
Catastrophic rhabdomyolysis following breast reconstruction operation using an abdominal flap: a case report
Highlight box
Key findings
• We report a rare case in a patient with postoperative rhabdomyolysis resulting from prolonged breast reconstruction using an abdominal flap.
What is known and what is new?
• Body regions in contact with the operating table during long-lasting surgery are subjected to high pressure when the patient’s position is not changed after anesthesia, substantially increasing the risk of postoperative rhabdomyolysis.
• No other cases of rhabdomyolysis caused by muscle injury in the buttock area following breast reconstruction have been reported, making the current report the first to share relevant information.
What is the implication, and what should change now?
• When performing a long-lasting operation without any position changes, the operator should assess possible pressure areas and understand that severe pressure sores can lead to rhabdomyolysis even with padding.
Introduction
Rhabdomyolysis is a potentially fatal clinical syndrome resulting from the damage or breakdown of skeletal muscle, which can also lead to permanent disabilities. When skeletal muscles are damaged, molecules such as myoglobin and creatinine kinase are released from the damaged tissues into the systemic circulation. Excessive myoglobin in circulation can accumulate in the renal tubules, blocking the passage of fluid and rapidly reducing kidney function, resulting in renal failure. Given that these impairments can lead to severe electrolyte imbalances (e.g., hyperkalemia) and hypovolemic shock, the fatal potential of rhabdomyolysis is a serious concern.
Various causes of rhabdomyolysis have been identified, the most common of which is muscle damage due to external trauma, medications, toxins, or infections (1,2). Compartment syndrome due to excessive pressure in the muscles, prolonged immobilization, or muscle compression due to improper patient positioning during prolonged surgery is also a common cause of rhabdomyolysis. Improper positioning during long-lasting surgery can cause a large amount of body weight to be focused on a specific region over a long period, leading to severe muscle damage. Studies published by researchers from various fields, including bariatric surgery, cardiac surgery, and neurosurgery, have demonstrated that body regions in contact with the operating table during long-lasting surgery are subjected to high pressure when the patient’s position does not change after anesthesia, substantially increasing the risk of postoperative rhabdomyolysis (3-5).
Prolonged surgical duration is a concern among patients undergoing breast reconstruction with an abdominal flap when microsurgery is required. In this report, we discuss a case of postoperative rhabdomyolysis resulting from prolonged breast reconstruction using an abdominal flap performed immediately after a skin-sparing mastectomy for breast cancer. We present this case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-8/rc).
Case presentation
Patient and procedure
A 57-year-old Asian patient with left breast cancer was referred to the Plastic Surgery Department of our institution for immediate breast reconstruction after a skin-sparing mastectomy with sentinel lymph node biopsy. Patient’s preoperative height and weight were 1.72 m and 82.3 kg, respectively, which were used to calculate a body mass index (BMI) of 29.41 kg/m2. Preoperative systolic blood pressure was approximately 124 mmHg, and preoperative laboratory values were normal [hemoglobin (Hb) =13.5 g/dL, hematocrit (Hct) =39.2%, mean corpuscular volume (MCV) =93.9 fL, red blood cell count (RBC) =4.18 mil/mm3, aspartate aminotransferase (AST) =26 U/L, alanine aminotransferase (ALT) =26 U/L, blood urea nitrogen (BUN) =12.4 mg/dL, serum creatinine (Cr) 0.62 mg/dL].
