abundant sources, biodegradability, compatibility, and no immune responses (15,24). Polysaccharide-based hemostatic materials have evolved with the development of technology and requirements in the clinic, and many researchers and clinicians have used these devices as topical hemostatic agents, tissue adhesives, and sealants (25,26). Due to biocompatibility, biodegradability, low toxicity, and relatively low cost, ORC has been widely used in medical products, such as hemostatic agents, wound dressings, and drug carriers (27). In the 1960s, Surgicel® (Johnson and Johnson, New Brunswick, NJ, USA) was introduced to the clinical market as the first ORC-derived hemostatic agent. Since then, ORC has evolved into a globally used topical hemostatic agents (28).
A recently developed and advanced form of ORC can aid in hemostasis through calcium and sodium ion interactions, acid-induced small vessel contraction, and sealant properties. In addition, ORC acts as a support matrix for the initiation and formation of the clot. These material-derived products can be molded into different shapes and sizes, and are compressible without loss of hemostatic ability (29). ORC has great potential with minimum cost, low rate of thrombotic complications, and low disease transmission risk. Moreover, it provides the benefit of a long shelf-life (13). ORC has also been applied for dressings, which are versatile and do not require wounds to be of a certain duration before application (30). Furthermore, the ORC not only shows an excellent hemostatic effect but has also emerged as an effective adhesion barrier over the past several years. In various abdominal surgeries, ORC and its derived products have proven to be effective and feasible for preventing postoperative adhesion events (31-33). According to a large cohort meta-analysis, ORC significantly reduced the incidence of adhesions, and no trials have reported data on reoperation for adhesive small bowel obstruction (34). When the sheet form of the ORC is placed to cover the surgical site, it changes into a gel form within 24 days, and the ORC is degraded by phagocytosis by macrophages. During tissue repair, fibroblasts, epithelial cells, and endothelial cells are stimulated to increase tissue-reinforcing efficacy, which is thought to act as an adhesion barrier (19,35).
In this study, we assessed the hemostatic effectiveness and satisfaction with user handling of SurgiGuard® from the perspective of a surgeon who confronted the surgical field. We previously reported the effectiveness and non-toxicity of this product in the porcine spleen and liver resection models. It was then important to verify the surgeon-oriented effectiveness for application in the real surgical field based on clinical data. The results of the current study confirmed that the surgeon’s preferred material type (SurgiGuard Non-woven®) regardless of surgery type. This result reflects the importance of ease of handling of the topical agent and how well it works on the treated site. Regardless of major or minor surgery, the effectiveness score was defined as how much the hemostatic effect of the SurgiGuard® satisfied the surgeon, which was higher in group A. In minor surgery, the most satisfied proportion (≥ grade 5) was smaller in group A than group B. However, the ‘extremely satisfied’ category (grade 6) in both effectiveness and satisfaction more frequently had patients in group A compared to group B. For the effectiveness grade, six patients in group B and one patient in group A were ‘somewhat/very dissatisfied’. Overall, no SurgiGuard®-related adverse effects were reported, but nine patients had postoperative complications.
Although randomized controlled trials have investigated the efficacy and safety of ORC as a topical hemostatic agent (36), the present study was based on a large cohort focusing on the clinical effectiveness of ORC-derived material in multiple clinical surgery departments. In previous studies, the SurgiGuard® shown to be effective and safe in porcine models (18,23). Based on these favorable results, a large-cohort multicenter collaborative study was designed and conducted. These results evaluated and reported from the perspective of surgeons who used topical hemostasis agents themselves may be a milestone for more surgeons who will use these materials in the future.
However, ORC hemostatic agents have several side effects. ORC has been reported to dissolve promptly at various sites in an animal experiment within 6 weeks (37). In contrast to the animal model, several case reports have presented that the residue of ORC could easily be mistaken for an abscess or granuloma on postoperative imaging (38). For this reason, some clinicians have suggested that ORC should be used in extreme care for rigid non-extensive anatomical structures and be removed after hemostasis as soon as possible (39).
Despite encouraging results, this study has certain limitations. First, this study was based on a survey that received responses from a surgeon who performed various surgeries. Thus, one of the main challenges of this study is that the results reflect subjective points of view and experiences. Second, due to the heterogeneity of the analyzed study group, we did not sufficiently investigate the unique characteristics of each surgery. In the same context, the degree of bleeding was not accurately assessed using the confirmed bleeding scale VIBe SCALE (validated intraoperative bleeding scale) (40). Third, this study was focused on a short-term outcome survey, and it was not possible to investigate long-term complications, such as abscess or mass-like foreign body, the most common complication of ORC-derived hemostatic agents. As various surgeries and divisions were included, the endpoint of this study was unclear. Further studies, such as head-to-head, randomized controlled cohorts, are required to investigate not only short-term but also long-term complications, taking into account the characteristics of each surgery. Moreover, research on which type of SurgiGuard® is useful and effective under what circumstances should be accompanied.
