
Revisiting Loré’s retrograde thyroidectomy from the perspective of preserving the external branch of the superior laryngeal nerve
Highlight box
Key findings
• The retrograde thyroidectomy (RT) approach effectively preserves external branch of superior laryngeal nerve (EBSLN) function without any additional notable surgical complications compared to conventional thyroidectomy (CT).
What is known and what is new?
• Injury to the EBSLN is a crucial factor in post-thyroidectomy dysphonia (PTD) in the absence of recurrent laryngeal nerve injury and may cause severe impairment.
• Compared to CT, RT had a better outcome in preserving the EBSLN, which was confirmed by postoperative electromyography.
What is the implication, and what should change now?
• RT technique may reduce the risk of EBSLN injury even without direct visual identification of the nerve.
Introduction
Thyroidectomy is a commonly performed procedure worldwide. Despite improvements in surgical knowledge and expertise, thyroidectomy-related problems continue to affect considerable number of patients. Post-thyroidectomy dysphonia (PTD) is a major concern for both patients and surgeons, as voice symptoms are more common than hypocalcemia or cosmetic issues (1). Recurrent laryngeal nerve (RLN) injury is the most well-known cause of PTD, leading to many studies to focus on preventing its injury during thyroid surgery (2). Although numerous other theoretically possible causes such as strap muscle or cricothyroid muscle injury, surgical adhesion, intubation-related trauma, laryngeal edema, and psychological distress have been proposed, injury to the external branches of the superior laryngeal nerve (EBSLN) has been under the spotlight as the underlying cause of PTD without RLN injury (3).
The EBSLN is vulnerable to injury during superior pole dissection during thyroidectomy. In response to this issue, anatomical classifications of the EBSLN have been recommended by a number of researchers (4-6), and there have been numerous initiatives to preserve the EBSLN. These include superior pole capsular dissection without identification (7), visual identification of the nerve prior to the ligation of superior thyroid vessels (8), and electrophysiological identification using a nerve stimulator (5,6), or intraoperative nerve monitoring (IONM) (3). These attempts were based on Kocher’s conventional thyroidectomy (CT) approach, which typically separates the Berry ligament from the tracheal cartilage as the final step, as it has been the prevailing and widely accepted technique for modern thyroidectomy (9). Alternatively, Loré described the retrograde thyroidectomy (RT) technique, which theoretically protects the EBSLN by facilitating complete thyroid mobilization and appropriate superior pole traction, and demonstrated his favorable results based on laryngoscopy, voice analyses, and detailed questionnaires (10). However, due to the lack of comprehensive data, evidence regarding the benefits of preserving the EBSLN is still inconclusive.
In this study, we aimed to compare the results of RT and CT with respect to EBSLN function, based on postoperative electromyography (EMG). We present this article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-21/rc).
Methods
Study population and ethical consideration
The preoperative, intraoperative, and postoperative clinical data of thyroid surgeries performed by the corresponding author (WC) were collected prospectively in the established datasheet forms and entered into a computerized database managed in Microsoft Access 2016 (Microsoft Corp., Redmond, WA, USA). We retrospectively reviewed consecutive thyroid surgeries conducted between December 2013 and December 2018. Among patients who underwent primary thyroid surgery with suffice postoperative EMG, patients with preoperative idiopathic vocal fold palsy; locally invasive thyroid cancer (T4a); poorly differentiated or undifferentiated thyroid carcinoma were excluded (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Under the approval of the Institutional Review Board of Pusan National University Hospital (No. 1810-010-071), all patient data was collected from the same institution where the corresponding author (WC) previously performed all surgical procedures as a faculty member. The requirement for informed consent of clinicopathologic data was waived due to the retrospective nature of this study.
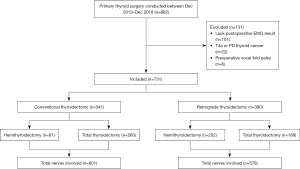
Study design
Patients were evaluated for age at operation, sex, clinicopathologic features of the disease, clinical information of the surgery, postoperative complications, and follow-up data. Patients were classified into two groups according to the surgical method: RT and CT. Postoperative bleeding events, drain amount, hypocalcemia, calcium replacement, RLN function, and EBSLN function were compared between the two groups. RLN function was assessed via laryngoscopy, and EBSLN function was determined by EMG conducted 2–3 months postoperatively.
