
Implications of isthmic location as a risk factor in papillary thyroid carcinoma
Highlight box
Key findings
• Clinicopathological characteristics of PTC located in thyroid isthmus are discussed.
What is known and what is new?
• PTC located in the isthmus generally known to have more aggressive pathological nature.
• Comparing PTCs located in unilateral lobe and isthmus, isthmic location is not an independent risk factor associated with aggressive clinicopathologic features and recurrence.
What is the implication, and what should change now?
• More conservative treatments like ‘isthmusectomy’ can be applied with oncological safety especially in early-stage lesions.
Introduction
Papillary thyroid carcinoma (PTC) located in the isthmus of the thyroid gland is relatively rare compared to PTC located in the two thyroid gland lobes (1-3). The isthmus is a thin thyroid parenchyma that connects both thyroid lobes anteriorly to the trachea. Isthmic PTC is more frequently associated with local invasion of adjacent tissues, bilateral lymph node involvement, and multifocality than PTC located in other parts of the thyroid gland (1-8). Therefore, many surgeons prefer total thyroidectomy and central compartment neck dissection (CCND) for primary treatment of PTC located in the isthmus (1,3,8-14).
Due to the indolent biologic behavior of PTCs and the steep increase of microcarcinoma, the treatment strategy of PTCs has become more conservative (15-18). Furthermore, the eighth edition of the tumor-node-metastasis (TNM) classification of the American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC) staging system has decreased the negative prognostic value of lymph node metastases and microscopic extrathyroidal extension (ETE), and these changes have led to the significant downstaging of PTC patients (19,20). In the case of PTC located unilaterally in a thyroid lobe without gross ETE or clinical nodal metastasis, thyroid lobectomy has become the standard surgical procedure and is gradually replacing total thyroidectomy (21,22). However, due to their rarity and unique clinicopathologic characteristics, no clear consensus has been established for the management of isthmic PTCs (18).
Recently, some researchers have proposed isthmusectomy alone or a combination of isthmusectomy and lobectomy for isthmic PTC management, but the evidence regarding oncologic safety is still limited (2,3,23-25). The aim of this study was to investigate the clinical significance of an isthmic tumor location and its prognostic value in PTC patients by comparing the clinicopathological characteristics of isthmic and unilateral-lobar PTCs. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-56/rc).
Methods
Patients
The medical records of PTC patients who underwent thyroidectomy at Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, from 2009 through 2013 were retrospectively reviewed. Patients with a single, dominant tumor, were categorized into two groups, isthmic or unilateral-lobe cancer, according to tumor location. Isthmic cancer was defined based on ultrasonography results when the center of the dominant tumor was located between two imaginary perpendicular lines extending from the most lateral point of the trachea. A dominant tumor located in a single thyroid lobe was categorized as unilateral-lobar cancer. Those with multiple cancers upon initial diagnosis were excluded. Of 175 single isthmic cancer patients who underwent total thyroidectomy, 160 were classical PTC subtypes and 15 were non-classical PTC subtypes including follicular, tall-cell, columnar-cell and diffuse sclerosing subtypes. Patients of all periods for follow up were included in this study.
The clinicopathological characteristics of isthmic cancer cases were compared with those of unilateral-lobar cancer cases in a PTC cohort at Seoul St. Mary’s Hospital from 2009 to 2012. To analyze the complete pathological information of PTC such as invasion, lymph node involvement, and tumor multifocality, the medical records of PTC patients who underwent total thyroidectomy were retrospectively reviewed. The consecutive patients of 1,527 with unilateral-lobar PTCs who underwent total thyroidectomy according to guideline recommendations 25 and 26 issued by the American Thyroid Association (ATA) in 2009 were identified (26). Of these, 1,340 patients were classical PTC subtypes and 187 were non-classical PTC subtypes.
Prophylactic CCND was performed in clinically node-negative patients, and therapeutic node dissection was performed in cN1a and cN1b patients. Patients were administered levothyroxine for thyroid stimulating hormone (TSH) suppression, and radioactive iodine therapy was performed within 6–8 weeks after surgery in patients who satisfied indications issued by the ATA guidelines (26). Serum thyroglobulin level measurements and neck ultrasonography were regularly performed every 6 or 12 months. None of the patients in this study had distant metastasis. Recurrence was defined as confirmed structural recurrence identified by image-guided cytology or histology.
