
Gender-affirming microvascular breast reconstruction
Introduction
Chest surgery in the transgender and gender nonbinary population is one of the most frequently performed gender-affirming surgeries (GAS) performed and continues to increase (1). Transgender women may seek feminizing chest surgery (FCS) in an effort to address gender dysphoria and enhance femininity by bridging their physical appearance with their gender identity. According to the 2015 United States Transgender survey, 51% of transgender women have had or someday would like to undergo FCS (2). FCS has been linked to increased breast satisfaction, psychosocial well-being, and sexual well-being among transgender women (3-5).
While hormonal replacement therapy (HRT) may contribute to overall feminization and aid in some degree of breast development, the effects vary and often are not enough for patients to overcome the dysphoria. Previous studies have demonstrated that despite HRT, up to 70% of transgender women desire surgical breast enhancement (6). It is recommended patients undergo a minimum of 12–24 months of HRT prior to FCS to maximize breast growth and improve the aesthetic result of any future breast procedures (7).
Options for FCS include using fat grafting techniques, implants, or autologous tissue, alone or in combination (8). While autologous tissue reconstruction is routinely offered to cisgender women after mastectomy, there are exceedingly few reports of transgender women undergoing this procedure. As in the cisgender patient population, there are sometimes transgender women who wish to avoid implants or are not ideal candidates for breast enhancement with prosthetics for any number of reasons (9,10). Additionally, due to significant differences in chest anatomy between cisgender and transgender women, implants may not always fulfill an individual’s aesthetic goals. In cases where both the skin envelope and breast volume are severely deficient, breast feminization may truly be considered “breast construction” based on the primary goals.
There are few cases of autologous breast reconstruction in transgender FCS cited in the literature. As surgeons recognize the needs of a growing and heterogeneous transgender patient population seeking breast feminization, the role of autologous reconstruction will likely become clearer in situations where implant-based reconstruction is not ideal. The objective of this study is to provide a comprehensive review of the current literature on microvascular surgical techniques in gender-affirming chest feminization and summarize the senior author’s experience in this emerging field.
Autologous tissue reconstruction in the literature
To our knowledge, there are only two published case reports describing the use of autologous breast reconstruction in chest feminization. Murariu et al. describe a transgender woman who presented with extensive breast fibrosis and inflammation after injecting liquid silicone for years (11). The authors reconstructed her breasts using autologous abdominal tissue, with a free muscle-sparing transverse rectus abdominis musculocutaneous (MS-TRAM) flap on one side and a deep inferior epigastric perforator (DIEP) flap on the other. A second stage was performed 5 months later to revise scars and perform nipple-areola reconstruction. Excellent results were reported at 11-month follow-up.
Majdak-Paredes et al. describe a unique case of Poland syndrome diagnosed in a transgender woman on HRT with significant breast asymmetry (12). The patient elected to undergo two-stage breast reconstruction, starting with tissue expander placement and followed by a free TRAM flap 5 months later. Six months later, she underwent another procedure for scar revision, fat grafting, and areolar tattooing for symmetry. The authors note that the patient sought a “natural” look and felt that this approach was the best of all options considered.
Claes et al., without much detail provided, briefly mention reconstruction of another transgender woman with Poland syndrome. Bilateral DIEP flaps were used for breast reconstruction and a third muscle flap was utilized for thoracic reconstruction, however the clinical outcome was not discussed (8).
With a paucity of literature and virtually no published technique, these are the only examples available outside of our experience. The first study describes free tissue reconstruction as a salvage option due to an unsatisfactory operative site. The two cases of congenital chest deformity provide complex regional anatomy and patient preference as additional indications for autologous breast reconstruction in transgender women.
Cisgender versus transgender breast feminization: anatomy and challenges
Hormone modulation using exogenous female sex hormones (estrogen, sometimes paired with a course of progesterone), combined with orchiectomy or chemical androgen blockade, aids in physiologic feminization of the individual. The resulting breast development is somewhat variable, and only occasionally leads to satisfactory glandular hypertrophy. Thus, HRT alone is usually not adequate to overcome the pre-existing anatomic dimorphism between the sexually mature transgender and cisgender woman.