Conclusions
In conclusion, SurgiGuard® had a higher effectiveness score when SurgiGuard® is used alone, and no direct adverse effects associated with SurgiGuard® use were reported. A prospective comparative study will be needed in the near future.
Acknowledgments
We would like to thank the following researchers for providing the clinical data: Yong Jin Kim, MD, PhD; Jeong Sig Kim, MD, PhD; Hyun-Young Kim, MD, PhD; Hee Seung Kim, MD, PhD; Kee Hyun Nam, MD, PhD; Sukh Que Park, MD, PhD; Jin-Sung Park, MD; Pyoungjae Park, MD, PhD; Jong Young Lee, MD, PhD; Hong Jun Jeon, MD, PhD; Bo-Hyun Jung, MD, PhD; Gi Hong Choi, MD, PhD; Dai Hoon Han, MD; Won Sik Ham, MD, PhD.
Funding: This work was supported by a research grant from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-675/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-675/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-675/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-675/coif). MJ is current employee of Samyang Biopharmaceuticals Corp. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Severance Hospital (Approval No. 4-2019-0576) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med 2007;356:2301-11. [Crossref] [PubMed]
- Lim C, Dokmak S, Farges O, et al. Reoperation for post-hepatectomy hemorrhage: increased risk of mortality. Langenbecks Arch Surg 2014;399:735-40. [Crossref] [PubMed]
- Correa-Gallego C, Brennan MF, D'Angelica MI, et al. Contemporary experience with postpancreatectomy hemorrhage: results of 1,122 patients resected between 2006 and 2011. J Am Coll Surg 2012;215:616-21. [Crossref] [PubMed]
- Song W, Yuan Y, Peng J, et al. The delayed massive hemorrhage after gastrectomy in patients with gastric cancer: characteristics, management opinions and risk factors. Eur J Surg Oncol 2014;40:1299-306. [Crossref] [PubMed]
- Golda T, Zerpa C, Kreisler E, et al. Incidence and management of anastomotic bleeding after ileocolic anastomosis. Colorectal Dis 2013;15:1301-8. [Crossref] [PubMed]
- Dyke C, Aronson S, Dietrich W, et al. Universal definition of perioperative bleeding in adult cardiac surgery. J Thorac Cardiovasc Surg 2014;147:1458-63.e1. [Crossref] [PubMed]
- McIlroy D, Murphy D, Kasza J, et al. Association of postoperative blood pressure and bleeding after cardiac surgery. J Thorac Cardiovasc Surg 2019;158:1370-9.e6. [Crossref] [PubMed]
- Jung S, Min GE, Chung BI, et al. Risk factors for postoperative hemorrhage after partial nephrectomy. Korean J Urol 2014;55:17-22. [Crossref] [PubMed]
- Jung SW, Hwang S, Namgoong JM, et al. Incidence and management of postoperative abdominal bleeding after liver transplantation. Transplant Proc 2012;44:765-8. [Crossref] [PubMed]
- Schuhmacher C, Pratschke J, Weiss S, et al. Safety and effectiveness of a synthetic hemostatic patch for intraoperative soft tissue bleeding. Med Devices (Auckl) 2015;8:167-74. [PubMed]
- Tasu JP, Vesselle G, Herpe G, et al. Postoperative abdominal bleeding. Diagn Interv Imaging 2015;96:823-31. [Crossref] [PubMed]
- Dagi TF. The management of postoperative bleeding. Surg Clin North Am 2005;85:1191-213. x. [Crossref] [PubMed]
- Behrens AM, Sikorski MJ, Kofinas P. Hemostatic strategies for traumatic and surgical bleeding. J Biomed Mater Res A 2014;102:4182-94. [Crossref] [PubMed]
- Levy JH, Tanaka KA. Management of surgical hemostasis: systemic agents. Vascular 2008;16:S14-21. [PubMed]
- Basu A, Kunduru KR, Abtew E, et al. Polysaccharide-Based Conjugates for Biomedical Applications. Bioconjug Chem 2015;26:1396-412. [Crossref] [PubMed]
- Frantz VK. Absorbable Cotton, Paper and Gauze: (Oxidized Cellulose). Ann Surg 1943;118:116-26. [Crossref] [PubMed]
- Yang X, Liu W, Li N, et al. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomater Sci 2017;5:2357-68. [Crossref] [PubMed]
- Kim SH, Kim SH, Yoon HS, et al. Efficacy of Oxidized Regenerated Cellulose, SurgiGuard®, in Porcine Surgery. Yonsei Med J 2017;58:195-205. [Crossref] [PubMed]