All clinic histological data were reviewed. ETE was categorized as no, microscopic, or gross ETE according to the eighth edition of the AJCC/UICC staging system (20). Incidental multifocality was classified according to the size of the largest satellite nodule. Satellite nodule larger than 0.3 cm were considered clinically multifocal, and 0.3 cm or smaller were categorized as occult multifocal. In terms of nodal risk, patients with more than five metastatic lymph nodes (MLNs) were classified as having higher-risk N1 disease, and those with five or fewer MLNs were classified as having lower-risk N1 disease (18,27). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Seoul St. Mary’s Hospital, The Catholic University of Korea (No. KC22RISI0058) and individual consent for this retrospective analysis was waived.
Statistical analysis
The clinicopathologic characteristics were analyzed to evaluate the prognostic significance of an isthmic tumor location. In order to reduce the bias of statistical analysis due to various subtypes with different prognoses, statistical analyzes were performed for classical subtypes which accounted for the majority. Propensity score matching (PSM) was used to adjust the baseline characteristics and reduce selection bias. Confounding variables, such as age, sex and tumor size, were included in the propensity score model. The isthmic group and the unilateral-lobar group were matched in 1:5 ratio. Continuous, quantitative data were expressed as mean ± standard deviation, and categorical, qualitative data were expressed as frequencies and percentages. The data were compared with the chi-square test, Mann-Whitney U test, or Student’s t-test. Multivariate analysis was performed using logistic regression. Recurrence data were analyzed via the Kaplan-Meier method, log-rank test, and Cox proportional hazards model. The statistical analyzes for non-classical subtypes were also performed, respectively. All statistical analyses were performed using the SPSS version 28.0 (IBM, Armonk, NY, USA) and the R Integration Package 4.0.0 (R-Core Team, 2020).
Results
Patient characteristics before and after PSM in the classical PTC
The clinicopathologic features and outcomes of 1,500 patients with classical subtype of PTC are described in Table 1. We compared 160 patients with isthmic cancer and 1,340 patients with unilateral-lobe cancer. The follow-up period was 104.4±31.8 (range, 0–151) months. The mean patient age was 47.1±12.4 years; the mean tumor size was 1.0±0.6 cm. The isthmic group was significantly older than the unilateral-lobar group (49.6 vs. 46.8 years, P=0.007). The mean tumor size was smaller in the isthmic group than in the unilateral-lobar group (0.8±0.4 vs. 1.0±0.7 cm, P<0.001). However, when tumors were grouped by size, no difference was observed between the two groups. The ETE status, incidence of multifocality, and clinical multifocality were not significantly different between the two groups. In terms of nodal status, the incidence of nodal metastasis and higher-risk N1 disease were not different between the two groups. However, lateral neck node metastasis (N1b) was more frequent in the unilateral-lobar group than in the isthmic group (10.8% vs. 2.5%, P<0.001), although bilateral neck node metastasis was more frequent in the isthmic group than in the unilateral-lobar group (75% vs. 12.4%, P<0.001). The rate of recurrence and the recurrence-free survival time were not significantly different between the two groups.
Table 1
| Variables | Total patients (n=1,500) | Isthmic group (n=160) | Unilateral-lobar group (n=1,340) | P value |
|---|---|---|---|---|
| Age (years), mean ± SD [min–max] | 47.1±12.4 [11–81] | 49.6±12.1 [21–76] | 46.8±12.4 [11–81] | 0.007 |
| Sex (male:female) | 264:1,236 | 21:139 | 243:1,097 | 0.116 |
| Tumor size (cm), mean ± SD [min–max] | 1.0±0.6 [0.1–8.5] | 0.8±0.4 [0.2–2.4] | 1.0±0.7 [0.1–8.5] | <0.001 |
| Tumor size (cm) in group, n (%) | 0.052 | |||
| ≤1 | 1,012 (67.5) | 123 (76.9) | 889 (66.3) | |
| >1 to 2 | 402 (26.8) | 32 (20.0) | 370 (27.6) | |