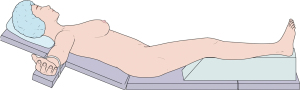
The patient underwent skin-sparing mastectomy with sentinel lymph node biopsy and immediate breast reconstruction using a free deep inferior epigastric artery perforator (DIEP) flap anastomosed to the internal mammary vessels. The procedure was performed under general orotracheal anesthesia with the patient placed in the supine position on a TRUMPF MARS operating table. The operating table had a thick cushion and an additional layer of sponge topped with an operating table cover for cushioning. In preparation for the planned DIEP flap surgery, the patient was placed with the knees in slight flexion using a brown cast. To optimize weight loading on the buttocks, the patient’s upper body was raised by 30°–45° prior to starting the procedure (Figure 1). The total procedure time was 18 h. The surgery exceeded the estimated time because, after anastomosis, severe congestion was observed in the flap due to an insufficiently ligated recipient vein. The surgical team eventually faced unexpected intraoperative challenges due to weak drainage into the vein and re-performed the reconstruction using a contralateral pedicled transverse rectus abdominis myocutaneous (TRAM). The patient remained hemodynamically stable throughout the procedure.
Postoperative status and course
The patient exhibited a high average pulse rate of 110–130 bpm 24 h after surgery. Systolic blood pressure ranged from 133 to 182 mmHg, whereas diastolic blood pressure ranged from 71 to 92 mmHg. Postoperative laboratory tests showed no significant abnormalities: BUN, 12.8 mg/dL; serum creatine kinase 0.62 mg/dL; modification of diet in renal disease (MDRD) eGFR 116 mL/min. The patient exhibited symptoms of delirium during the night following the surgery, intermittently displaying vigorous movements on the bed. After consulting a psychiatrist, the patient was prescribed haloperidol and closely monitored. The patient experienced mild fever four times during the first 24 h after surgery, which resolved each time after the intravenous injection of propacetamol. In a subsequent examination, the patient exhibited satisfactory wound conditions without signs of infection or abnormal symptoms, in addition to a short-lasting fever.
The patient experienced tolerable pain at the surgical site. Although she did not have strong complaints of pain or discomfort at the pressure points that had been in contact with the operating table during the procedure, the reports of her subjective experience were considered with caution, given the presence of severe delirium in the early postoperative period. Considering the possibility of abdominal tension at the donor site, we instructed the patient to maintain knee flexion and engage in absolute bed rest. Despite taking medications, the patient experienced even more severe delirium beginning on postoperative 2 day (POD 2), following which she received two doses of 1 mg risperidone and was placed in a body brace for safety. However, the patient’s condition did not improve; the patient was consulted the Department of Psychiatry for postoperative delirium care.
On POD 3, the patient developed sudden shock, with systolic blood pressure ranging from 70 to 80 mmHg, diastolic blood pressure ranging from 50 to 60 mmHg, and an increased heart rate ranging from 104 to 117 bpm. The patient was deemed to have experienced hypovolemic shock and was admitted to the intensive care unit (ICU) accordingly. A complete laboratory workup was performed, including analyses of complete blood count, serum electrolytes, blood urea nitrogen, serum creatinine, blood creatine kinase, urine myoglobin, and urinalysis. The following results were obtained: urine myoglobin, 11,135.4 mcg/L; creatine kinase >7,800 U/L; serum lactate dehydrogenase (LDH) >4,500 U/L; BUN, 41.9 mg/dL; serum creatinine, 1.33 mg/dL; sodium, 129 mmol/L; potassium >10 mmol/L; AST, 681 U/L; ALT, 409 U/L; Hb 9.0 g/dL; Hct, 26.5% (Figure 2). On the basis of these laboratory results, the patient was diagnosed with rhabdomyolysis.
