
Application of fluorescence endoscopy with methylene blue dye and indocyanine green dual-tracer method in sentinel lymph node biopsy for women with breast cancer
Highlight box
Key findings
• The fluorescence endoscopy method assisted with dual tracers facilitates sentinel lymph node detection with a comparatively short procedure duration and low complication rate.
What is known and what is new?
• Indocyanine green allows for the real-time visualization of lymphatic drainage and provides favorable performance for sentinel lymph node mapping in the traditional open approach of sentinel lymph node biopsy for breast cancer.
• Fluorescence endoscopy with the dual-tracer method using indocyanine green and methylene blue provides a subcutaneous and in vivo view of lymphatic vessels, which makes sentinel lymph node detection easier, especially for overweight or obese patients. This minimally invasive procedure is completed with 3 minor incisions, which avoids obvious incisions in the axilla while achieving better cosmetic results.
What is the implication, and what should change now?
• More high-quality, large-scale randomized controlled clinical trials and long-term follow-up are needed to assess the oncological outcomes and confirm our findings.
Introduction
According to the latest global cancer data published by the International Agency for Research on Cancer, 2.3 million new cases of breast cancer occurred in 2020 (1). Breast cancer is the most frequently diagnosed malignancy and the leading cause of cancer-related death for women worldwide. Currently, sentinel lymph node biopsy (SLNB) has become a standard procedure for evaluating metastasis to lymph nodes in patients with early-stage breast cancer with clinically nonsuspicious nodes (2,3). Blue dye and radioactive colloids are recommended for the sentinel lymph node (SLN) mapping of breast cancer. However, radioactive colloids have not been widely used in China due to radioactive contamination and complicated restrictions on radioactive materials (4).
Indocyanine green (ICG) is radiation-free and allows for the real-time visualization of lymphatic drainage. ICG in combination with methylene blue dye (MBD) for open SLNB has been proven to be safe and feasible (5) and thus may offer an alternative means to avoiding the issues of the radioisotope and dye methods. Recently, ICG-assisted fluorescence endoscopy, which integrates fluorescence imaging and minimally invasive endoscopy, has been increasingly applied in clinical work. Several studies have demonstrated that fluorescence endoscopy contributes to the detection of lymph nodes (6-8).
We therefore sought to further apply fluorescence endoscopy in SLNB for women with breast cancer. In this study, we retrospectively analyzed 117 cases of SLNB using fluorescence endoscopy. The SLN detection results and surgical outcomes were analyzed to evaluate the safety and accuracy of fluorescence endoscopy assisted with MBD and ICG in SLNB. We present this article in accordance with the TREND reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-469/rc).
Methods
Ethical approval of the study protocol
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhujiang Hospital of Southern Medical University (No. 2021-JS-001-01), and individual consent for this retrospective analysis was waived. The available treatment methods and risks of each surgical procedure were explained to all patients, all of whom provided written informed consent for surgery.
Inclusion and exclusion criteria
The inclusion criteria were the following: (I) a confirmed diagnosis of breast cancer by histology (preoperative needle-core biopsy or resection) and (II) no suspicious signs of metastasis to the axillary lymph node (ALN) found upon physical examination and imaging.
The exclusion criteria were the following: (I) a history of surgery or radiotherapy to the axillary area; (II) distant metastasis; (III) allergy to iodine agents or ICG; (IV) with inflammatory breast carcinomas; (V) patients unsuited for surgery or who refused surgery; and (VI) pregnant and lactating women.
Study cohort
Breast cancer patients who underwent surgical treatment including SLNB in our department between November 2020 and September 2021 were selected as the study participants. A total of 117 patients were finally included for analysis according to the abovementioned inclusion and exclusion criteria. Preoperatively, evaluation of tumor and ALN status were conducted through physical examination, mammography, ultrasound, and chest computed tomography. Assessment of tumor growth included the evaluations of tumor type, size, and distant metastasis. ALN status included an assessment of the location, number, size, and shape of the ALNs. Both the breast and axilla were observed with ultrasound and computed tomography (CT). Patients scheduled for breast-conserving surgery underwent magnetic resonance imaging (MRI) to further assess the condition of the breast and ALNs. All surgical procedures were performed by the same team of surgeons, and a uniform method was completed across all patients.