| >2 to 4 | 82 (5.5) | 5 (3.1) | 77 (5.7) | |
| >4 | 4 (0.3) | 0 | 4 (0.3) | |
| ETE, n (%) | 0.964 | |||
| None | 724 (48.3) | 76 (47.5) | 648 (48.4) | |
| Microscopic | 693 (46.2) | 76 (47.5) | 617 (46.0) | |
| Gross ETE | 83 (5.5) | 8 (5.0) | 75 (5.6) | |
| Multifocality, n (%) | 0.949 | |||
| Absent | 934 (62.3) | 100 (62.5) | 834 (62.2) | |
| Present | 566 (37.7) | 60 (37.5) | 506 (37.8) | |
| Occult multifocality | 277 (48.9) | 34 (56.7) | 243 (48.0) | 0.205 |
| Clinical multifocality | 289 (51.1) | 26 (43.3) | 263 (52.0) | |
| Node metastasis, n (%) | 0.477 | |||
| No | 696 (46.4) | 70 (43.8) | 626 (46.7) | |
| Yes | 804 (53.6) | 90 (56.3) | 714 (53.3) | |
| 1 ≤ MLN ≤ 5 | 583 (72.5) | 72 (80.0) | 511 (71.6) | 0.091 |
| MLN >5 | 221 (27.5) | 18 (20.0) | 203 (28.4) | |
| Lateral neck node metastasis, n (%) | ||||
| Absent | 1,351 (90.1) | 156 (97.5) | 1,195 (89.2) | |
| Present (N1b) | 149 (9.9) | 4 (2.5) | 145 (10.8) | <0.001 |
| Ipsilateral | 128 (85.9) | 1 (25.0) | 127 (87.6) | |
| Bilateral | 21 (14.1) | 3 (75.0) | 18 (12.4) | <0.001 |
| Recurrence | 55 (3.7) | 2 (1.3) | 53 (4.0) | 0.085 |
| RFS (months) [95% CI] | 146.3 [145.0–147.5] | 149.2 [146.7–151.7] | 145.9 [144.6–147.3] | 0.093 |
PTC, papillary thyroid carcinoma; SD, standard deviation; ETE, extrathyroidal extension; MLN, the numbers of metastatic lymph node; RFS, recurrence-free survival; CI, confidence interval.
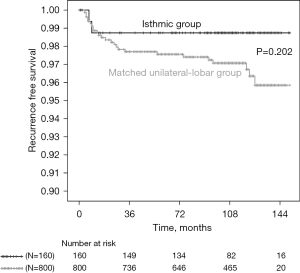
After matching the isthmic group and the unilateral-lobar group using a 1:5 ratio according to age, sex and tumor size, 160 isthmic PTCs and 800 matched unilateral-lobar PTCs were compared (Table 2). The changes in the standard mean differences of factors in both groups before and after PSM are 0.148 to 0.037 for sex ratio, 0.231 to 0.052 for age, and 0.372 to 0.008 for tumor size. Age, sex and tumor size were comparable between the two groups after matching. There were no differences in terms of tumor size category, ETE, multifocality, or the rate of clinical multifocality between the two groups. Nodal metastasis and the proportion of patients with higher-risk N1 disease were similar in both groups. However, N1b cases were more frequent in the unilateral-lobar group than in the isthmic group (7.0% vs. 2.5%, P=0.032), whereas bilateral N1b cases were more common in the isthmic groups than in the unilateral-lobar group, even after PSM (75% vs. 5.4%, P<0.001). The recurrence rate and recurrence-free survival time did not differ between the two groups (Table 2 and Figure 1).
Table 2
| Variables | Isthmic group (n=160) | Unilateral-lobar group (n=800) | P value |
|---|---|---|---|
| Age (years), mean ± SD [min–max] | 49.6±12.1 [21–76] | 49.0±12.1 [18–78] | 0.549 |
| Sex (male:female) | 21:139 | 95:705 | 0.658 |
| Tumor size (cm), mean ± SD [min–max] | 0.8±0.4 [0.2–2.4] | 0.8±0.4 [0.1–2.8] | 0.931 |
| Tumor size (cm) in group, n (%) | 0.515 | ||
| ≤1 | 123 (76.9) | 607 (75.9) | |
| >1 to 2 | 32 (20.0) | 178 (22.3) | |
| >2 to 4 | 5 (3.1) | 15 (1.9) | |
| ETE, n (%) | 0.583 | ||
| None | 76 (47.5) | 396 (49.5) | |
| Microscopic | 76 (47.5) | 377 (47.1) | |
| Gross ETE | 8 (5.0) | 27 (3.4) | |
| Multifocality, n (%) | 0.696 | ||
| Absent | 100 (62.5) | 513 (64.1) | |
| Present | 60 (37.5) | 287 (35.9) | |
| Occult multifocality | 34 (56.7) | 139 (48.4) | 0.246 |
| Clinical multifocality | 26 (43.3) | 148 (51.6) | |
| Node metastasis, n (%) | 0.126 | ||
| No | 70 (43.8) | 403 (50.4) | |
| Yes | 90 (56.3) | 397 (49.6) | |
| 1 ≤ MLN ≤ 5 | 72 (80.0) | 315 (79.3) | 0.890 |
| MLN >5 | 18 (20.0) | 82 (20.7) | |
| Lateral neck node metastasis, n (%) | |||
| Absent | 156 (97.5) | 744 (93.0) | |
| Present (N1b) | 4 (2.5) | 56 (7.0) | 0.032 |
| Ipsilateral | 1 (25.0) | 53 (94.6) | |
| Bilateral | 3 (75.0) | 3 (5.4) | <0.001 |
| Recurrence | 2 (1.3) | 25 (3.1) | 0.19 |
| RFS (months) [95% CI] | 149.2 [146.7–151.7] | 147.1 [145.5–148.6] | 0.202 |
PTC, papillary thyroid carcinoma; SD, standard deviation; ETE, extrathyroidal extension; MLN, the numbers of metastatic lymph node; RFS, recurrence-free survival; CI, confidence interval.