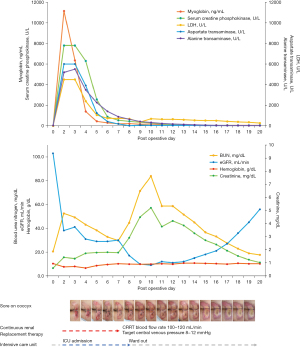
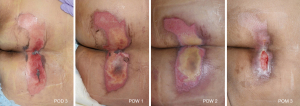
The patient developed ischemia which led to shock on POD 3, and was diagnosed with metabolic acidosis secondary to rhabdomyolysis. After consultation with the Department of Nephrology in the ICU, the patient was managed with continuous renal replacement therapy (CRRT) for 5 days in accordance with the latest guidelines (6,7). CRRT was performed at a flow rate of 100–120 mL/min and target central venous pressure (CVP) of 8–12 mmHg. Myoglobin, LDH, AST, and ALT levels improved over the course of the CRRT. We suspected a release of muscle enzymes from the site of the direct incision and dissection of the rectus muscle due to the use of the pedicled TRAM flap. However, removing the rectus muscle, from which the pedicle arose would have defeated the purpose of the procedure. Furthermore, the area of the reconstructed breast most distal to the pedicled TRAM flap region exhibited satisfactory blenching, bleeding, and warmth. Therefore, we continued our efforts to identify the cause of the rhabdomyolysis by monitoring the patient. Although she had difficulty describing her symptoms due to delirium, we noticed the patient’s hand repeatedly reaching out to her buttocks and confirmed stage II pressure sores on the left coccyx and heel as well as wide and deep pressure sores on the regions of both buttocks that bore the greatest weight in the operating position. The patient was then treated with a foam dressing and tangential debridement followed by continuous wound management; complete healing was eventually observed (Figure 3). We believe that rhabdomyolysis resulted from prolonged pressure on the large gluteus maximus muscle located below the site of the pressure sore.
The patient’s renal function began to normalize, and her delirium began to improve 72 h after the initiation of CCRT. Serial labs were drawn every few days. CRRT was discontinued on POD 8, and the patient was discharged on POD 9. Her BUN, creatinine, sodium, and potassium levels were stable on POD 13 (BUN/Cr, 44.9/3.26), with her BUN/Cr reaching 22.8/1.51 POD 17. The patient exhibited continued clinical improvement and was discharged 20 days postoperatively without further complications. In post operative month (POM) 1, her blood creatinine and eGFR level became fully normalized (Cr 0.65 mg/dL, eGFR 94 mL/min).
A follow-up outpatient assessment was performed using POM 1, which confirmed satisfactory general and wound conditions (Figure 4).

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent for publication and accompanying images was obtained from the patient. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
During rhabdomyolysis, various intracellular muscle components, such as myoglobin, creatine kinase, and LDH, are released from the damaged muscle into the bloodstream and extracellular fluid and eventually into the circulatory system. This can result in disturbances in electrolyte balance, metabolic acidosis, hyperthermia, hypovolemic shock, disseminated intravascular coagulation, and acute kidney injury (8). Renal failure is a common complication of rhabdomyolysis and is reported in 13–50% of patients with rhabdomyolysis (9,10). Rhabdomyolysis accompanied by acute kidney injury can be fatal, with a mortality rate of 20% (11,12). Therefore, early diagnosis, management, treatment, and strategies for preventing rhabdomyolysis are essential.
Since it is difficult to suspect rhabdomyolysis in patients with good general condition immediately after surgery, as in the present case, the need to assess creatine kinase may not be immediately evident. As the patient exhibited delirium and was actively ambulated after surgery, postoperative rhabdomyolysis was not immediately suspected. On POD 3, the patient was admitted to the ICU due to hypovolemic shock, resulting in unmanaged systolic blood pressure (BP). Laboratory tests revealed serum myoglobin levels peaking above 11,000 ng/mL and creatine kinase levels peaking above 7,800 U/L. These levels remained above the upper limit of normal (>1,000 mg/dL) until POD 5. The BUN level peaked above 4,000 mg/dL on POD 3. Although it continued to decrease until POD 7, the measurements were not necessarily accurate as they were obtained during CRRT. The BUN level began to increase again after POD 7, peaking at 6,500 mg/dL on POD 10, which was a more accurate reflection of the patient’s BUN level. CRRT was discontinued based on a mutual decision in consultation with the Department of Cardiology after observing improvements in renal function parameters such as urine output. Although hepatic injury was suspected based on AST and ALT levels above 5,000 U/L on POD 3, no unusual findings other than benign liver cysts on CT were observed, confirming that the increases in AST and ALT levels were related to the release of muscle enzymes due to rhabdomyolysis. The patient’s AST and ALT levels gradually normalized after clearance of muscle enzymes via CRRT.