Surgical techniques: CT and RT technique
All operations were performed by the corresponding author (WC) with the routine use of surgical loupes (SurgiTel®, Ann Arbor, MI, USA) with ×3.5 magnification. In our study, IONM was not consistently used, as its application was restricted to select cases approved by National Health Insurance policies. For these selected cases, neurostimulation was administered after completing thyroidectomy to verify the EBSLN integrity.
CT was performed, beginning with dissection of the lateral thyroid area and division of the middle thyroid vein. The inferior parathyroid gland was usually identified during capsular dissection, and the RLN was identified deep to the inferior parathyroid gland. The superior thyroid vessels were carefully distinguished and ligated at the terminal branches using capsular dissection. The thyroid lobe was medially rotated after the dissection of the superior and inferior poles. Finally, with meticulous RLN preservation, the Berry ligament was separated from the tracheal cartilage.
RT was performed with initial division of the thyroid isthmus. Along with retracting the isthmus laterally, the pretracheal fascia between the cricotracheal structure and the medial surface of the thyroid was meticulously dissected. Then, with medial traction of the thyroid, the sternothyroid muscle was detached from the thyroid. The thick and tough ligament of Berry was easily identified during dissection of the pretracheal fascia between the thyroid and tracheal wall. The branches of the inferior thyroidal vein and the adjacent tissues were dissected and ligated adjacent to the thyroid capsule. During dissection of the inferior pole, the inferior parathyroid gland could be identified and preserved with attachment to the visceral fascia. The RLN was identified in a triangle bounded laterally by the medial surface of the Zuckerkandl tubercle, medially by the trachea, and superiorly by the Berry ligament. Under direct visualization of the RLN, the visceral fascia and Berry ligament were carefully dissected with traction in the inferior-to-superior direction. After the Berry ligament was completely divided, the thyroid was fully mobilized from the cricotracheal complex. Finally, the terminal branches of the superior thyroid artery were meticulously ligated with proper tension on the thyroid. Visual identification of the EBSLN was not intentionally pursued. However, during upper pole dissection, some EBSLNs were inadvertently encountered and visually recognized over the inferior constrictor (Figure 2).
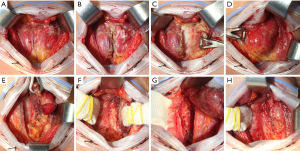
Postoperative EMG of cricothyroid muscle
In our study, patients underwent bilateral CT muscle EMG examinations 2–3 months postoperatively at our hospital’s rehabilitation medicine department. EMG was performed using Cadwell Sierra Summit EMG unit (Cadwell Industries, Inc., Kennewick, WA, USA). A single electrophysiologist (Prof. Jae Hyeok Chang) conducted all exams without knowledge of operative findings. Patients were positioned supine with their head slightly hyperextended, and the skin between the cricoid and thyroid cartilage was anesthetized with 1% lidocaine. A 37 mm concentric disposable EMG needle electrode was inserted through the anesthetized skin, 2 mm lateral to the midline, tangential to the cricoid arch’s upper border, in a superior and lateral direction. The patient vocalized the vowel “e” at both the lowest and highest pitches possible to confirm proper needle location with clear, crisp-sounding motor unit signals. Additionally, the patient was asked to elevate their head from the table, during which only distant electrical activity should be observed (11). The presence of abnormal spontaneous activity such as fibrillation potentials, positive sharp waves, or complex repetitive discharge was thoroughly evaluated. The motor unit action potential (MUAP) morphology and interference pattern were also analyzed.
EMG findings were systematically evaluated in terms of insertional activity, resting potential, single MUAP morphology, and recruitment pattern. The absence of any electrical activity either on electrode insertion or on attempted voluntary motion is called “electrical silence” (12). The EMG findings and results were reported as “normal” when there were no abnormal findings in the systematic evaluation and were reported as “abnormal” if presenting with any abnormal findings. Electrophysiologic findings were categorized as Grade I (neuropraxia), Grade II (mild to moderate axonotmesis or neurotmesis), Grade III (severe axonotmesis or neurotmesis), and Grade IV (electrical silence) according to the four main characteristics mentioned above. Grade I required diagnostic criteria for the detection of a decreased recruitment pattern on voluntary action, the absence of abnormal resting potentials, and no changes in MUAP morphology. Grade II was defined as the presence of abnormal resting potentials or abnormal MUAP morphology and a normal recruitment pattern. Grade III was assigned based on the presence of abnormal resting potentials or abnormalities in MUAP morphology and a decreased recruitment pattern. Finally, Grade IV, representing complete destruction of the entire nerve structure over its full diameter, was given for electrical silence on EMG.