Risk factors associated with aggressive features
Multivariate logistic regression was performed to identify independent risk factors associated with aggressive clinical features in matched isthmic and unilateral-lobar cancer patients (Table 3). Gross ETE showed a positive correlation with the patient age, larger tumor size category, and more than five MLNs. Higher-risk N1 disease, which was defined as more than five MLNs, increased in patients with male sex, younger age, larger tumor size, gross ETE, and multifocality. The presence of multifocality was only correlated with more than five MLNs. However, isthmic location was not correlated with gross ETE, higher-risk N1 disease, or multifocality. Cox regression analysis was performed to compare recurrence-free survival between the two groups (Table 4). Younger age and having more than five MLNs increased the risk of recurrence. However, an isthmic tumor location was not significantly correlated with recurrence-free survival time.
Table 3
| Variables | Gross ETE | Multifocality | MLN >5 | |||||
|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | P value | HR [95% CI] | P value | HR [95% CI] | P value | |||
| Isthmic location | 1.607 [0.673–3.833] | 0.285 | 1.086 [0.763–1.547] | 0.647 | 1.141 [0.634–2.054] | 0.66 | ||
| Patients’ age | 1.033 [1.004–1.062] | 0.026 | 1.002 [0.991–1.013] | 0.746 | 0.949 [0.932–0.968] | <0.001 | ||
| Male sex | 0.369 [0.101–1.352] | 0.132 | 0.787 [0.517–1.197] | 0.263 | 2.532 [1.445–4.438] | 0.001 | ||
| Tumor size, cm | ||||||||
| ≤1 | Ref. | Ref. | Ref. | |||||
| >1 to 2 | 8.314 [3.535–19.555] | <0.001 | 1.295 [0.93–1.803] | 0.126 | 4.085 [2.542–6.566] | <0.001 | ||
| >2 to 4 | 20.322 [5.21–79.278] | <0.001 | 0.793 [0.296–2.128] | 0.645 | 3.137 [1.001–9.834] | 0.05 | ||
| Gross ETE | N/A | N/A | 0.689 [0.324–1.464] | 0.333 | 5.121 [2.262–11.59] | <0.001 | ||
| Multifocality | 0.697 [0.325–1.494] | 0.353 | N/A | N/A | 1.695 [1.076–2.67] | 0.023 | ||
| MLN >5 | 5.135 [2.256–11.686] | <0.001 | 1.675 [1.069–2.624] | 0.024 | N/A | N/A | ||
PTC, papillary thyroid carcinoma; ETE, extrathyroidal extension; MLN, the numbers of metastatic lymph node; HR, hazard ratio; CI, confidence interval; Ref., reference; N/A, not applicable.
Table 4
| Variables | HR [95% CI] | P value |
|---|---|---|
| Isthmic location | 0.412 [0.097–1.753] | 0.23 |
| Patients’ age | 0.938 [0.906–0.972] | <0.001 |
| Male sex | 0.938 [0.32–2.75] | 0.908 |
| Tumor size, cm | ||
| ≤1 | Ref. | |
| >1 to 2 | 1.181 [0.5–2.792] | 0.704 |
| >2 to 4 | 1.681 [0.217–13.016] | 0.619 |
| Gross ETE | 1.433 [0.308–6.67] | 0.646 |
| Multifocality | 1.1 [0.499–2.423] | 0.813 |
| MLN >5 | 4.397 [1.85–10.452] | 0.001 |
PTC, papillary thyroid carcinoma; HR, hazard ratio; CI, confidence interval; Ref., reference; ETE, extrathyroidal extension; MLN, the numbers of metastatic lymph node.
