
Percutaneous microwave ablation combined with endocrine therapy versus standard therapy for elderly patients with HR-positive and HER2-negative invasive breast cancer: a propensity score-matched analysis of a multi-center, prospective cohort study
Highlight box
Key findings
• MWA combined with endocrine therapy and standard surgery combined with adjuvant therapy for elderly breast cancer patients achieved similar outcomes.
• MWA combined with endocrine therapy may be a feasible treatment strategy for elderly patients with HR-positive and HER2-negative invasive breast cancer.
• MWA had much shorter length of hospital stay than standard surgery.
What is known and what is new?
• As a minimally invasive thermal therapy, MWA has been attempted to treat breast cancer of small lesions.
• The precise and optimal treatment strategy remains unknown for elderly patients with early-stage breast cancer, especially for HR-positive and HER2-negative breast cancer.
What is the implication, and what should change now?
• MWA combined with endocrine therapy and standard surgery combined with adjuvant therapy for elderly breast cancer patients achieved similar outcomes. MWA combined with endocrine may be a feasible treatment strategy for elderly patients with HR-positive and HER2-negative invasive breast cancer.
Introduction
Female breast cancer has surpassed lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases in 2020 (1). And approximately 30% of breast cancer cases occur in women aged 70 years and older (2). Compared with young patients, less aggressive treatment has been performed for elderly patients (3-5), due to their physiologic alterations, presumed short life expectancy, comorbid diseases, and inability to tolerate active therapy. But locoregional surgery cannot be omitted for these elderly patients because of a very high rate of local progression and presumed shorter life expectancy without local-regional treatment (3,6). Locoregional surgery has undergone a dramatic evolution over the past several decades from radical mastectomy to breast-conserving surgery (BCS) and sentinel lymph node biopsy (SLNB), providing less aggressive treatment for patients with early-stage breast cancer (7).
Apart from these surgeries, minimally invasive therapies, including radiofrequency ablation (RFA), microwave ablation (MWA), laser therapy, and cryotherapy, have been attempted to treat breast cancer of small lesions in several ablation-resection feasibility studies (8-13). However, the relatively long-term local efficacy of ablation in the treatment of breast cancer has only been reported in limited case series (14,15) with small sample sizes. The ICE3 study has shown that cryoablation is effective for breast cancer of small lesions ≤1.5 cm (16). MWA shows several advantages compared to other minimally invasive therapies (8,17-19), including larger ablation volumes, shorter ablation times and improved convection profile. Only one retrospective comparative study with a small sample size reported MWA of breast cancer ≤5 cm without subsequent excision in comparison to nipple sparing mastectomy (20). However, all of the 21 patients treated with MWA included different molecular subtypes; and the efficacy analysis has been influenced by comprehensive treatments, including chemotherapy, endocrine therapy, radiotherapy and targeted therapy.
For elderly patients with hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative early-stage breast cancer, usually receive endocrine therapy only, and there is a trend to omit chemotherapy for these patients (3,4,6,21,22). Additionally, there is a growing trend to apply minimally invasive local treatments for elderly patients with early-stage breast cancer (23), such as omit SLNB (24) and radiotherapy after lumpectomy for elderly patients with low risk of recurrence (25). The precise and optimal treatment strategy remains unknown for elderly patients with early-stage breast cancer, especially for HR-positive and HER2-negative breast cancer.
In this multi-center prospective cohort study, we aimed to determine the efficacy of MWA combined with endocrine therapy (MWA group) versus standard surgery combined with adjuvant therapy (surgery group) in the treatment of primary tumors (≤3 cm) for elderly patients diagnosed with HR-positive and HER2-negative invasive breast cancer. To the best of our knowledge, this is the first study to compare these two groups for elderly patients with HR-positive and HER2-negative early-stage breast cancer. We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-33/rc).
Methods
Study design
This prospective, non-randomized, multi-center study was conducted with the approval from the Institutional Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (No. 2010-SR-003), and all patients had given written informed consent for participation. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). From January 2016 to July 2021, elderly female patients diagnosed with invasive breast cancer were included in this study from three hospitals, The First Affiliated Hospital with Nanjing Medical University, Sir Run Run Hospital Nanjing Medical University, and The Fourth Affiliated Hospital of Nanjing Medical University.