Statistical analysis
Continuous variables were presented as means ± standard deviations. An unpaired t-test was used to assess differences in continuous variables between the CT and RT groups for normally distributed data, and Mann-Whitney U tests for data not normally distributed. Pearson’s chi-square test and Fisher’s exact test were used to analyze categorical variables and to determine nonrandom associations between the postoperative results and surgical approach. All statistical analyses were performed using IBM SPSS v. 20.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P<0.05.
Results
Between December 2013 and December 2018, 862 patients underwent primary thyroid surgeries. After a thorough review, 131 patients [8 patients with preoperative idiopathic vocal fold palsy, 22 with locally invasive thyroid cancer (T4a) and poorly differentiated or undifferentiated thyroid carcinoma, and 101 without sufficient postoperative EMG results] were excluded. In total, 731 patients were included in this study (Figure 1). CT was solely performed on consecutive patients from December 2013 to October 2015, whereas RT was exclusively conducted from October 2015 to December 2018.
Clinical characteristics and surgical details of the patients are presented in Table 1. A total of 731 patients (133 men and 598 women; male-to-female ratio, 1:4.5) aged 18–84 years (median 51 years) underwent total thyroidectomy (n=448) or hemithyroidectomy (n=283) under general endotracheal anesthesia. In 277, 291, 19, and 144 patients, the tumor was located on the left, right, isthmus, and bilaterally, respectively. Overall, 675 (92.3%) patients had well-differentiated thyroid carcinoma or other malignant lesions, such as medullary thyroid carcinoma or Hurthle cell carcinoma, and 56 patients (7.7%) were treated for benign lesions, such as follicular adenoma or benign thyroid follicle. Central neck dissection (CND) was followed on 696 patients and among these patients, lateral neck dissection was also performed in 40 patients. The CT and RT groups were similar in most clinical characteristics and surgical data; however, total thyroidectomy was performed more frequently in the CT group, and more time was required for CT when performing hemithyroidectomy with CND.
Table 1
| Variable | Total (n=731) | Conventional (n=341) | Retrograde (n=390) | P |
|---|---|---|---|---|
| Age (years) | 50.5±12.3 | 47.6±12.5 | 53.1±11.6 | 0.326 |
| Sex | 0.496 | |||
| Female | 598 (81.8) | 283 (83.0) | 315 (80.8) | |
| Male | 133 (18.2) | 58 (17.0) | 75 (19.2) | |
| Pathology | 0.216 | |||
| Benign | 56 (7.7) | 21 (6.2) | 35 (9.0) | |
| DTC | 672 (91.9) | 320 (93.8) | 352 (90.3) | |
| Other malignancy | 3 (0.4) | 0 (0.0) | 3 (0.8) | |
| Tumor side | 0.440 | |||
| Left | 277 (37.9) | 138 (40.5) | 139 (35.6) | |
| Right | 291 (39.8) | 128 (37.5) | 163 (41.8) | |
| Isthmus | 19 (2.6) | 7 (2.1) | 12 (3.1) | |
| Bilateral | 144 (19.7) | 68 (19.9) | 76 (19.5) | |
| Tumor location | 0.762 | |||
| Upper | 143 (19.6) | 64 (18.8) | 79 (20.3) | |
| Mid | 509 (69.6) | 242 (71.0) | 267 (68.5) | |
| Lower | 79 (10.8) | 35 (10.3) | 44 (11.3) | |
| Dimension (cm) | 1.02±1.04 | 0.97±0.96 | 1.06±1.11 | 0.250 |
| ETE | 0.113 | |||
| No | 584 (79.9) | 281 (82.4) | 303 (77.7) | |
| Yes | 147 (20.1) | 60 (17.6) | 87 (22.3) | |
| Multifocality | 0.548 | |||
| No | 539 (73.7) | 255 (74.8) | 284 (72.8) | |
| Yes | 192 (26.3) | 86 (25.2) | 106 (27.2) | |
| T staging (DTC) | 0.119 | |||
| 1 | 501 (74.5) | 248 (77.5) | 253 (71.9) | |
| 2 | 18 (2.7) | 10 (3.1) | 8 (2.3) | |
| 3 | 153 (22.8) | 62 (19.4) | 91 (25.9) | |
| N staging (DTC) | 0.140 | |||
| 0 | 443 (65.9) | 220 (68.8) | 223 (63.4) | |
| 1 | 229 (34.1) | 100 (31.3) | 129 (36.6) | |
| Overall staging (DTC) | 0.207 | |||