Patient characteristics in non-classical subtypes
We analyzed 15 patients with isthmic cancer and 187 patients with unilateral-lobe cancer which had non-classical subtypes of PTC (Table 5). The follow-up period was 95.4±26.2 (range, 14–140) months. There was no statistical difference in the proportion of non-classical subtypes in the two groups (15/175 in the isthmic group vs. 187/1,527 in the unilateral lobar group, P=0.155). The mean tumor size was smaller in the isthmic group than in the unilateral-lobar group (0.7±0.3 vs. 1.4±1.1 cm, P<0.001), and this difference continued to the tumor size in group (P=0.028). The mean patients’ age, sex ratio, ETE, incidence of multifocality and nodal factors were not significantly different between the two groups. The eight recurrences occurred in only unilateral lobar group of non-classical subtypes of PTC and the rate of recurrence were not significantly different between the two groups.
Table 5
| Variables | Total patients (n=202) | Isthmic group (n=15) | Unilateral-lobar group (n=187) | P value |
|---|---|---|---|---|
| The proportion of patients with non-classical subtypes | 11.9% (202/1,702) | 8.6% (15/175) | 12.2% (187/1,527) | 0.155 |
| Subtypes*, n (%) | 0.641 | |||
| Follicular | 124 (58.2) | 11 (73.3) | 113 (57.1) | |
| Tall cell | 53 (24.9) | 4 (26.7) | 49 (24.7) | |
| Columnar cell | 7 (3.3) | 0 (0.0) | 7 (3.5) | |
| Oncocytic | 6 (2.8) | 0 (0.0) | 6 (3.0) | |
| Diffuse sclerosing | 1 (0.5) | 0 (0.0) | 1 (0.5) | |
| Others | 22 (10.3) | 0 (0.0) | 22 (11.1) | |
| Age (years), mean ± SD [min–max] | 48.9±12.4 [19–81] | 47.47±11.7 [28–73] | 48.96±12.4 [19–81] | 0.653 |
| Sex (male:female) | 37:165 | 3:12 | 34:153 | 0.861 |
| Tumor size (cm), mean ± SD [min–max] | 1.34±1.1 [0.1–6.5] | 0.7±0.3 [0.4–1.4] | 1.4±1.1 [0.1–6.5] | <0.001 |
| Tumor size (cm) in group, n (%) | 0.028 | |||
| ≤1 | 114 (56.4) | 14 (93.3) | 100 (53.5) | |
| >1 to 2 | 55 (27.2) | 1 (6.7) | 54 (28.9) | |
| >2 to 4 | 25 (12.4) | 0 | 25 (13.4) | |
| >4 | 8 (4.0) | 0 | 8 (4.3) | |
| ETE, n (%) | 0.703 | |||
| None | 128 (63.4) | 8 (53.3) | 120 (64.2) | |
| Microscopic | 63 (31.2) | 6 (40.0) | 57 (30.5) | |
| Gross ETE | 11 (5.4) | 1 (6.7) | 10 (5.3) | |
| Multifocality, n (%) | 0.739 | |||
| Absent | 116 (57.4) | 8 (53.3) | 108 (57.8) | |
| Present | 86 (42.6) | 7 (46.7) | 79 (42.2) | |
| Node metastasis, n (%) | 0.529 | |||
| No | 110 (54.5) | 7 (46.7) | 103 (55.1) | |
| Yes | 92 (45.5) | 8 (53.3) | 84 (44.9) | |
| 1 ≤ MLN ≤ 5 | 61 (66.3) | 4 (50.0) | 57 (67.9) | 0.307 |
| MLN >5 | 31 (33.7) | 4 (50.0) | 27 (32.1) | |
| Lateral neck node metastasis, n (%) | ||||
| Absent | 183 (90.6) | 15 (100.0) | 168 (89.8) | |
| Present (N1b) | 19 (9.4) | 0 | 19 (10.2) | 0.195 |
| Ipsilateral | 19 (100.0) | N/A | 19 (100.0) | |
| Bilateral | 0 | N/A | 0 | N/A |
| Recurrence | 8 (4.0) | 0 | 8 (4.3) | 0.414 |
| RFS (months) [95% CI] | 136.3 [134.0–139.2] | N/A | 136.3 [133.6–139.1] | 0.421 |
*, cases with different subtypes in a single patient were also counted separately. PTC, papillary thyroid carcinoma; SD, standard deviation; ETE, extrathyroidal extension; MLN, the numbers of metastatic lymph node; RFS, recurrence-free survival; CI, confidence interval; N/A, not applicable.