The inclusion criteria included the following: (I) female patients with the age over 70 years old diagnosed with HR-positive and HER2-negative invasive breast cancer; (II) a solitary mass confirmed by using both US and mammography; (III) a tumor outside the nipple-areola area with any distance to the skin and chest wall but not infiltrated to the skin and pectoralis major muscle; (IV) estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki67 were determined by using immunohistochemical analysis of tissue from a core-needle biopsy specimen; (V) breast cancer 3.0 cm or less in greatest diameter confirmed by using ultrasonography (US); (VI) clinical negative axillary lymph node status confirmed by using US. Exclusion criteria were patients who received any previous surgery, radiotherapy or systemic anti-tumor treatment before enrollment or with distant metastasis.
Allocation of treatments
This prospective cohort study was based on patients’ self-selected treatments: patients who chose to undergo MWA combined with endocrine therapy or standard surgery followed by chemotherapy, radiotherapy or endocrine therapy if necessary. Patients who chose MWA, would be arranged for MWA under local anesthesia without axillary lymph node surgery (biopsy or dissection). No patient received radiotherapy or chemotherapy in the MWA group. All of the included patients received aromatase inhibitors (AIs) for endocrine therapy. Patients who chose surgery, would receive standard surgeries under general anesthesia, including mastectomy or BCS for breast, and axillary lymph node dissection (ALND) or SLNB for axillary lymph node. And radiotherapy and chemotherapy were recommended after surgery according to operation procedures (BCS or mastectomy), clinical-pathological characteristics, patients’ physical conditions and decisions. And endocrine therapy was recommended for all patients.
MWA procedure
All MWA procedures were performed by one surgeon with 10 years of experience in breast intervention. After enrollment, MWA was performed to targeted tumor under local anesthesia. US was used to guide MWA and monitor the procedure. Local anesthesia was induced with 10 mL of 1% lidocaine. And about 20 mL of 0.5% lidocaine was injected into both subcutaneous space and retromammary space and hydrodissection was performed (26). The antenna (14 G) was placed into the tumors along the largest axis. The microwave irradiation frequency of the system (Nanjing Yigao Microwave Electric Institute, Nanjing, China) was 2,450 MHz with the output power set at 40 W. After testing the cycling system with cold water, MWA was started immediately to cover the entire tumor.
According to our previous experiences, MWA for at least 2 minutes was recommended to achieve complete ablation for tumor larger than 2 cm, and at least 1 min for tumor smaller than 2 cm (27). Complete ablation was defined as the tumor disappearing completely on US. The whole MWA procedure was completed in half an hour. After ablation, patients were monitored for half an hour in the hospital where they received therapy for immediate complications. Technical effectiveness of MWA was defined as complete ablation at follow-up enhanced imaging 1 month after MWA.
Follow-up
All the patients were followed completely in the outpatient clinic. For the MWA group, breast US was recommended 1 week after ablation, breast magnetic resonance imaging (MRI) and contrast-enhanced US (CEUS) were recommended 1 month after ablation and then every year during follow-up. Complete ablation was defined as no focal enhancement within or at the periphery of the tumor confirmed by CEUS or MRI during follow-up (14). For both groups, breast US was recommended at 3-month intervals during the first year after treatment and then at 6-month intervals thereafter. Laboratory and other imaging examinations (e.g., chest and abdominal computed X-ray tomography, abdominal ultrasound, gynecological ultrasound) for metastases screening were performed at 6-month intervals within 2 years after treatment and then every year.
Clinical outcomes
Endpoints for the comparisons between the two groups were disease-free survival (DFS), overall survival (OS) and length of hospital stay (LOS) of the first hospitalization. The LOS was calculated as the difference between admittance and discharge dates, including pre-treatment examination, core-needle biopsy, waiting for pathological results and primary treatment (MWA or surgery). The post-operation LOS was calculated as the difference between primary treatment (MWA or surgery) and discharge dates.