- Dimitrijevich SD, Tatarko M, Gracy RW, et al. Biodegradation of oxidized regenerated cellulose. Carbohydr Res 1990;195:247-56. [Crossref] [PubMed]
- Momin M, Mishra V, Gharat S, et al. Recent advancements in cellulose-based biomaterials for management of infected wounds. Expert Opin Drug Deliv 2021;18:1741-60. [Crossref] [PubMed]
- Dimitrijevich SD, Tatarko M, Gracy RW, et al. In vivo degradation of oxidized, regenerated cellulose. Carbohydr Res 1990;198:331-41. [Crossref] [PubMed]
- Spangler D, Rothenburger S, Nguyen K, et al. In vitro antimicrobial activity of oxidized regenerated cellulose against antibiotic-resistant microorganisms. Surg Infect (Larchmt) 2003;4:255-62. [Crossref] [PubMed]
- Kim SH, Yoon HS, In CH, et al. Efficacy evaluation of SurgiGuard® in partially hepatectomized pigs. Korean J Hepatobiliary Pancreat Surg 2016;20:102-9. [Crossref] [PubMed]
- Wagenhäuser MU, Mulorz J, Ibing W, et al. Oxidized (non)-regenerated cellulose affects fundamental cellular processes of wound healing. Sci Rep 2016;6:32238. [Crossref] [PubMed]
- Lewis KM, Atlee H, Mannone A, et al. Efficacy of hemostatic matrix and microporous polysaccharide hemospheres. J Surg Res 2015;193:825-30. [Crossref] [PubMed]
- Chiara O, Cimbanassi S, Bellanova G, et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg 2018;18:68. [Crossref] [PubMed]
- Mertaniemi H, Escobedo-Lucea C, Sanz-Garcia A, et al. Human stem cell decorated nanocellulose threads for biomedical applications. Biomaterials 2016;82:208-20. [Crossref] [PubMed]
- Sundaram CP, Keenan AC. Evolution of hemostatic agents in surgical practice. Indian J Urol 2010;26:374-8. [Crossref] [PubMed]
- Pereira BM, Bortoto JB, Fraga GP. Topical hemostatic agents in surgery: review and prospects. Rev Col Bras Cir 2018;45:e1900. [PubMed]
- Wu S, Applewhite AJ, Niezgoda J, et al. Oxidized Regenerated Cellulose/Collagen Dressings: Review of Evidence and Recommendations. Adv Skin Wound Care 2017;30:S1-S18. [Crossref] [PubMed]
- Okubo S, Shindoh J, Kobayashi Y, et al. Safety of Use of a Sheet-Type Adhesion Barrier (Interceed(®)) During Liver Surgery. World J Surg 2020;44:4214-20. [Crossref] [PubMed]
- Okubo S, Shindoh J, Kobayashi Y, et al. Safety of a new spray-type adhesion barrier (AdSpray(®)) in liver surgery. J Hepatobiliary Pancreat Sci 2020;27:648-54. [Crossref] [PubMed]
- Reid RL, Hahn PM, Spence JE, et al. A randomized clinical trial of oxidized regenerated cellulose adhesion barrier (Interceed, TC7) alone or in combination with heparin. Fertil Steril 1997;67:23-9. [Crossref] [PubMed]
- Ten Broek RPG, Stommel MWJ, Strik C, et al. Benefits and harms of adhesion barriers for abdominal surgery: a systematic review and meta-analysis. Lancet 2014;383:48-59. [Crossref] [PubMed]
- Cheng F, Wu Y, Li H, et al. Biodegradable N, O-carboxymethyl chitosan/oxidized regenerated cellulose composite gauze as a barrier for preventing postoperative adhesion. Carbohydr Polym 2019;207:180-90. [Crossref] [PubMed]
- Develle R, Schaerf R, Najibi S, et al. Efficacy and safety of regenerated cellulose topical gauze haemostats in managing secondary haemostasis: a randomised control trial. J Wound Care 2020;29:670-7. [Crossref] [PubMed]
- Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs 2005;8:137-42. [Crossref] [PubMed]
- Franceschini G. Internal surgical use of biodegradable carbohydrate polymers. Warning for a conscious and proper use of oxidized regenerated cellulose. Carbohydr Polym 2019;216:213-6. [Crossref] [PubMed]
- Piozzi GN, Reitano E, Panizzo V, et al. Practical Suggestions for Prevention of Complications Arising from Oxidized Cellulose Retention: A Case Report and Review of the Literature. Am J Case Rep 2018;19:812-9. [Crossref] [PubMed]
- Lewis KM, Li Q, Jones DS, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery 2017;161:771-81. [Crossref] [PubMed]