Discussion
The increasing incidence of small thyroid cancer over time and the accumulation of cancer outcome data have led to more detailed interpretations of various prognostic factors (18,20,26). Microscopic ETE is no longer considered an independent prognostic factor for disease recurrence or survival. Therefore, microscopic ETE is considered as an intrathyroidal lesion in the eighth edition of the AJCC/TNM cancer staging system (18,28). The frequent, hidden, microscopic multifocal lesions of PTC have been used to justify performing ‘total thyroidectomy’ or ‘completion thyroidectomy after thyroid lobectomy’ (26,29,30). However, recently, the current approach is to perform total thyroidectomy only when clear evidence supports the removal of the contralateral thyroid gland, and the completion thyroidectomy is recommended only when total thyroidectomy would have been recommended if the diagnosis had been available before the initial surgery (18). Nodal risk is stratified according to the number of MLNs, the size of the metastatic foci, and the presence of extranodal extension (18,27). Accordingly, the treatment of PTC is gradually becoming more conservative than in the past (18,20,21,31).
The anatomic location of the isthmus contributes to frequent ETE, bilateral lymphatic spread, and multiple PTC lesions (1-8). Due to these clinicopathologic features, many surgeons insist on performing a total thyroidectomy with CCND to treat isthmic PTC (1,3,8-14). However, most evidence remains based on past prognostic factors or stages (1,4,6,9-12). For select patients, researchers suggested isthmusectomy as a feasible treatment option for isthmic PTC, although this remains controversial (2,3,23-25).
As isthmic cancer is rare, no studies have analyzed the above-mentioned, up-to-date prognostic factors for isthmic PTC. In this study, we analyzed the clinicopathological features of isthmic PTCs compared with those of unilateral-lobar PTCs. Furthermore, we investigated whether an isthmic location is related to PTC recurrence.
In isthmic cancer, the adjacent structures, including strap muscles and the trachea, are vulnerable to invasion because of the thin thyroid parenchyma. Chang et al. and Lee et al. reported that isthmic cancer showed more frequent thyroid capsule invasion than uni-lobular cancer (1,9,32). By contrast, Lyu et al., Li et al., and Song et al. reported no significant difference in the incidence of ETE (7,11,12). However, as any invasions beyond the thyroid capsule were defined as ETE in these studies, the actual incidence of gross ETE in isthmic PTCs cannot be accurately assessed. In this study, the isthmic and unilateral-lobar groups did not show significant differences in microscopic or gross ETE before or after PSM. Furthermore, an isthmic tumor location was not an independent risk factor for gross ETE in the multivariate analysis, whereas older age, lager tumor size, and more than five MLNs were associated with gross ETE. Most isthmic cancers were intrathyroidal or exhibited capsular invasion. Only 5% (n=8) of the isthmic group showed clinically significant gross invasion to strap muscles, and no tracheal invasion was observed in this study. Chung et al. reported that the anterior replacement of the strap muscle by thyroid cancer or obtuse angles between the thyroid cancer and trachea in ultrasonography are highly predictive of gross extrathyroidal invasion (33). Therefore, gross ETE of isthmic PTCs can be detected effectively in the preoperative evaluation.
Tumor multifocality is one of the features that advocated total thyroidectomy in isthmic PTC (1,5,10). However, Lee et al. reported that multifocality and bilaterality did not differ between isthmic and non-isthmic PTCs (1). Similarly, in our study, multifocality did not differ between the isthmic and unilateral-lobar groups before or after PSM. In the multivariate analysis, an isthmic location was not an independent risk factor for multifocality. To further analyze the characteristics of multiple incidental lesions, we classified satellite nodules according to their size. Since high-resolution ultrasonography can detect thyroid malignancies as small as 2 mm, satellite cancers with a size larger than 0.3 cm were considered clinically significant, and 0.3 cm or smaller were considered occult (34). Only 26 (16.3%) patients with isthmic PTCs exhibited clinical multifocality, and these clinical satellite lesions may be detected by careful preoperative ultrasonography.
The central location of the isthmus and its lymphatic drainage contribute to the bilateral nodal spread of PTC (6). Many reports have described frequent cervical node involvement in isthmic cancer, especially in the central compartment (7-9,11,12,32). An isthmic tumor location was described as a strong independent risk factor for N1 disease (11,12). In a recent meta-analysis study about PTC located in the isthmus, Lyu et al. reported a significantly high rate of central lymph node metastasis in isthmic PTC (7). However, these studies analyzed risk factors only according to the presence or absence of node metastasis and did not classify the nodal risk according to the revised initial risk stratification of the ATA (18,27). The present study showed that the incidence of nodal metastasis and higher-risk N1 disease (more than five MLNs) were similar in both the isthmic and unilateral-lobar groups before and after PSM. In the multivariate analysis, higher-risk N1 disease was correlated with male sex, younger age, larger tumor size, gross ETE, and multifocality, but not with isthmic location. Lateral neck node metastasis was more frequent in the unilateral-lobar groups both before and after PSM. However, the rate of bilateral N1b disease was significantly higher in the isthmic group than in the unilateral-lobar group, likely due to bilateral lymphatic spread. Therefore, careful preoperative examination of both lateral neck nodes is recommended for isthmic PTCs.