Statistical analysis
Median, percentiles, range, mean and standard deviation were analyzed for numerical data as appropriate. In order to avoid potential selection bias and reduce the influence of confounding factors, propensity score matching analysis was performed. The proportion of included patients in the surgery group and MWA group is 3:1. According to the previous studies, the variables used for this procedure were date of diagnosis, age, tumor histology, tumor size, ER status, PR status and Ki-67 (28-30). Differences between the two groups were analyzed with the chi-square test or Fisher’s exact test for categorical variables and the Student’s t-test for continuous variables. Wilcoxon test was used to compare the difference of LOS and postoperative hospital stay between the two groups. Hazard ratios and 95% confidence interval (CI) were calculated using a stratified Cox proportional hazards model. The median follow-up and survival were analyzed with the Kaplan-Meier method. All P values were two-tailed with 5% significance levels. All statistical analyses were performed by using software STATA version 13.0 (StataCorp, College Station, TX, USA) or R version 4.1.1.
Results
Basic characteristics
From January 2016 to July 2021, 246 patients were finally eligible for inclusion in the study. A flow diagram of the study participants is shown in Figure 1. Of these included 246 patients, 33 patients underwent MWA combined with endocrine therapy (MWA group), and other 213 patients underwent standard surgeries combined with standard adjuvant therapies (surgery group) (Table 1). To avoid potential selection bias and reduce the influence of confounding factors, propensity score matching analysis was performed. After 1:3 propensity score matching for date of diagnosis, age, histology, tumor size, ER status, PR status and Ki-67, 99 patients were finally identified as control in the surgery group.
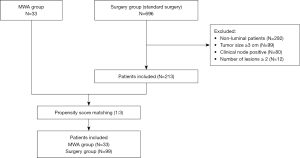
Table 1
| Characteristic | N |
|---|---|
| Age, years | |
| ≤75 | 129 |
| >75 | 84 |
| Histology | |
| Invasive ductal carcinoma | 173 |
| Invasive lobular carcinoma | 16 |
| Other invasive carcinoma | 24 |
| Clinical tumor size, cm | |
| T1 | 116 |
| T2 | 95 |
| T3 | 2 |
| Estrogen receptor | |
| <80% | 14 |
| 80–90% | 130 |
| >90% | 69 |
| Progesterone receptor | |
| <20% | 45 |
| ≥20% | 168 |
| Ki67 status | |
| ≤14% | 50 |
| >14% | 163 |
| Breast surgery | |
| Breast-conserving surgery | 15 |
| Mastectomy | 198 |
| Axillary surgery | |
| SLNB | 154 |
| ALND | 59 |
| Radiotherapy | |
| Yes | 6 |
| No | 207 |
| Chemotherapy | |
| Yes | 33 |
| No | 180 |
| Endocrine therapy | |
| Yes | 207 |
| No | 6 |
HR, hormone receptor; HER2, human epidermal growth factor receptor2; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
The mean age of the patients was 77.9 (range, 70–94) years for the overall patients; and 79.4 (range, 70–94), 77.4 (range, 70–92) years old for patients in the MWA group and in the surgery group, respectively. The baseline characteristics of patients in two groups are summarized in Table 2.
Table 2
| Characteristic | MWA group (n=33) | Surgery group (n=99) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 79.4±7.6 | 77.4±5.5 | 0.103† |
| Histology, n | 0.147‡ | ||
| Invasive ductal carcinoma | 31 | 80 | |
| Invasive lobular carcinoma | 1 | 4 | |
| Other invasive carcinoma (mucinous carcinoma) | 1 | 15 | |
| Clinical tumor size (cm), n | 0.948‡ | ||
| <1 | 2 | 7 | |
| 1–2 | 18 | 57 | |
| >2 | 13 | 35 | |
| Estrogen receptor, n | 1.000‡ | ||
| <90% | 2 | 8 | |
| ≥90% | 31 | 91 | |
| Progesterone receptor, n | 0.619‡ | ||
| <20% | 8 | 19 | |
| ≥20% | 25 | 80 | |
| Ki67 status, n | 0.681‡ | ||
| ≤14% | 13 | 35 | |
| >14% | 20 | 64 |
†, Student’s t-test; ‡, Fisher’s exact test. MWA, microwave ablation; SD, standard deviation.