Surgical procedures
MBD injection
After the induction of anesthesia, 2 mL of MBD (20 mg; Jumpcan Pharmaceutical Group, Taixing, China) was administered (i.d.) into the periareolar region in 4 quadrants of the breast. Light massage of the breast for 2 to 3 min was undertaken to facilitate MBD mobility in lymphatic vessels.
SLN localization with ICG
Upon the initiation of surgery, 2 mL of ICG (2.5 mg/mL; i.e., an ICG vial of 25 mg diluted with 10 mL of sterile water; Eisai Co., Ltd., Tokyo, Japan) was injected (i.d.) into the areola of the affected side at multiple points (Figure 1). Subsequently, real-time observations of lymphatic drainage from the breast were undertaken under the “standard” or “pseudo-color” fluorescence modes of a high-definition fluorescence endoscope (OPTO-2100; OptoMedic Technologies, Foshan, China). The location of each SLN was marked on the body surface 2 cm from the terminal fluorescent lymphatic vessels in the axillary direction (Figure 2).

High-definition fluorescence endoscopy system
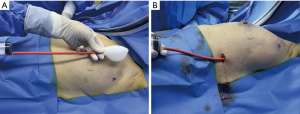
To establish the area of surgical exploration and operating space, a self-made balloon was applied to a mark on the body surface (Figure 3A,3B). Three trocars and endoscopic instruments were then placed at the corresponding positions.
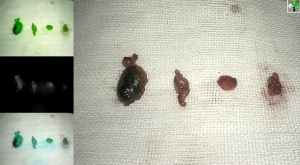
Upon the placement of the endoscope, blue-stained lymphatic vessels and lymph nodes were searched along the lateral border of the pectoralis major in standard white-light mode. The switch to fluorescence mode revealed the visualized lymphatic vessels and lymph nodes emitting a strong green fluorescence, which enabled them to be distinguished from surrounding tissues (Figure 4). The fluorescent or blue-stained lymphatic vessels were separated with an ultrasonic scalpel, and the stained nodes that these lymphatic vessels flowed into were removed and frozen rapidly for subsequent pathology (Figure 5).

Histopathology of lymph nodes
Intraoperative examination of frozen sections was conducted to rapidly diagnose the metastasis to SLNs. Patients with negative SLNs were exempted from ALN dissection; otherwise, ALN dissection was required. For patients who met the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial or Axillary Radiation Therapy in Treating Women With Invasive Breast Cancer (AMAROS) trial inclusion criteria, ALN dissection is not considered necessary if only 1 or 2 positive SLNs are present, while radiotherapy should be included as part of postoperative treatment. All lymph nodes removed during surgery underwent a paraffin pathology examination.
Treatment and follow-up
Postoperatively, all patients received standard treatment (chemotherapy, radiotherapy, and endocrine therapy) according to the National Comprehensive Cancer Network guidelines. Surgery-related complications, such as upper limb lymphedema, paresthesia, reduced arm mobility, and wound infections, were observed and recorded during follow-up. The follow-up was based on outpatient visits every 3 months, supplemented by monthly telephone visits. During the telephone visit, we asked and recorded whether patients experienced pain, numbness, or discomfort in their upper limbs, and whether they felt asymmetry or swelling in either upper limb to inform the assessment of the patient at the next outpatient follow-up visit. Every 3 months after surgery, each patient underwent outpatient tests for sensation and mobility in the arm, measurements of the circumference of the upper arm, and searches for infections and axillary effusions in the surgical incisions. A flexible tape measure was used for upper limb circumferences. Measurements were taken at the palm, wrist, and elbow, and every 4 cm from the wrist to the axilla. Lymphedema was defined as a difference of more than 2 cm in circumference between the 2 arms at any measurement point. The follow-up duration was a minimum of 6 months.
Statistical analysis
The primary endpoint of this study was the detection of SLNs with fluorescence endoscopy with the MBD and ICG dual tracer, including the identification rate and the number of SLNs identified per patient. The secondary endpoints were surgical outcomes, including the time to reach the first the SLN and total procedure duration, and postoperative morbidity including upper limb lymphedema, paresthesia, reduced arm mobility, and wound infection. The processing and analysis of data were conducted using SPSS 26.0 (IBM, Armonk, NY, USA). Measurement data are expressed as the mean ± standard deviation (SD) or median (P25, P75), and were analyzed with the Student t-test. Enumeration data are expressed as percentages and were analyzed using the chi-squared test or Fisher exact test. P<0.05 was considered significant.