In our study, 55 patients had recurrent disease, and the two groups showed similar recurrence rates. Younger patient age and higher-risk N1 disease were related to recurrence. However, an isthmic tumor location was not related to recurrence after PSM. These findings indicate that an isthmic location alone is not a poor prognostic factor for PTCs. Our results support evidence that isthmusectomy may a feasible treatment options in patients with small isthmic PTC. Our study has several strengths. This study is the first to analyze the clinicopathologic characteristics of isthmic PTCs by applying the most recent prognostic factors. Our study included a relatively large number of isthmic cancer patients compared with previous studies. All patients underwent total thyroidectomy and nodal dissection, therefore, histologic data, including multifocality and nodal involvement, were available. Furthermore, we performed the PSM to reduce selection bias between the two groups. However, our study also has limitations. This study is a retrospective study performed at a single institution. The nodal risk was assessed only by the number of MLNs, and the size of the metastatic foci or the presence of extranodal extension was not evaluated. Also, due to the limitation of the number of patients, only the classical PTC subtype was fully analyzed. Since preoperative ultrasound or cytological characteristics cannot predict the histologic subtype of PTC before surgery, additional research according to the non-classical PTC subtype located in the isthmic location is needed. Additionally, most patients in this cohort had early-stage disease with a small tumor size. Therefore, large-scale studies with patients in various disease stages are required to analyze the clinical implication of an isthmic PTC location.
Conclusions
An isthmic location alone is not an independent risk factor for aggressive clinicopathologic features and is not related to PTC recurrence in early-stage lesions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-56/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-56/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-56/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Seoul St. Mary’s Hospital, The Catholic University of Korea approved the study (No. KC22RISI0058) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee YS, Jeong JJ, Nam KH, et al. Papillary carcinoma located in the thyroid isthmus. World J Surg 2010;34:36-9. [Crossref] [PubMed]
- Nixon IJ, Palmer FL, Whitcher MM, et al. Thyroid isthmusectomy for well-differentiated thyroid cancer. Ann Surg Oncol 2011;18:767-70. [Crossref] [PubMed]
- Vasileiadis I, Boutzios G, Karalaki M, et al. Papillary thyroid carcinoma of the isthmus: Total thyroidectomy or isthmusectomy? Am J Surg 2018;216:135-9. [Crossref] [PubMed]
- Huang H, Liu SY, Ni S, et al. Treatment Outcome of Papillary Carcinoma Confined to the Thyroid Isthmus. J Cancer Ther 2016;7:963-9. [Crossref]
- Karatzas T, Charitoudis G, Vasileiadis D, et al. Surgical treatment for dominant malignant nodules of the isthmus of the thyroid gland: A case control study. Int J Surg 2015;18:64-8. [Crossref] [PubMed]
- Hahn SY, Han BK, Ko EY, et al. Ultrasound findings of papillary thyroid carcinoma originating in the isthmus: comparison with lobe-originating papillary thyroid carcinoma. AJR Am J Roentgenol 2014;203:637-42. [Crossref] [PubMed]
- Lyu YS, Pyo JS, Cho WJ, et al. Clinicopathological Significance of Papillary Thyroid Carcinoma Located in the Isthmus: A Meta-Analysis. World J Surg 2021;45:2759-68. [Crossref] [PubMed]
- Shuai Y, Yue K, Duan Y, et al. Surgical Extent of Central Lymph Node Dissection for Papillary Thyroid Carcinoma Located in the Isthmus: A Propensity Scoring Matched Study. Front Endocrinol (Lausanne) 2021;12:620147. [Crossref] [PubMed]
- Chang YW, Lee HY, Kim HS, et al. Extent of central lymph node dissection for papillary thyroid carcinoma in the isthmus. Ann Surg Treat Res 2018;94:229-34. [Crossref] [PubMed]