| 1 | 543 (80.8) | 265 (82.8) | 278 (79.0) | |
| 2 | 129 (19.2) | 55 (17.2) | 74 (21.0) | |
| Operation type | <0.001* | |||
| HT | 283 (38.7) | 81 (23.8) | 202 (51.8) | |
| TT | 448 (61.3) | 260 (76.2) | 188 (48.2) | |
| Management of neck | 0.205 | |||
| Not done | 35 (4.8) | 15 (4.4) | 20 (5.1) | |
| Only CND | 656 (89.7) | 302 (88.6) | 354 (90.8) | |
| CND and LND | 40 (5.5) | 24 (7.0) | 16 (4.1) | |
| Operation time (min) | ||||
| HT without CND | 113.3±44.1 | 110.8±29.9 | 114.8±53.8 | 0.460 |
| HT with CND | 104.3±30.6 | 110.2±28.4 | 102.1±31.2 | 0.011* |
| TT without CND | 184.4±33.0 | 195.0±37.7 | 178.0±32.5 | 0.541 |
| TT with CND | 145.3±41.1 | 143.1±36.9 | 148.2±46.2 | 0.535 |
| TT with CND and LND | 336.4±106.4 | 345.6±120.6 | 322.5±82.5 | 0.699 |
Continuous variables are presented as mean ± SD, and categorical variables are presented as n (%). *, statistically significant results of P value less than 0.05. DTC, differentiated thyroid cancer; ETE, extrathyroidal extension; HT, hemithyroidectomy; TT, total thyroidectomy; CND, central neck dissection; LND, lateral neck dissection; SD, standard deviation.
Postoperative results are demonstrated in Table 2. The two surgical methods yielded similar postoperative findings with respect to bleeding events and drain amount. Postoperative hypocalcemia—as identified by subjective symptoms, ionized calcium level measured less than 2 days postoperatively, and calcium replacement—was similar between the two groups, and the occurrence of permanent hypocalcemia was also comparable between CT (2.7%) and RT (1.1%). A total of 1,179 RLN and EBSLNs were at risk, of which 601 were involved in CT and 578 in RT. Among them, 14 cases of temporary vocal fold paralysis (6 on CT and 8 on RT) eventually improved, leading to the absence of permanent vocal fold paralysis among the nerves analyzed.
Table 2
| Variable | Total (n=731) | Conventional (n=341) | Retrograde (n=390) | P |
|---|---|---|---|---|
| Postoperative bleeding | 1.000 | |||
| No | 725 (99.2) | 338 (99.1) | 387 (99.2) | |
| Yes | 6 (0.8) | 3 (0.9) | 3 (0.8) | |
| Total drain amount (mL) | ||||
| HT without CND | 84.3±71.2 | 81.3±54.3 | 86.7±84.1 | 0.732 |
| HT without CND | 67.3±58.0 | 71.7±53.1 | 65.6±59.9 | 0.167 |
| TT without CND | 162.5±71.2 | 165.0±60.6 | 161.0±83.8 | 0.761 |
| TT with CND | 101.2±71.4 | 93.8±65.0 | 111.6±78.6 | 0.075 |
| TT with CND and LND | 306.2±294.8 | 281.9±208.0 | 342.8±396.4 | 0.923 |
| Postoperative temporary hypocalcemia | ||||
| Subjective symptom | 0.062 | |||
| No | 316 (70.5) | 174 (66.9) | 142 (75.5) | |
| Yes | 132 (29.5) | 86 (33.1) | 46 (24.5) | |
| Ionized calcium (<1.0 mmol/L) | 1.000 | |||
| No | 379 (84.6) | 220 (84.6) | 159 (84.6) | |
| Yes | 69 (15.4) | 40 (15.4) | 29 (15.4) | |
| Oral replacement during hospitalization | 0.299 | |||
| No | 311 (69.4) | 175 (67.3) | 136 (72.3) | |
| Yes | 137 (30.6) | 85 (32.7) | 52 (27.7) | |
| IV replacement during hospitalization | 0.387 | |||
| No | 372 (83.0) | 212 (81.5) | 160 (85.1) | |
| Yes | 76 (17.0) | 48 (18.5) | 28 (14.9) | |
| Postoperative permanent hypocalcemia | 0.384 | |||
| No | 439 (98.0) | 253 (97.3) | 186 (98.9) | |
| Yes | 9 (2.0) | 7 (2.7) | 2 (1.1) | |
| Total nerves involved | 1,179 | 601 | 578 | 0.886 |
| Right | 591 (50.1) | 303 (50.4) | 288 (49.8) | |
| Left | 588 (49.9) | 298 (49.6) | 290 (50.2) | |
| Recurrent laryngeal nerve | N/A | |||
| Identification | 1,179 (100.0) | 601 (100.0) | 578 (100.0) | |
| Preservation | 1,179 (100.0) | 601 (100.0) | 578 (100.0) | |
| Temporary vocal fold paralysis | 1.000 | |||
| No | 1,165 (98.8) | 595 (99.0) | 570 (98.6) | |
| Yes | 14 (1.2) | 6 (1.0) | 8 (1.4) | |
| Permanent vocal fold paralysis | 1.000 | |||