Body shape and fat distribution
Natal males exposed to testosterone during and after puberty have broader shoulders, a wider sternum, shorter nipple to inframammary fold (IMF) distances, hypertrophied pectoralis major muscles, and smaller nipple-areola complexes (NACs) compared to mature natal females (13). The broader sternums and chests of transgender women offered implant-based breast enhancement may necessitate the need for larger implants to achieve certain aesthetic goals such as cleavage, however, the breast skin envelope can limit the achievable breast volume and shape (14).
Typical fat distribution also differs between cisgender and transgender women. The cisgender female body is more likely to have an ectomorphic or mesomorphic shape, as opposed to the transgender female habitus that is typically endomorphic (15). Natal males tend to carry excess subcutaneous fat along the abdomen and flanks. On the other hand, natal females possess around 10% higher body fat composition than natal males, with the highest concentration in the lower body region (16). Due to these differences, if autologous breast reconstruction is being considered, fat distribution should help guide the decision of flap selection (10).
Breast envelope and pectoralis major musculature
Transgender women have less breast ptosis due to a more limited breast skin envelope compared to cisgender women (15). Some surgeons use a multi-staged approach to breast enhancement, employing tissue expanders to achieve a greater breast envelope before placing implants. Even with this approach, inelastic skin and scars may prevent the desired skin expansion prior to permanent subglandular implant placement. Transgender women also tend to have larger, bulkier pectoralis musculature. Without release of the medial pectoralis major muscle insertion, implants tend to sit wide apart in these patients. Aggressive muscle release, however, contributes to animation deformity (14).
Nipple-areolar complex (NAC)
The shape and position of the NAC is an important component of chest feminization. The NACs of transgender women are smaller and more laterally spaced. In contrast to the round or vertically-stretched elliptical shape of those in cisgender women, the natal male NAC has more of a horizontally-oriented oval shape with less nipple projection (13,14).
Inframammary fold (IMF)
Cisgender women may have a lower IMF with an increased nipple to IMF distance when compared to that of transgender women. This can affect the vertical positioning of the implant, resulting in a more superior placement (14). Surgeons typically lower the IMF, but this sometimes leads to an amorphous, effaced fold with varying degrees of bottoming-out. Revision procedures to reconstruct the IMF may be needed when this occurs.
Congenital and acquired defects of the chest
Congenital deformity and surgical scars are also important to consider when considering the optimal approach to chest feminization. Anatomical deformity, such as that associated with Poland syndrome, pectus excavatum, or pectus carinatum pose challenges to achieving symmetry and an aesthetic breast augmentation. Acquired issues with the breasts and chest are also important to consider, as in the example of a patient with extensive silicone granulomatosis. Mastectomy for breast cancer would be another. Scars from prior sternotomy, thoracotomy or subcostal incisions may render implant-based chest feminization impractical. Patients who suffer failed breast augmentation [due to infection, seroma, recurrent capsular contracture or breast implant-associated anaplastic large cell lymphoma (BIA-ALCL)] also represent a cohort who may seek alternatives to prosthetic breast enhancement.
The case for autologous breast feminization
Although there are often aesthetic advantages to autologous breast reconstruction, the authors would like to emphasize that this should be an option only after excluding the utility of implants due to an individual’s circumstances. Table 1 lists the main advantages of each approach. Table 2 summarizes potential indications for autologous reconstruction.
Table 1
| Advantages of implant-based reconstruction |
| Shorter anesthesia time |
| Lower cost of index surgery |
| Requires a single surgical site |
| Does not require a donor site (less scar) |
| Less invasive, microsurgical skill not required |
| Does not require hospital admission |
| Advantages of autologous reconstruction |
| More natural-appearing breast shape and ptosis |
| Long-term similar or lower cost due to fewer reoperations, no need for staged approach (e.g., tissue expansion) (17-20) |
| Eliminates risk of animation deformity |
| May be able to conceal aspects of transgender female anatomy more than implant-based reconstruction (IMF placement, nipple to IMF distance, laterally placed nipples) |
| Less challenging than implant-based reconstruction in patients with surgical scars or other anatomic deformity |
| Eliminates risk of BIA-ALCL, BII, implant infection, implant rupture, implant migration, capsular contracture |
IMF, inframammary fold; BIA-ALCL, breast implant-associated anaplastic large cell lymphoma; BII, breast implant illness.