- Lei J, Zhu J, Li Z, et al. Surgical procedures for papillary thyroid carcinoma located in the thyroid isthmus: an intention-to-treat analysis. Onco Targets Ther 2016;9:5209-16. [Crossref] [PubMed]
- Li G, Lei J, Peng Q, et al. Lymph node metastasis characteristics of papillary thyroid carcinoma located in the isthmus: A single-center analysis. Medicine (Baltimore) 2017;96:e7143. [Crossref] [PubMed]
- Song CM, Lee DW, Ji YB, et al. Frequency and pattern of central lymph node metastasis in papillary carcinoma of the thyroid isthmus. Head Neck 2016;38:E412-6. [Crossref] [PubMed]
- Xiang D, Xie L, Xu Y, et al. Papillary thyroid microcarcinomas located at the middle part of the middle third of the thyroid gland correlates with the presence of neck metastasis. Surgery 2015;157:526-33. [Crossref] [PubMed]
- Wang J, Sun H, Gao L, et al. Evaluation of thyroid isthmusectomy as a potential treatment for papillary thyroid carcinoma limited to the isthmus: A clinical study of 73 patients. Head Neck 2016;38:E1510-4. [Crossref] [PubMed]
- Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 2009;18:784-91. [Crossref] [PubMed]
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [Crossref] [PubMed]
- Ito Y, Nikiforov YE, Schlumberger M, et al. Increasing incidence of thyroid cancer: controversies explored. Nat Rev Endocrinol 2013;9:178-84. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Shaha AR, Migliacci JC, Nixon IJ, et al. Stage migration with the new American Joint Committee on Cancer (AJCC) staging system (8th edition) for differentiated thyroid cancer. Surgery 2019;165:6-11.
- Lamartina L, Grani G, Arvat E, et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: what to expect (ITCO#2). Endocr Relat Cancer 2018;25:L7-L11.
- Bosset M, Bonjour M, Castellnou S, et al. Long-Term Outcome of Lobectomy for Thyroid Cancer. Eur Thyroid J 2021;10:486-94. [Crossref] [PubMed]
- Nixon IJ, Ganly I, Patel SG, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery 2012;151:571-9. [Crossref] [PubMed]
- Park H, Harries V, McGill MR, et al. Isthmusectomy in selected patients with well-differentiated thyroid carcinoma. Head Neck 2020;42:43-9. [Crossref] [PubMed]
- Kwon O, Lee S, Bae JS, et al. Thyroid Isthmusectomy with Prophylactic Central Compartment Neck Dissection is a Feasible Approach for Papillary Thyroid Cancer on the Isthmus. Ann Surg Oncol 2021;28:6603-12. [Crossref] [PubMed]
- Gui Z, Wang Z, Xiang J, et al. Comparison of Outcomes Following Thyroid Isthmusectomy, Unilateral Thyroid Lobectomy, and Total Thyroidectomy in Patients with Papillary Thyroid Microcarcinoma of the Thyroid Isthmus: A Retrospective Study at a Single Center. Med Sci Monit 2020;26:e927407. [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [Crossref] [PubMed]
- Bortz MD, Kuchta K, Winchester DJ, et al. Extrathyroidal extension predicts negative clinical outcomes in papillary thyroid cancer. Surgery 2021;169:2-6. [Crossref] [PubMed]
- Pacini F, Elisei R, Capezzone M, et al. Contralateral papillary thyroid cancer is frequent at completion thyroidectomy with no difference in low- and high-risk patients. Thyroid 2001;11:877-81. [Crossref] [PubMed]
- Gemsenjäger E, Heitz PU. Contralateral papillary thyroid cancer-high incidence in therapeutic completion thyroidectomy. Thyroid 2002;12:345-6. [Crossref] [PubMed]
- Brito JP, Ito Y, Miyauchi A, et al. A Clinical Framework to Facilitate Risk Stratification When Considering an Active Surveillance Alternative to Immediate Biopsy and Surgery in Papillary Microcarcinoma. Thyroid 2016;26:144-9. [Crossref] [PubMed]
- Lee YC, Na SY, Chung H, et al. Clinicopathologic characteristics and pattern of central lymph node metastasis in papillary thyroid cancer located in the isthmus. Laryngoscope 2016;126:2419-21. [Crossref] [PubMed]
- Chung SR, Baek JH, Choi YJ, et al. Sonographic Assessment of the Extent of Extrathyroidal Extension in Thyroid Cancer. Korean J Radiol 2020;21:1187-95. [Crossref] [PubMed]
- Ito Y, Hirokawa M, Fukushima M, et al. Occult papillary thyroid carcinoma: diagnostic and clinical implications in the era of routine ultrasonography. World J Surg 2008;32:1955-60. [Crossref] [PubMed]