Results
Patient characteristics
From November 2020 to September 2021, 117 patients admitted to the Department of Breast Surgery within Zhujiang Hospital were enrolled based on the inclusion and exclusion criteria. Of these, 59 were in clinical stage I, 47 were in stage II, and 11 were unable to be accurately assessed in terms of tumor size due to previous tumor resection. These patients ranged in age from 27 to 78 years, with 49 younger than 50 years and 68 older than 50 years. The lowest and highest body mass index (BMI) in our study were 16.8 and 32.8 kg/m2, respectively. Of the participants, 67 had a normal BMI, while 3 were underweight and 47 were overweight. The clinical and pathological characteristics of patients are presented in Table 1.
Table 1
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| <50 | 49 (41.88) |
| ≥50 | 68 (58.12) |
| BMI (kg/m2) | |
| ≤18.5 | 3 (2.56) |
| 18.6–23.9 | 67 (57.27) |
| ≥24.0 | 47 (40.17) |
| T stage | |
| Tis | 12 (10.26) |
| T1 | 47 (40.17) |
| T2 | 43 (36.75) |
| T3 | 4 (3.42) |
| Tx | 11 (9.40) |
| Tumor location | |
| Outer upper quadrant | 46 (39.32) |
| Outer lower quadrant | 20 (17.09) |
| Inner upper quadrant | 28 (23.93) |
| Inner lower quadrant | 11 (9.40) |
| Nipple or areolar region | 12 (10.26) |
| Histology type | |
| In situ | 14 (11.96) |
| Invasive carcinoma | |
| No special type | 93 (79.49) |
| Specified carcinoma | 10 (8.55) |
| ER | |
| Positive | 79 (67.52) |
| Negative | 38 (32.48) |
| PR | |
| Positive | 58 (49.57) |
| Negative | 59 (50.43) |
| HER-2 | |
| Positive | 42 (35.90) |
| Negative | 75 (64.10) |
| Ki-67 (%) | |
| ≤15 | 22 (18.80) |
| 15–30 | 31 (26.50) |
| ≥30 | 64 (54.70) |
BMI, body mass index; ER, estrogen receptor; PR, progesterone receptors; HER-2, human epidermal growth factor receptor 2.
SLN detection and surgical outcomes
Among the 117 patients who underwent endoscopic SLNB using ICG combined with MBD, ≥1 fluorescent or blue-stained lymph node was detected in 116 patients (99.15%). Among them, blue-stained SLNs were found in 99 patients (84.62%), and fluorescent SLNs were detected in 112 patients (95.73%) during the surgery. A total of 34 patients (29.06%) had positive SLNs. All positive SLNs in these 34 patients were either blue-stained and/or fluorescent. In 6 cases (5.13%), the positive SLNs were only stained with ICG fluorescence but not with blue dye. Meanwhile, in 1 case (0.85%), the positive SLNs were only blue-stained without fluorescence staining. Of the 34 patients with positive SLNs, 31 underwent ALN dissection, and only 1 positive SLN was found in the remaining 3 patients who underwent breast-conserving surgery and expected postoperative radiotherapy. A total of 599 SLNs were resected, with an average of 5.12±1.87 per patient.
Adverse reactions and complications related to tracing agents (e.g., allergy and skin necrosis) did not occur in any patient. The mean duration between endoscope placement and initial identification of an SLN was 7.14±6.31 min. From skin incision to SLN resection, the mean duration of the endoscopic SLNB was 37.75±16.94 min (Table 2). The mean duration of endoscopic SLNB refers to the time between the skin incision and the removal of SLNs from the body, which includes the entire surgical process of SLNB.
Table 2
| Measure | n/N (%) or mean ± SD |
|---|---|
| Identification rate | 116 of 117 (99.15) |
| Number of SLNs identified per patient | 5.12±1.87 |
| Time to reach the first SLN (min) | 7.14±6.31 |
| Total time for SLNB (min) | 37.75±16.94 |
SLN, sentinel lymph node; SD, standard deviation; SLNB, sentinel lymph node biopsy.