| No | 1,179 (100.0) | 601 (100.0) | 578 (100.0) | |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| EBSLN identification | <0.001* | |||
| No | 1,152 (97.7) | 576 (95.8) | 576 (99.7) | |
| Yes | 27 (2.3) | 25 (4.2) | 2 (0.3) | |
| Electromyography (EBSLN) | <0.001* | |||
| Normal | 1,122 (95.2) | 554 (92.2) | 568 (98.3) | |
| Abnormal | 57 (4.8) | 47 (7.8) | 10 (1.7) | |
| Electromyography grade (EBSLN) | <0.001* | |||
| I | 7 (12.3) | 0 (0.0) | 7 (70.0) | |
| II | 40 (70.2) | 37 (78.7) | 3 (30.0) | |
| III | 10 (17.5) | 10 (21.3) | 0 (0.0) | |
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Continuous variables are presented as mean ± SD, and categorical variables are presented as numbers, or n (%). *, statistically significant results of P value less than 0.05. HT, hemithyroidectomy; CND, central neck dissection; TT, total thyroidectomy; LND, lateral neck dissection; IV, intravenous; N/A, not applicable; EBSLN, external branch of the superior laryngeal nerve; SD, standard deviation.
The EBSLN was more likely to be exposed and identified in the surgical field during CT (4.2%) than during RT (0.3%). However, according to the postoperative EMG results, the abnormality rate was significantly higher after CT (7.8%) than that after RT (1.7%). In the CT group, 3 out of 25 patients with visually identified EBSLN had abnormal postoperative EMG results. In contrast, none of the 2 patients in the RT group with visually identified EBSLN exhibited abnormal EMG findings, Additionally, the CT group also presented a higher grade of abnormal EMG findings. Of the 47 patients in the CT group, 37 presented with Grade II (78.7%) and 10 with Grade III (21.3%), and for the 10 RT group, 7 presented with Grade I (70.0%) and 3 with Grade II (30.0%).
Discussion
This study presents the clinical outcomes of RT and compares the results with those of CT, which demonstrate the competency of RT, especially for preserving the EBSLN based on postoperative EMG. Our results provide clinical evidence of possible benefits of Loré’s technique; thus, it may support clinical decisions in the appropriate approach for thyroidectomy customized to each patient.
Modern thyroidectomy techniques have significantly progressed since Kocher established CT, reducing morbidity and mortality rates (9). Subsequently, surgeons like Halsted and Delbridge developed methods such as capsular dissection to preserve parathyroid gland vascular supply and minimize complications like RLN palsy and hypoparathyroidism (13,14). As the CT approach represents a widely accepted technique for thyroidectomy, relevant anatomy and landmarks for this approach have been extensively studied (15-18), and this method is considered to offer a high level of safety for handling RLN, with consistent results among numerous surgeons.
Throughout the progress of thyroid surgery, the EBSLN has been attributed much less clinical significance and was even referred to as the “neglected” nerve (19). Injury to the EBSLN may result in significant disability, as witnessed in the case of the famous opera soprano Amelita Galli-Curci, who underwent thyroid surgery and suffered EBSLN injury with distressing consequences (20). Nevertheless, it is seldom ignored since the actual prevalence of vocal impairment caused by EBSLN injury is uncertain, alterations to the natural speaking voice can be minor, and laryngeal findings are typically ambiguous and inconspicuous (21). Although voice therapy is a potential treatment strategy for EBSLN injury, no current treatment’s efficacy has been proven. Therefore, prevention is more critical than post-injury rehabilitation. The optimal tactics for preserving the EBSLN are still debatable, even though several of the previously described strategies are commonly suggested to prevent EBSLN damage (3,5-8,21).