Table 2
| Poor skin envelope elasticity despite attempt at expansion |
| Prior chest wall scars or trauma obviating implants |
| Unable to achieve patient goals with implant-based reconstruction |
| Unable to achieve symmetry due to pre-existing anatomic deformity |
| Repeated implant failure, capsular contracture or silicone granulomatosis |
Patient reported outcomes
Breast reconstruction using autologous tissue can provide a more natural-appearing ptotic breast in both cisgender and transgender women. Toyserkani et al. conducted a review using the highly validated Breast-Q survey, comparing patient reported outcomes of cisgender women who underwent implant-based versus autologous breast reconstruction after mastectomy. The authors found that patients who had undergone autologous tissue-based breast reconstruction had greater satisfaction with the surgical outcome, as well as better scores in psychosocial well-being and sexual well-being (21). Additionally, some studies have found no significant difference in overall complication rate between implant-based and autologous reconstruction (22).
Cost
Lemaine et al. evaluated the difference in cost between implant-based versus reconstruction with autologous tissue and found that even though the index operation for implant reconstruction is less expensive, it was associated with higher subsequent healthcare costs. By 2 years, the resulting total cost was similar (17). Other studies and meta-analyses have come to similar conclusions (18-20).
Controversy
The counterpoint will be made that despite the issues of cost and patient satisfaction, it may not be appropriate to compare cisgender women undergoing mastectomy for cancer or prophylaxis and transgender women seeking breast feminization. As a society, we have placed a priority on making a patient whole by giving her breasts back after they are removed, by choice or due to malignancy. While cost consciousness is an ever-growing necessity in this time of scarce medical resources and declining reimbursement, it is important to consider that transgender women probably comprise less than 1% of the overall population and only a small fraction of these patients will be a candidate for, or desire, autologous reconstruction (23).
Technique
While the majority of FCS performed are implant-based, an individualized approach should be taken to optimize the patient’s goals. The senior author has performed two FCS using autologous free tissue reconstruction. Our approach to FCS begins with stating the patient’s goals and evaluating her anatomy. If the overall chest anatomy is favorable (i.e., none of the contraindications are identified) and the breast envelope is adequate for an implant that will achieve the patient’s goals, an implant-based reconstruction is performed. If not, the skin elasticity is evaluated. If there is adequate elasticity present, a multi-staged approach using a tissue expander with subsequent implant placement is performed. If there is poor elasticity or other significant contraindications exist, autologous options are discussed with the patient.
If autologous reconstruction is being considered, potential donor sites are evaluated. Patients with sufficient abdominal soft tissues may be candidates for DIEP or MS-TRAM flaps. Otherwise, some combination of other donor site(s) may be considered, including that of the transverse upper gracilis (TUG), profunda artery perforator (PAP), superior gluteal artery perforator (SGAP), or lumbar artery perforator (LAP) flaps. If none of these are ideal options to achieve the patient’s goals, a latissimus dorsi musculocutaneous flap with an implant may also be considered.
The senior author’s approach to breast reconstruction in two transgender women is described below. In one case, a 53-year-old patient with body mass index (BMI) 35 kg/m2 had a remote history of silicone injection into her breasts, and more than 30 years hence sought a breast reduction. On screening mammography, the breasts were completely filled with silicone granulomas. Faced with the options of keeping her breasts, breast amputation or autologous reconstruction, she opted for the latter. The second patient was a 66-year-old transgender woman with BMI of 30 kg/m2 who wanted natural-appearing ptotic breasts and did not want breast implants. HRT (estradiol, progesterone and an androgen blocker) did not provide adequate breast growth and her broad chest and tight upper pole skin envelope was insufficient to give her the aesthetic result she wanted with implants alone. She also elected to have autologous reconstruction. Both patients had sufficient abdominal soft tissue to achieve the size and shape breasts they desired.
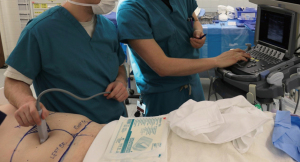
On the day of surgery, color Doppler sonography is used at the bedside to identify dominant deep inferior epigastric perforators. These are marked on the skin (Figure 1).