Follow-up and complications
Patients were followed up for 6 to 14 months. The median duration of follow-up was 10 months. Of the 117 patients, 5 (4.27%) experienced upper-limb lymphoedema during the follow-up after surgery, all of whom underwent ALN dissection. Their condition improved gradually after administration of therapeutic exercises and medications, as the swelling appeared to decrease. In addition, 3 patients experienced from pain and discomfort in the upper limbs. In other patients, complications such as abnormal swelling, limitation of limb movement, or wound infection were not observed during the follow-up period. By the end of follow-up, tumor recurrence to lymph nodes or distant metastasis had not occurred in any patient.
Discussion
In this study, 117 patients underwent endoscopic SLNB using ICG combined with MBD. Biopsied SLNs were identified in 116 patients (99.15%), with an average of 5.12±1.87 per patient. Blue-stained SLNs were found in 99 patients (84.62%), and fluorescent SLNs in 112 patients (95.73%). In 6 cases (5.13%), the positive SLNs were only stained with ICG fluorescence, while in 1 case (0.85%) they were only blue-stained without fluorescence staining. The mean time taken for identification of the first SLN was 7.14±6.31 min, while that for endoscopic SLNB was 37.75±16.94 min. Five cases (4.27%) of upper-limb lymphoedema were observed during follow-up.
ICG is a newly emerging near-infrared fluorescent dye approved by the US Food and Drug Administration for clinical applications. After administration, ICG binds tightly to plasma proteins, which can become excited and emit fluorescence under irradiation of near-infrared light at ~820 nm (9). This fluorescence signal can be captured by a special device equipped with an imaging system. This system enables the visualization of the shape of lymphatic vessels and the flow toward lymph nodes, which is helpful for the detection and dissection of malignant nodes. This method is being increasingly employed in minimally invasive surgery for melanoma and ovarian, cervical, and gastrointestinal cancers (10-13).
However, the fluorescence-tracing method in the traditional open approach of SLNB for breast cancer has not achieved the desired results. In 2005, Kitai et al. (14) proposed a novel method using fluorescence imaging based on ICG to detect SLNs of women with breast cancer. In this study, SLNs were identified in 17 of 18 cases (94.4%). Subsequently, Murawa et al. (15) reported a prevalence of SLN identification of 96.7% (29/30) with a false-negative prevalence of 9.5% (2/21) in 30 patients with breast cancer using ICG as a tracer. Moreover, in a study by Guo et al., 200 patients underwent SLNB with a combination of ICG and MBD (16), and fluorescent flow was not visible through the skin in 16 of these cases.
The limited tissue-penetration ability of near-infrared fluorescence might explain the failure of biopsies. In traditional open surgery, superficial lymphatic vessels may be visible in most cases if detected from the body surface. However, in patients with a high BMI, lymphatic vessels and nodes may be difficult to observe because of the barrier of thick subcutaneous adipose tissue. This situation may lead to the nonidentification of deep-seated SLNs, which would affect the accuracy of detection and cause false-negative results.
Here, we describe the technical details of fluorescence endoscopy with a dual-tracer method using ICG and MBD in SLNB for breast cancer and discuss the feasibility and further applications of this method in minimally invasive surgery.
The fluorescence endoscopy system we used is equipped with 4 imaging modes: standard white light, standard fluorescence, pseudo-color fluorescence, and multi-display. These modes can be switched to conveniently by pressing the camera button to meet the demands of different surgical conditions. After ICG administration, real-time visualization of lymphatic drainage was displayed in the standard fluorescence mode. In the standard white-light mode, blue-stained lymphatic vessels and lymph nodes could be observed directly under the high-definition field of view. In the fluorescence mode, lymphatic vessels and nodes emitting strong green fluorescence appeared stark contrast to the surrounding tissues, which was conducive to the rapid identification of SLNs. Employment of fluorescence endoscopy to examine lymph nodes within loose axillary tissue solves the problem of inaccurate location of SLNs or the inability to detect SLNs directly due to the fluorescence attenuation caused by the barrier of skin and subcutaneous tissue during open surgery. More importantly, this method simplifies the process of searching for SLNs and reduces the probability of omission. We found that the tracing method using ICG could be carried out even in patients with a high BMI if the SLNB was performed under endoscopy.