Treatments
In the MWA group, MWA was successfully performed to all 33 cases under local anaesthesia (Table 3). The mean ablation time was 2.64±0.59 minutes, with a range from 1.67 to 4.5 minutes. The exact duration of MWA for each patient is shown in Table 4. After the first assessment 1 week after ablation, second ablation was given to one case for a little residual on the edge of the lesion under ultrasound. The remaining 32 cases received ablation only once, and ultrasound 1 week later showed no residual lesions. One month after ablation, no enhancement of the ablated tumor was observed by using MRI (Figure 2) and CEUS (Figure 3) in all cases. Of these 33 cases, technical effectiveness (complete ablation at follow-up enhanced imaging 1 month after MWA) was achieved in all 33 cases (100%; 95% CI, 89.4–100.0%). No skin burn, haematoma, infection or other ablation-related adverse events were observed during or after the procedure.
Table 3
| Variables | MWA group (n=33) | Surgery group (n=99) | P value |
|---|---|---|---|
| The procedure of MWA | – | ||
| MWA time (minutes), mean ± SD | 2.64±0.59 | – | |
| Complete ablation after 1 week, n | 32 | – | |
| Complete ablation after 1 month, n | 33 | – | |
| Breast surgery, n | – | ||
| Breast-conserving surgery | – | 8 | |
| Mastectomy | – | 91 | |
| Axillary surgery, n | <0.001‡ | ||
| SLNB | 0 | 76 | |
| ALND | 0 | 23 | |
| No | 33 | 0 | |
| Radiotherapy, n | 0.200‡ | ||
| Yes | 0 | 8 | |
| No | 33 | 91 | |
| Chemotherapy, n | 0.200‡ | ||
| Yes | 0 | 8 | |
| No | 33 | 91 | |
| Endocrine therapy, n | – | ||
| Yes | 33 | 99 | |
| No | 0 | 0 |
‡, Fisher’s exact test. MWA, microwave ablation; SD, standard deviation; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
Table 4
| Case | Tumor size | Ablation duration |
|---|---|---|
| 1 | 2.6 cm | 3 min |
| 2 | 2.7 cm | 3.5 min |
| 3 | 1.7 cm | 3 min |
| 4 | 1.5 cm | 2.5 min |
| 5 | 1.7 cm | 3 min |
| 6 | 2.1 cm | 3.5 min |
| 7 | 1.7 cm | 2.5 min |
| 8 | 2.3 cm | 2.25 min |
| 9 | 1.33 cm | 2 min |
| 10 | 2.3 cm | 3 min |
| 11 | 0.7 cm | 1 min 40 s |
| 12 | 1.2 cm | 2.5 min |
| 13 | 2.4 cm | 2 min |
| 14 | 3.0 cm | 2 min 40 s |
| 15 | 2.3 cm | 2 min |
| 16 | 1.3 cm | 2 min |
| 17 | 1.7 cm | 4.5 min |
| 18 | 1.7 cm | 2 min 20 s |
| 19 | 1.8 cm | 2.5 min |
| 20 | 1.5 cm | 2 min 10 s |
| 21 | 1.4 cm | 3 min |
| 22 | 2.2 cm | 3.5 min |
| 23 | 2.6 cm | 2.5 min |
| 24 | 1.5 cm | 2.5 min |
| 25 | 2.91 cm | 2 min 20 s |
| 26 | 1.8 cm | 2 min 20 s |
| 27 | 0.7 cm | 2 min |
| 28 | 1.5 cm | 3 min |
| 29 | 1.4 cm | 2 min 20 s |
| 30 | 2.3 cm | 2.5 min |
| 31 | 1.9 cm | 2 min 20 s |
| 32 | 2.9 cm | 3 min 37 s |
| 33 | 2.0 cm | 2 min 40 s |
MWA, microwave ablation.