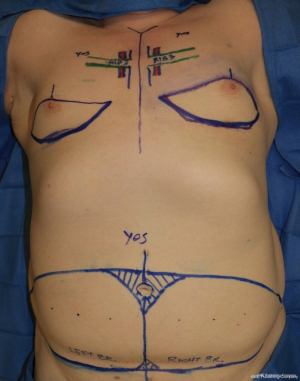
Flap elevation proceeds in a manner similar to that used in conventional cisgender breast reconstruction, though in both cases described, virtually all of the flap skin was used. The flaps were designed to optimize the skin envelope, orienting the flaps and rounding the corners to allow the flap to completely line the neo-IMF (Figure 2). The curvilinear neo-IMF is drawn at or below the native IMF, around the 5th to 6th rib. The skin from this line to just above the NAC is excised to allow for the entire lower pole, and some of the upper pole, to be recreated (Figure 2). In the midline of each breast, a vertical extension of the upper incision may be made to further open up the upper pole to accommodate the upper edge of the flap. The NACs are de-epithelialized and reattached as a skin graft following final flap inset (Figure 3).
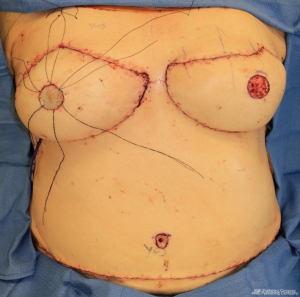
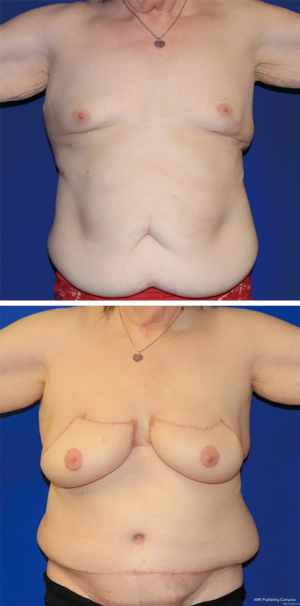
Before and after photos of the second patient with bilateral DIEP flaps is shown in Figure 4. Follow-up was at 2 months post-op. Excellent ptosis and expanded upper poles were achieved. An endomorphic body shape may result in excess skin and fat above the DIEP donor site that can be addressed with a combination of lipectomy and skin excision. The patient was grateful for the natural shape, preservation of her NACs through skin grafting and the removal of her abdominal panniculus. She elected to avoid any further revisions to address truncal donor site contour.
Conclusions
While implant-based breast reconstruction remains the most common approach to FCS and often produces very good results, there are occasional reasons to consider other options. If a patient’s anatomy favors it and overall goals of surgery cannot be achieved with implants alone, autologous tissue reconstruction should be considered. In the transgender population, autologous reconstruction has been used to address severe asymmetry and in salvage situations that prohibit implant-based reconstruction. An individualized approach should be taken when determining what type of reconstruction is best for the patient based on the same considerations in cisgender breast reconstruction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ketan M. Patel and Ara A. Salibian) for the series “Advances in Microsurgical Breast Reconstruction” published in Gland Surgery. The article has undergone external peer review.