The importance of using the capsular dissection of the upper pole in terms of protecting the EBSLN was initially emphasized by Coller and Boyden (22). They recommended avoiding mass ligation and advocated individual ligation of superior thyroid vessels’ terminal branches in terms of preserving EBSLN. One of the most compelling techniques of thyroidectomy was introduced by Loré, also known for the “Loré’s RLN triangle”. Loré described his technique separating the Berry ligament prior to dissecting the upper pole at the end of surgery. Through this method, he demonstrated a low incidence of EBSLN injury (0.1%), which was confirmed by laryngoscopy (23). He stated that his retrograde technique provides the advantage of proper traction and capsular dissection of the upper pole, which should reduce the risk of EBSLN injury (24,25). Loré’s technique is clearly distinguished from CT, which is performed in the lateral-to-medial aspect. However, evidence of the advantages of RT has not yet been thoroughly established.
Our study offers a significant amount of clinical data on the two thyroid surgery techniques, Loré’s RT and CT. Our study identified favorable outcomes in terms of the incidence of EBSLN injury based on the postoperative EMG of the RT group (1.7% vs. 7.8% in the CT group; P<0.001). This suggests that capsular dissection and proper traction of the thyroid facilitated by RT may be beneficial for preserving the EBSLN. To our knowledge, there is a shortage of English-language studies that have compared the effectiveness of CT and RT in preventing injury to the EBSLN.
In our study, EBSLN function was evaluated based on the EMG of the cricothyroid muscles. Needle EMG of the cricothyroid muscle is considered as the most accurate tool and the gold standard for the evaluating the integrity of the EBSLN (6,21). Most clinicians have used and preferred the indirect method to diagnose EBSLN injuries due to invasiveness, inconvenience, and a high barrier to entry in the technique and interpretation of EMG (3). Therefore, patients’ subjective voice change, perceptual voice quality assessment using the GRBAS scale, patient-reported questionnaires, acoustic analysis, and laryngoscopy or stroboscopy evaluation of the vocal folds have been used in most studies evaluating EBSLN injury as an alternative method (26). Despite being a retrospective study, the credibility of our data is strengthened by the use of postoperative EMG, which was conducted with informed consent from all patients. To ensure the reliability of our EMG technique, we followed established protocols and engaged an experienced electrophysiologist blinded to operative findings. We adhered to widely accepted literature for needle electrode insertion, patient positioning, and thoroughly evaluated abnormal spontaneous activity and MUAP morphology. Although inherent challenges in differentiating between EBSLN injury and direct muscle damage, our study carefully ensured the preservation of the cricothyroid muscle during thyroidectomy, which led us to infer that abnormal EMG findings on the cricothyroid muscle could potentially indicate EBSLN injury.
Another interesting finding of our study is that the intraoperative visual identification rate of EBSLN is low. Only 2.3% of the total nerves involved (27 of 1,179) were visually identified, which was significantly lower in the RT group than that in the CT group (0.3% vs. 4.2%, respectively; P<0.001). Despite the lower identification rate, the RT group demonstrated higher preservation rate of the EBSN, indicating that actively visualizing and identifying the EBSLN during thyroidectomy may not be mandatory for preserving the nerve. Notably, the only 3 patients with visually identified EBSLN and abnormal postoperative EMG were from the CT group, indicating a potential difference in the efficacy of nerve preservation between the two surgical approaches. However, the sample size was too small for clinical inference. The benefits of active visualization and identification of EBSLN are a subject of debate among surgeons. Following the traditional principles of surgery, the identification of the EBSLN along with individual ligation of the superior thyroid vessels was suggested as the exceptional method to preserve the nerve (8). However, some argue that pure visual identification without electrophysiological confirmation can also elevate the risk of nerve injury due to the invasiveness of the procedure and confusion during the process (27,28). Although this topic may still remain controversial, our result provides a considerable evidence to this debate.
Recent thyroidectomy practices widely recognize the use of IONM as the ideal method for EBSLN identification, with various studies reporting over 90% identification rates using IONM (5,29). This is particularly important considering the EBSLN variations that could increase the risk of injury during thyroidectomy (5,29-31). Notably, popular variations such as Cernea 2a or 2b, and Friedman type I, which are considered to be at greater risk of injury, are known to comprise a significant proportion in the average of roughly 40% among different classification studies (30,31). Although routine identification with IONM is undoubtedly ideal and effective in preserving EBSLN, the RT technique can support EBSLN preservation by facilitating the release of the superior pole, thereby providing better traction for meticulous capsular dissection. This technique is further supported by the typical anatomical plane location of the EBSLN, which resides deep below the inferior constrictor muscle or the false thyroid capsule and remains unaffected by capsular dissection (26). The discrepancy between the anatomical plane of the EBSLN and the procedure plane may reduce the risk of encountering the EBSLN during surgery. While IONM serves as the ideal method for EBSLN identification, RT offers a beneficial surgical approach in preserving the EBSLN. Additionally, our results imply that when IONM is unavailable for intraoperative use, employing the RT approach could contribute to achieving favorable outcomes in thyroidectomy.