In the surgery group, 8 (8.1%) patients received BCS, 91 (91.9%) patients received mastectomy, 76 (76.8%) patients received SLNB, and 23 (23.2%) patients received ALND. Besides, 8 (8.1%) patients received radiotherapy, and 8 (8.1%) patients received chemotherapy. All of the patients in the two groups received AIs for endocrine therapy (Table 3).
DFS and OS
With a median follow-up of 31 months (range, 2–74 months), DFS among patients in the MWA group and surgery group was not significantly different (hazard ratio, 0.536; 95% CI: 0.128–2.249; P=0.38) (Figure 4). Only 1 (1/33) patient in the MWA group had local recurrence 23 months after MWA, to whom modified radical mastectomy was performed. This patient was alive without any second recurrence at the end of follow-up. No patient in the MWA group developed distant metastasis. For the surgery group, tumor progression occurred in 3 (3/99) patients. One patient developed recurrent breast cancer involving chest wall at 23 months and was treated with further surgery. And this patient died 34 months after surgery. One patient was diagnosed with lung metastasis at 12 months after surgery and a third patient developed liver metastasis at 31 months after surgery. These two patients have received first-line endocrine therapy and still alive at the last follow-up.

No significant difference in OS was observed between the two groups of patients treated with MWA combined endocrine therapy and standard surgery combined with adjuvant therapies ((hazard ratio, 0.537; 95% CI: 0.089–3.235; P=0.49) (Figure 4).
The 1-, 3-year OS rates were 97.0% and 92.6% for patients in the MWA group and 100.0% and 96.1% for patients in the surgery group, respectively.
LOS
Among patients in the MWA group, there were 9 patients received MWA on an outpatient basis without hospitalization. In comparison to LOS for patients received standard surgeries, the mean LOS for patients received MWA was approximately 5 days shorter (7.1 versus 13.0 days, P<0.001) (Figure 5). And the post-operative LOS was still shorter for patients in the MWA group than those in the surgery group (1.3 versus 6.1 days, P<0.001) (Figure 5).

Discussion
We firstly report follow-up outcomes of MWA combined with endocrine therapy in the treatment of HR-positive and HER2-negative invasive breast cancer with small lesions. We found that the local efficacy and survival of patients received MWA were not significantly different from patients receiving standard surgeries. Among 33 cases during follow-up, local recurrence was observed in only one case 23 months after MWA, which might be attributed to endocrine therapy resistance. Our results suggested that MWA might be a reliable local therapy for elderly patients with breast cancer of small lesions. And MWA combined with adjuvant endocrine therapy may be a feasible treatment strategy for elderly patients with HR-positive and HER2-negative early-stage invasive breast cancer. In addition, MWA can be performed under local anesthesia, with less invasiveness, shorter operative time and hospitalization time compared with standard surgical approach. And it is of feasibility and safety to perform MWA on outpatient basis, resulting in potential cost savings and perhaps a higher quality of life.
For elderly patients, there is a trend to treat early-stage breast cancer with less aggressive therapies (4). However, local surgery cannot be omitted for elderly patients treated with adjuvant endocrine therapy (6,31). Minimally invasive therapies have been attempted to treat early breast cancer (14,15). MWA in the treatment of breast cancer has been reported in several feasibility studies (8,19), and a high rate of complete ablation has been found. Different from the margin status assessment of BCS (23), MRI and CEUS were applied to assess the ablation efficacy of MWA in the treatment of breast cancer in previous studies (11,14,15,32), which were effective and convenient approaches for follow-up after MWA. In the current study, we found excellent efficacy dependent on MRI or CEUS and favorable survival among patients receiving MWA with low risk of local recurrence.
Radiotherapy is also indispensable for local control after BCS, even for old patients at low risk of local recurrence (25), and sentinel node biopsy is the standard therapy for early breast cancer with a clinically negative axilla (33,34). Importantly, there is a trend to omit radiotherapy and SLNB for early breast cancer with very low risk of local-regional recurrence (24,25). In the current study, no patient received axillary lymph node surgery or radiotherapy in the MWA group. Moreover, no patient received chemotherapy in this study, even for patients with a high Ki67 index. Only one local recurrence was observed, and then radical mastectomy was performed on this patient, who was alive without any recurrence after surgery. For these elderly patients with HR-positive and HER2-negative breast cancer, minimally invasive thermal therapies such as MWA should be proposed to patients who have contraindication for surgery or who decline the surgical treatment even with comprehensive explanations.