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-133/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-133/coif). The series “Advances in Microsurgical Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cuccolo NG, Kang CO, Boskey ER, et al. Epidemiologic Characteristics and Postoperative Complications following Augmentation Mammaplasty: Comparison of Transgender and Cisgender Females. Plast Reconstr Surg Glob Open 2019;7:e2461. [Crossref] [PubMed]
- James SE, Herman JL, Rankin S, et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
- Weigert R, Frison E, Sessiecq Q, et al. Patient satisfaction with breasts and psychosocial, sexual, and physical well-being after breast augmentation in male-to-female transsexuals. Plast Reconstr Surg 2013;132:1421-9. [Crossref] [PubMed]
- Javier C, Crimston CR, Barlow FK. Surgical satisfaction and quality of life outcomes reported by transgender men and women at least one year post gender-affirming surgery: A systematic literature review. Int J Transgend Health 2022;23:255-73. [Crossref] [PubMed]
- Almazan AN, Keuroghlian AS. Association Between Gender-Affirming Surgeries and Mental Health Outcomes. JAMA Surg 2021;156:611-8. [Crossref] [PubMed]
- Wierckx K, Gooren L. T'Sjoen G. Clinical review: Breast development in trans women receiving cross-sex hormones. J Sex Med 2014;11:1240-7. [Crossref] [PubMed]
- Coleman E, Radix AE, Bouman WP, et al. Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. Int J Transgend Health 2022;23:S1-S259. [Crossref] [PubMed]
- Claes KEY, D'Arpa S, Monstrey SJ. Chest Surgery for Transgender and Gender Nonconforming Individuals. Clin Plast Surg 2018;45:369-80. [Crossref] [PubMed]
- Miller TJ, Wilson SC, Massie JP, et al. Breast augmentation in male-to-female transgender patients: Technical considerations and outcomes. JPRAS Open 2019;21:63-74. [Crossref] [PubMed]
- Morrison SD, Massie JP, Kneib CJ, et al. The Use of Autologous Tissue for Chest Feminization in Gender-Affirming Surgery. Plast Reconstr Surg 2020;145:228e-9e. [Crossref] [PubMed]
- Murariu D, Holland MC, Gampper TJ, et al. Illegal silicone injections create unique reconstructive challenges in transgender patients. Plast Reconstr Surg 2015;135:932e-3e. [Crossref] [PubMed]
- Majdak-Paredes EJ, Shafighi M, Fatah F. Unilateral hypoplastic breast in a male-to-female transsexual with Poland syndrome after gender reassignment--reconstructive considerations. J Plast Reconstr Aesthet Surg 2009;62:398-401. [Crossref] [PubMed]
- Bekeny JC, Zolper EG, Manrique OJ, et al. Breast augmentation in the transgender patient: narrative review of current techniques and complications. Ann Transl Med 2021;9:611. [Crossref] [PubMed]
- Morrison SD, Wilson SC, Mosser SW. Breast and Body Contouring for Transgender and Gender Nonconforming Individuals. Clin Plast Surg 2018;45:333-42. [Crossref] [PubMed]
- Nauta AC, Baltrusch KM, Heston AL, et al. Differences in Chest Measurements between the Cis-female and Trans-female Chest Exposed to Estrogen and Its Implications for Breast Augmentation. Plast Reconstr Surg Glob Open 2019;7:e2167. [Crossref] [PubMed]
- Karastergiou K, Smith SR, Greenberg AS, et al. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ 2012;3:13. [Crossref] [PubMed]
- Lemaine V, Schilz SR, Van Houten HK, et al. Autologous Breast Reconstruction versus Implant-Based Reconstruction: How Do Long-Term Costs and Health Care Use Compare? Plast Reconstr Surg 2020;145:303-11. [Crossref] [PubMed]
- Fischer JP, Wes AM, Nelson JA, et al. Propensity-matched, longitudinal outcomes analysis of complications and cost: comparing abdominal free flaps and implant-based breast reconstruction. J Am Coll Surg 2014;219:303-12. [Crossref] [PubMed]
- Sando IC, Momoh AO, Chung KC, et al. The Early Years of Practice: An Assessment of Operative Efficiency and Cost of Free Flap and Implant Breast Reconstruction at an Academic Institution. J Reconstr Microsurg 2016;32:445-54. [Crossref] [PubMed]
- Khajuria A, Prokopenko M, Greenfield M, et al. A Meta-analysis of Clinical, Patient-Reported Outcomes and Cost of DIEP versus Implant-based Breast Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2486. [Crossref] [PubMed]
- Toyserkani NM, Jørgensen MG, Tabatabaeifar S, et al. Autologous versus implant-based breast reconstruction: A systematic review and meta-analysis of Breast-Q patient-reported outcomes. J Plast Reconstr Aesthet Surg 2020;73:278-85. [Crossref] [PubMed]
- Stefura T, Rusinek J, Wątor J, et al. Implant vs. autologous tissue-based breast reconstruction: A systematic review and meta-analysis of the studies comparing surgical approaches in 55,455 patients. J Plast Reconstr Aesthet Surg 2023;77:346-58. [Crossref] [PubMed]
- Meerwijk EL, Sevelius JM. Transgender Population Size in the United States: a Meta-Regression of Population-Based Probability Samples. Am J Public Health 2017;107:e1-8. [Crossref] [PubMed]