The retrospective design of the study and the lack of proper randomization of the two groups for treatment allocation are major limitations of our study. To minimize potential bias, consecutive patients undergoing thyroidectomies within a specific time frame were included, and the rehabilitation physician who performed the EMG was blinded to the operation details. Another potential limitation was that all procedures were performed by a single surgeon, which may have resulted in better postoperative outcomes in the RT group due to the surgeon’s improving experience and knowledge over time. Additionally, including patients who underwent central or lateral neck dissection in our analysis could potentially introduce bias in the comparison. However, we believe that this bias is minimized since there were no significant differences in the distribution of patients who underwent different types of neck dissection, and the impact of neck dissection on EBSLN function is presumed to be minimal. Despite these limitations, our study retains its integrity in that the two approaches were conducted in accordance with an accepted protocol, and no intentional attempts were made to identify the EBSLN by the protocol. However, a larger-scale multicenter study would be necessary to generalize our results.
Conclusions
Loré’s demonstrated a low rate of EBSLN injury using the RT technique, highlighting the crucial role of surgical technique in preserving the EBSLN. Our study demonstrates that the incidence of EBSLN injury using RT was 1.7%, which was significantly lower than that for CT. The results were obtained using postoperative EMG, which is considered the most reliable measurement method. Our study suggest that the meticulous capsular dissection and appropriate traction of the thyroid facilitated by RT can provide benefits in preserving the EBSLN, even without direct visual identification of the nerve. Our findings can assist thyroid surgeons in determining the appropriate approach in various cases, refining their technique to preserve the EBSLN.
Acknowledgments
Funding: This study was supported by the National Research Foundation of Korea grant funded by the Korean Government (Ministry of Science and ICT) (No. 2022R1F1A1074487).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-21/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-21/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-21/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-21/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Under the approval of the Institutional Review Board of Pusan National University Hospital (IRB No. 1810-010-071), all patient data was collected from the same institution where the corresponding author (WC) previously performed all surgical procedures as a faculty member. The requirement for informed consent of clinicopathologic data was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grover G, Sadler GP, Mihai R. Morbidity after thyroid surgery: patient perspective. Laryngoscope 2013;123:2319-23. [Crossref] [PubMed]
- Henry BM, Graves MJ, Vikse J, et al. The current state of intermittent intraoperative neural monitoring for prevention of recurrent laryngeal nerve injury during thyroidectomy: a PRISMA-compliant systematic review of overlapping meta-analyses. Langenbecks Arch Surg 2017;402:663-73. [Crossref] [PubMed]
- Barczyński M, Konturek A, Stopa M, et al. Randomized controlled trial of visualization versus neuromonitoring of the external branch of the superior laryngeal nerve during thyroidectomy. World J Surg 2012;36:1340-7. [Crossref] [PubMed]
- Cernea CR, Nishio S, Hojaij FC. Identification of the external branch of the superior laryngeal nerve (EBSLN) in large goiters. Am J Otolaryngol 1995;16:307-11. [Crossref] [PubMed]
- Friedman M, LoSavio P, Ibrahim H. Superior laryngeal nerve identification and preservation in thyroidectomy. Arch Otolaryngol Head Neck Surg 2002;128:296-303. [Crossref] [PubMed]
- Selvan B, Babu S, Paul MJ, et al. Mapping the compound muscle action potentials of cricothyroid muscle using electromyography in thyroid operations: a novel method to clinically type the external branch of the superior laryngeal nerve. Ann Surg 2009;250:293-300. [Crossref] [PubMed]
- Bellantone R, Boscherini M, Lombardi CP, et al. Is the identification of the external branch of the superior laryngeal nerve mandatory in thyroid operation? Results of a prospective randomized study. Surgery 2001;130:1055-9. [Crossref] [PubMed]
- Lennquist S, Cahlin C, Smeds S. The superior laryngeal nerve in thyroid surgery. Surgery 1987;102:999-1008. [PubMed]
- Sarkar S, Banerjee S, Sarkar R, et al. A Review on the History of ‘Thyroid Surgery’. Indian J Surg 2016;78:32-6. [Crossref] [PubMed]
- Loré JM Jr, Kokocharov SI, Kaufman S, et al. Thirty-eight-year evaluation of a surgical technique to protect the external branch of the superior laryngeal nerve during thyroidectomy. Ann Otol Rhinol Laryngol 1998;107:1015-22. [Crossref] [PubMed]
- Dumitru D, Zwarts MJ. Focal cranial neuropathies. In: Electrodiagnostic medicine. 2nd edition. Philadelphia, PA, USA: Hanley & Belfus; 2002:653-712.