According to previous reports (17,18), ICG provides favorable performance for SLN mapping. As shown in Figure 2, this technique is particularly useful in visualizing lymphatic flow to the axilla through the skin and helps to estimate the possible SLN location prior to skin incision. In addition, fluorescence endoscopy provides a subcutaneous or in vivo view of lymphatic vessels, which makes SLN detection easier, especially for overweight or obese patients. Real-time observations of lymphatic drainage from the breast are performed prior to surgery initiation to predict the location of the SLN, as previously reported (19). Then, based on the mark on the body surface, endoscopic surgery is performed to subcutaneously explore the lymphatic vessels and remove the SLNs. On the basis of endoscopy techniques, we use fluorescence endoscopy for SLNB, aiming to complete SLNB and nipple-sparing mastectomy (NSM) through 3 minor incisions. This minimally invasive procedure avoids additional incisions in the axilla and breast while achieving excellent cosmetic results.
None of the 117 patients suffered anaphylaxis or adverse reactions related to the tracer agent, which suggested that ICG could be applied safely for lymphatic tracing in the SLNB of breast cancer. Fluorescent or blue-stained SLNs were acquired in 116 cases (99.15%), which is equivalent to the standard method used for radioisotopes and dyes reported previously (20) but slightly higher than that of navigation using ICG fluorescence in open surgery (21-23). SLNs were not identified by our dual-tracer method in 1 case. The tumor in this patient was located in the upper outer quadrant of the right breast. Prior to breast cancer surgery, an excisional biopsy of the tumor was performed through an areolar incision. The lymphatic drainage channel to the axilla might have been damaged and destroyed due to biopsy, resulting in the absence of the fluorescent or blue-stained SLN. Based on the anatomical position of the SLN, we were able to find and remove 3 lymph nodes under endoscopy during the operation, with negative results confirmed by frozen section examination. Remarkably, in 18 patients (15.38%), blue-stained SLNs and lymphatic vessels were not detected under the white-light mode, whereas SLNs with green fluorescence were observed after switching to the fluorescence mode, which suggested that these patients would have experienced a poor surgical outcome if the dye method were used alone. Similarly, of the 34 patients with SLN metastasis, 6 patients had positive SLNs that were only fluorescent and not blue-stained, all of which were detected with ICG. We speculate that if methylene blue were used alone for SLN mapping, positive SLNs might have been omitted in 5.13% of patients, leading to false-negative results. Therefore, the MBD and ICG dual-tracer method under fluorescence endoscopy yields a high visualization rate of SLNs, avoids missing positive SLNs, and reduces the false-negative rate.
Studies have shown that the prevalence of false-negative results decreases with an increase in the number of SLNs detected (24). Removal of only 1 or 2 SLNs may increase the probability of missing positive nodes, and resection of >4 may reduce the prevalence of false-negative results significantly (25). The average number of SLNs detected by a combination of ICG and MBD under fluorescence endoscopy was 5.12 in each patient, which is more than that documented in previous studies (26). Hence, a combination of ICG and MBD under fluorescence endoscopy could increase the number of SLN acquisitions and the chance of identifying positive SLNs. This strategy would help reduce the prevalence of false-negative results and improve the accuracy of assessment of ALN status.
Under direct vision of the fluorescence endoscope, the intricate structures of axillary tissue were magnified, and the tiny lymphatic vessels and small nodes were displayed clearly with an adequate surgical field. In this study, the mean time required for the discovery of the first lymph node was 7.14±6.31 min, whereas the first SLN recognition time of the dual-tracer method with ICG and MBD in open surgery was 17±5 min (27). The average duration of the surgical procedure of this group of patients was 37.75±16.94 min, and that of endoscopic SLNB using MBD alone in our center previously was 39±11 min (28), which is shorter than that for traditional open surgery (29). These results showed that under the multiple imaging modalities of fluorescence endoscopy, the tracing effects of ICG and MBD complemented one another. Our method can help surgeons to locate, identify, and remove SLNs rapidly which, in turn, would shorten the procedure time of SLNB. In addition, switching the exploration mode under fluorescence endoscopy did not involve the elimination of interference by visible light. That is, there was no need to interrupt the surgical procedure and switch off the illuminating lights (30), as the desired effect could be realized by adjusting the camera button. Real-time fluorescence positioning and resection could be carried out simultaneously, thus increasing the convenience of the procedure.