One of the most important goals of rhabdomyolysis treatment is to avoid acute kidney injury, for which proper intravenous fluid resuscitation is essential (13,14). Since hypovolemia resulting from the accumulation of fluid in muscular compartments can occur in the event of rhabdomyolysis, our patient was subjected to massive fluid resuscitation with CRRT in the ICU. Following treatment, the patient’s blood pressure and laboratory results were rapidly improved.
Although our patient had undergone a prolonged operation that lasted 18 h, our attention was primarily placed on the patient’s wound and general condition immediately after surgery. Prior to this case, we did not observe severe pressure sores, even in patients who had undergone longer operations. We reviewed other case reports and collected references on POD 3 during our ICU care of the patient. While there have been case reports of rhabdomyolysis following DIEP breast reconstruction, the present patient reported pain in the leg, which allowed us to localize the site of rhabdomyolysis (15,16). One reason we did not observe severe pressure sores prior to the present case is that we operated on patients in the supine position, meaning that pressure was distributed throughout the region in contact with the operating table rather than focusing on a specific region, preventing ischemic injury. However, the patient underwent breast reconstruction in the sitting position to ensure a symmetrical and natural breast shape resembling its original state. Additionally, a brown splint was placed underneath both legs to keep the hip and knees flexed to ensure donor-site closure when using an abdominal-based flap. We hypothesize that this positioning was the root cause of the rhabdomyolysis in this patient.
Based on these results, our study shows that it is essential to identify the patient’s risk factors for rhabdomyolysis, monitor vital signs and serum biochemistry after a prolonged operation, and palpate or visually assess regions other than the surgical site. The known risk factors for rhabdomyolysis include prolonged surgery (greater than 7 h) without adequate repositioning, BMI greater than 40 kg/m2, and accompanying diseases such as diabetes, hypertension, and peripheral vascular disease (17-19). The patient did not have any risk factors for rhabdomyolysis, as she had a BMI of 29.41 kg/m2 and no underlying diseases. Intraoperative records from the Department of Anesthesiology revealed that the patient’s blood pressure was monitored through the atrial line and that she did not become hypotensive. Phenylephrine, a vasopressor, was maintained at a standard dose. Despite confirming brain hypoxia using a cerebral oximeter, the patient’s cerebral perfusion and oxygen saturation levels remained normal. Moreover, during the prolonged operation, she did not exhibit signs of hypovolemia, such as hypotension or tachycardia. Based on this information, the only possible root cause of rhabdomyolysis was the lack of positional changes during prolonged surgery. The current case emphasizes that when performing a long-lasting operation without any position changes, the operator should assess possible pressure areas and understand that severe pressure sores can lead to rhabdomyolysis even if padding is added to the operating table. Additionally, multidisciplinary collaboration is necessary to ensure an optimal treatment protocol and personalized treatment in accordance with the latest guidelines (20). In the present case, a breast surgeon was responsible for diagnosing shock and providing ICU care. Acute renal injury related to rhabdomyolysis was assessed in collaboration with the Department of Cardiology. This collaborative effort enabled the early initiation of CRRT to filter out muscle enzymes, thereby significantly improving the patient’s condition.
Through multidisciplinary care, the medical staff consistently explained the treatment progress to the patient and her guardians, providing constant reassurance and maintaining a high rapport with the patient. Based on our review of studies on rhabdomyolysis after prolonged surgeries, no other cases of rhabdomyolysis caused by muscle injury in the buttock area following breast reconstruction have been reported. This makes the current report the first to share information related to patient conditions and treatment progress in such cases.