- Sittel C, Stennert E, Thumfart WF, et al. Prognostic value of laryngeal electromyography in vocal fold paralysis. Arch Otolaryngol Head Neck Surg 2001;127:155-60. [Crossref] [PubMed]
- Halsted WS, Evans HM. I. The Parathyroid Glandules. Their Blood Supply and their Preservation in Operation upon the Thyroid Gland. Ann Surg 1907;46:489-506. [Crossref] [PubMed]
- Delbridge L, Reeve TS, Khadra M, et al. Total thyroidectomy: the technique of capsular dissection. Aust N Z J Surg 1992;62:96-9. [Crossref] [PubMed]
- Sasou S, Nakamura S, Kurihara H. Suspensory ligament of Berry: its relationship to recurrent laryngeal nerve and anatomic examination of 24 autopsies. Head Neck 1998;20:695-8. [Crossref] [PubMed]
- Serpell JW, Grodski S, Yeung M, et al. Hemithyroidectomy: a heuristics perspective. ANZ J Surg 2008;78:1122-7. [Crossref] [PubMed]
- Serpell JW. New operative surgical concept of two fascial layers enveloping the recurrent laryngeal nerve. Ann Surg Oncol 2010;17:1628-36. [Crossref] [PubMed]
- Tan YH, Du GN, Xiao YG, et al. The false thyroid capsule: new findings. J Laryngol Otol 2013;127:897-901. [Crossref] [PubMed]
- Delbridge L. The ‘neglected’ nerve in thyroid surgery: the case for routine identification of the external laryngeal nerve. ANZ J Surg 2001;71:199. [Crossref] [PubMed]
- Marchese-Ragona R, Restivo DA, Mylonakis I, et al. The superior laryngeal nerve injury of a famous soprano, Amelita Galli-Curci. Acta Otorhinolaryngol Ital 2013;33:67-71. [PubMed]
- Potenza AS, Araujo Filho VJF, Cernea CR. Injury of the external branch of the superior laryngeal nerve in thyroid surgery. Gland Surg 2017;6:552-62. [Crossref] [PubMed]
- Coller FA, Boyden AM. The development of technique of thyroidectomy: presentation of method used in university hospital. Surg Gynecol Obstet 1937;65:495.
- Lore JM Jr. An Atlas of Head and Neck Surgery. LWW; 1990.
- Naraynsingh V, Cawich SO, Maharaj R, et al. Retrograde thyroidectomy: a technique for visualization and preservation of the external branch of superior laryngeal nerve. Int J Surg Case Rep 2014;5:122-5. [Crossref] [PubMed]
- Naraynsingh V, Cawich S, Hassranah D, et al. Retrograde Thyroidectomy for preservation of the External Branch of the Superior Laryngeal Nerve: A case series. Int J Surg Case Rep 2018;53:517-21. [Crossref] [PubMed]
- Sung ES, Chang JH, Kim J, et al. Is cricothyroid muscle twitch predictive of the integrity of the EBSLN in Thyroid Surgery? Laryngoscope 2018;128:2654-61. [Crossref] [PubMed]
- Jonas J, Bähr R. Neuromonitoring of the external branch of the superior laryngeal nerve during thyroid surgery. Am J Surg 2000;179:234-6. [Crossref] [PubMed]
- Hwang SB, Lee HY, Kim WY, et al. The anatomy of the external branch of the superior laryngeal nerve in Koreans. Asian J Surg 2013;36:13-9. [Crossref] [PubMed]
- Papachristos AJ, Glover A, Sywak M, et al. Thyroidectomy in Australia 2022: lessons from 21,000 consecutive cases. ANZ J Surg 2022;92:1626-30. [Crossref] [PubMed]
- Cheruiyot I, Kipkorir V, Henry BM, et al. Surgical anatomy of the external branch of the superior laryngeal nerve: a systematic review and meta-analysis. Langenbecks Arch Surg 2018;403:811-23. [Crossref] [PubMed]
- Morton RP, Whitfield P, Al-Ali S. Anatomical and surgical considerations of the external branch of the superior laryngeal nerve: a systematic review. Clin Otolaryngol 2006;31:368-74. [Crossref] [PubMed]