Limitations
Several limitations existed in the current study. First, the current study was not a randomized study. Thus, propensity score matching analysis was performed to avoid potential selection bias and imbalance of clinical characteristics of patients in two groups. Second, the ablation efficacy after MWA was not evaluated by surgery. MRI or CEUS was applied to confirm the efficacy of MWA for all of the patients during follow-up, in which accumulative experience is needed in the judgment. Besides, follow-up was with imaging alone and hence microscopic tumor cannot be excluded, albeit probably rare. Third, because of limited participating patients and follow-up, future randomized clinical trials with large sample size are needed to test this treatment strategy.
Conclusions
Of all patients enrolled, very few (one patient in the MWA group, and three in the surgery group) experienced recurrence or died within the observed time frame. MWA combined with adjuvant endocrine therapy and standard surgery combined with adjuvant therapies for elderly patients with breast cancer achieved similar outcomes. MWA of breast cancer of small lesions is efficient in elderly patients. MWA combined with adjuvant endocrine therapy may be a feasible treatment strategy for elderly patients with HR-positive and HER2-negative breast cancer, although future studies with long-term follow-up are still needed for adoption into everyday practice.
Acknowledgments
Funding: This work was supported in part by the National Natural Science Foundation of China (Nos. 81771953 and 82172683), the Natural Science Foundation of Jiangsu Province (No. BK20180108), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-33/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-33/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-33/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-33/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of the First Affiliated Hospital with Nanjing Medical University (No. 2010-SR-003) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Lodi M, Scheer L, Reix N, et al. Breast cancer in elderly women and altered clinico-pathological characteristics: a systematic review. Breast Cancer Res Treat 2017;166:657-68. [Crossref] [PubMed]
- Fennessy M, Bates T, MacRae K, et al. Late follow-up of a randomized trial of surgery plus tamoxifen versus tamoxifen alone in women aged over 70 years with operable breast cancer. Br J Surg 2004;91:699-704. [Crossref] [PubMed]
- Gennari R, Curigliano G, Rotmensz N, et al. Breast carcinoma in elderly women: features of disease presentation, choice of local and systemic treatments compared with younger postmenopasual patients. Cancer 2004;101:1302-10. [Crossref] [PubMed]
- Eaker S, Dickman PW, Bergkvist L, et al. Differences in management of older women influence breast cancer survival: results from a population-based database in Sweden. PLoS Med 2006;3:e25. [Crossref] [PubMed]
- Mustacchi G, Ceccherini R, Milani S, et al. Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: long-term results of the phase III randomized controlled multicenter GRETA trial. Ann Oncol 2003;14:414-20. [Crossref] [PubMed]
- Black DM, Mittendorf EA. Landmark trials affecting the surgical management of invasive breast cancer. Surg Clin North Am 2013;93:501-18. [Crossref] [PubMed]
- Zhou W, Zha X, Liu X, et al. US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology 2012;263:364-73. [Crossref] [PubMed]
- Fornage BD, Sneige N, Ross MI, et al. Small (< or = 2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology 2004;231:215-24. [Crossref] [PubMed]
- Burak WE Jr, Agnese DM, Povoski SP, et al. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer 2003;98:1369-76. [Crossref] [PubMed]