Conclusions
Given the possibility of prolonged procedure time in patients undergoing breast reconstruction, the current case emphasizes the need to identify each patient’s risk factors for rhabdomyolysis and to prepare for possible rhabdomyolysis in advance. Consideration should be given to changing the patient’s position or placing a sufficient layer of padding on the operating table to distribute pressure points and prevent ischemic injuries to large muscles. In addition, appropriate fluid control should be ensured to reduce the risk of complications such as hypovolemic shock.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-8/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-8/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent for publication and accompanying images was obtained from the patient. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature. Neuromuscul Disord 2014;24:651-9. [Crossref] [PubMed]
- Kim YH, Choi JH, Kim J, et al. Fasciotomy in compartment syndrome from snakebite. Arch Plast Surg 2019;46:69-74. [Crossref] [PubMed]
- Bueno BT, Gencarelli P Jr, Nasra MH, et al. Postoperative Rhabdomyolysis in the Bilateral Shoulder Areas After Cardiac Surgery. Cureus 2021;13:e18522. [Crossref] [PubMed]
- Chakravartty S, Sarma DR, Patel AG. Rhabdomyolysis in bariatric surgery: a systematic review. Obes Surg 2013;23:1333-40. [Crossref] [PubMed]
- De Tommasi C, Cusimano MD. Rhabdomyolysis after neurosurgery: a review and a framework for prevention. Neurosurg Rev 2013;36:195-202; discussion 203. [Crossref] [PubMed]
- Tandukar S, Palevsky PM. Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest 2019;155:626-38. [Crossref] [PubMed]
- Shahbazov R, Fox M, Alejo JL, et al. A case of rhabdomyolysis after kidney transplantation successfully managed with intensive continuous dialysis. J Surg Case Rep 2018;2018:rjy078. [Crossref] [PubMed]
- Slater MS, Mullins RJ. Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: a review. J Am Coll Surg 1998;186:693-716. [Crossref] [PubMed]
- Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med 2010;48:749-56. [Crossref] [PubMed]
- Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care 2014;18:224. [Crossref] [PubMed]
- Merino I, Borrat X, Balust J, et al. Rhabdomyolysis after bariatric surgery: a potentially fatal complication. Br J Anaesth 2009;102:283-4. [Crossref] [PubMed]
- Better OS, Abassi ZA. Early fluid resuscitation in patients with rhabdomyolysis. Nat Rev Nephrol 2011;7:416-22. [Crossref] [PubMed]
- Wichova H, Subbarayan R, Muelleman T, et al. Rhabdomyolysis in a Morbidly Obese Patient After Oral Cavity Free Flap Reconstruction. Indian J Otolaryngol Head Neck Surg 2019;71:752-4. [Crossref] [PubMed]
- Glassman DT, Merriam WG, Trabulsi EJ, et al. Rhabdomyolysis after laparoscopic nephrectomy. JSLS 2007;11:432-7. [PubMed]
- Reid AW, Malata CM. Postoperative rhabdomyolysis in deep inferior epigastric perforator flap breast reconstruction. Breast Reconstruction 2016;1389-96.
- Segaran A, Reid AW, Malata CM. Post-operative rhabdomyolysis in a bilateral immediate DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:1297-9. [Crossref] [PubMed]
- Lagandré S, Arnalsteen L, Vallet B, et al. Predictive factors for rhabdomyolysis after bariatric surgery. Obes Surg 2006;16:1365-70. [Crossref] [PubMed]
- El-Abdellati E, Eyselbergs M, Sirimsi H, et al. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: acute kidney injury. Ann Intensive Care 2013;3:8. [Crossref] [PubMed]
- Gelpi-Hammerschmidt F, Tinay I, Allard CB, et al. The Contemporary Incidence and Sequelae of Rhabdomyolysis Following Extirpative Renal Surgery: A Population Based Analysis. J Urol 2016;195:399-405. [Crossref] [PubMed]
- Splendiani G, Mazzarella V, Cipriani S, et al. Dialytic treatment of rhabdomyolysis-induced acute renal failure: our experience. Ren Fail 2001;23:183-91. [Crossref] [PubMed]