- Simmons RM, Ballman KV, Cox C, et al. A Phase II Trial Exploring the Success of Cryoablation Therapy in the Treatment of Invasive Breast Carcinoma: Results from ACOSOG (Alliance) Z1072. Ann Surg Oncol 2016;23:2438-45. [Crossref] [PubMed]
- Roubidoux MA, Sabel MS, Bailey JE, et al. Small (< 2.0-cm) breast cancers: mammographic and US findings at US-guided cryoablation--initial experience. Radiology 2004;233:857-67. [Crossref] [PubMed]
- Dowlatshahi K, Francescatti DS, Bloom KJ. Laser therapy for small breast cancers. Am J Surg 2002;184:359-63. [Crossref] [PubMed]
- Palussière J, Henriques C, Mauriac L, et al. Radiofrequency ablation as a substitute for surgery in elderly patients with nonresected breast cancer: pilot study with long-term outcomes. Radiology 2012;264:597-605. [Crossref] [PubMed]
- Cazzato RL, de Lara CT, Buy X, et al. Single-Centre Experience with Percutaneous Cryoablation of Breast Cancer in 23 Consecutive Non-surgical Patients. Cardiovasc Intervent Radiol 2015;38:1237-43. [Crossref] [PubMed]
- Fine RE, Gilmore RC, Dietz JR, et al. Cryoablation Without Excision for Low-Risk Early-Stage Breast Cancer: 3-Year Interim Analysis of Ipsilateral Breast Tumor Recurrence in the ICE3 Trial. Ann Surg Oncol 2021;28:5525-34. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25:S69-83. [Crossref] [PubMed]
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol 2011;79:124-30. [Crossref] [PubMed]
- Zhou W, Jiang Y, Chen L, et al. Image and pathological changes after microwave ablation of breast cancer: a pilot study. Eur J Radiol 2014;83:1771-7. [Crossref] [PubMed]
- Yu J, Han ZY, Li T, et al. Microwave Ablation Versus Nipple Sparing Mastectomy for Breast Cancer ≤5 cm: A Pilot Cohort Study. Front Oncol 2020;10:546883. [Crossref] [PubMed]
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Pan H, Zhang K, Wang M, et al. Palliative Local Surgery for Locally Advanced Breast Cancer Depending on Hormone Receptor Status in Elderly Patients. Clin Breast Cancer 2019;19:e247-60. [Crossref] [PubMed]
- Fornage BD, Hunt KK. Image-guided Percutaneous Ablation of Small Breast Cancer: Which Technique is Leading the Pack? Technol Cancer Res Treat 2015;14:209-11. [Crossref] [PubMed]
- Gentilini O, Botteri E, Dadda P, et al. Physical function of the upper limb after breast cancer surgery. Results from the SOUND (Sentinel node vs. Observation after axillary Ultra-souND) trial. Eur J Surg Oncol 2016;42:685-9. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. Erratum in: Lancet Oncol 2015;16:e105. [Crossref] [PubMed]
- Zhou W, Wang R, Liu X, et al. Ultrasound-guided microwave ablation: a promising tool in management of benign breast tumours. Int J Hyperthermia 2017;33:263-70. [Crossref] [PubMed]
- Pan H, Qian M, Chen H, et al. Precision Breast-Conserving Surgery With Microwave Ablation Guidance: A Pilot Single-Center, Prospective Cohort Study. Front Oncol 2021;11:680091. [Crossref] [PubMed]
- Wan A, Liang Y, Chen L, et al. Association of Long-term Oncologic Prognosis With Minimal Access Breast Surgery vs Conventional Breast Surgery. JAMA Surg 2022;157:e224711. [Crossref] [PubMed]
- Warschkow R, Güller U, Tarantino I, et al. Improved Survival After Primary Tumor Surgery in Metastatic Breast Cancer: A Propensity-adjusted, Population-based SEER Trend Analysis. Ann Surg 2016;263:1188-98. [Crossref] [PubMed]
- Li WP, Gao HF, Ji F, et al. The role of adjuvant chemotherapy in stage I-III male breast cancer: a SEER-based analysis. Ther Adv Med Oncol 2020;12:1758835920958358. [Crossref] [PubMed]
- Arriagada R, Lê MG, Guinebretière JM, et al. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol 2003;14:1617-22. [Crossref] [PubMed]
- Merckel LG, van den Bosch MA. Imaging-guided breast cancer ablation. Radiology 2012;265:322-3; author reply 323. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 1997;349:1864-7. [Crossref] [PubMed]