Xiong and colleagues (31) described the advantages of endoscopic-assisted SLNB, during which the relationship between SLNs and surrounding tissues could be observed under direct vision to allow intervention and reduce damage. Avoiding obvious surgical scars in the axilla, this minimally invasive endoscopy method also reduced the risk of complications, such as incision-site infection, postoperative pain, paresthesia, limited movement, and lymphedema of the upper limb. Goldberg et al. (32) reported a lymphedema prevalence of 3% in 600 patients who underwent SLNB and a higher prevalence of patient-perceived lymphedema that was associated with a higher number of lymph nodes removed. Meanwhile, Ashikaga et al. reported a prevalence of lymphedema after conventional SLNB of 7% to 9% (33). In our study, 5 patients (4.27%) developed upper-limb lymphedema in the 6 months after surgery. Our data suggest that endoscopic SLNB does not result in the extensive resection of lymph nodes and does not appear to be associated with an increased risk of lymphedema.
Our research had some limitations. First, all patients were from a single institution, and the study cohort was relatively small. Second, the follow-up duration was short. Therefore, more high-quality, large-scale, randomized controlled clinical trials and long-term follow-up are needed to assess oncological outcomes and confirm our findings.
Conclusions
Our fluorescence endoscopy method promoted the rapid search for, accurate identification, and precise resection of SLNs while additionally minimizing the difficulty of SLN exploration, shortening the duration of the surgical procedure, and reducing the size of skin incisions, all representing obvious advantages over the current SLNB navigation technique. Therefore, the application of fluorescence endoscopy combined with a double-tracer method may provide a new method for SLNB in patients with breast cancer.
Acknowledgments
Funding: This work was supported by the New Medical Technology Project of Zhujiang Hospital of Southern Medical University (No. 202101001), the Beijing Xinrui Cancer Support Therapy Research for Commonweal Project (No. cphcf-2022-076 to Pusheng Zhang), and the Guangzhou Clinical Characteristic Technology Project in 2022 (No. 2023P-TS23 to Yunfeng Luo).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-469/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-469/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-469/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Zhujiang Hospital of Southern Medical University (No. 2021-JS-001-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer. World Cancer Day: Breast cancer overtakes lung cancer in terms of number of new cancer cases worldwide. IARC showcases key research projects to address breast cancer. Lyon, France. Available online: www.iarc.who.int/pressrelease/
- Brackstone M, Baldassarre FG, Perera FE, et al. Management of the Axilla in Early-Stage Breast Cancer: Ontario Health (Cancer Care Ontario) and ASCO Guideline. J Clin Oncol 2021;39:3056-82. [Crossref] [PubMed]
- Straver ME, Meijnen P, van Tienhoven G, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol 2010;17:1854-61. [Crossref] [PubMed]
- Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 2014;15:e351-62. [Crossref] [PubMed]
- Baeten IGT, Hoogendam JP, Braat AJAT, et al. Fluorescent Indocyanine Green versus Technetium-99m and Blue Dye for Bilateral SENTinel Lymph Node Detection in Stage I-IIA Cervical Cancer (FluoreSENT): protocol for a non-inferiority study. BMJ Open 2022;12:e061829. [Crossref] [PubMed]
- Zhong Q, Chen QY, Huang XB, et al. Clinical implications of Indocyanine Green Fluorescence Imaging-Guided laparoscopic lymphadenectomy for patients with gastric cancer: A cohort study from two randomized, controlled trials using individual patient data. Int J Surg 2021;94:106120. [Crossref] [PubMed]
- Watanabe J, Ota M, Suwa Y, et al. Real-time indocyanine green fluorescence imaging-guided complete mesocolic excision in laparoscopic flexural colon cancer surgery. Dis Colon Rectum 2016;59:701-5. [Crossref] [PubMed]
- Keller DS, Joshi HM, Rodriguez-Justo M, et al. Using fluorescence lymphangiography to define the ileocolic mesentery: proof of concept for the watershed area using real-time imaging. Tech Coloproctol 2017;21:757-60. [Crossref] [PubMed]
- Egloff-Juras C, Bezdetnaya L, Dolivet G, et al. NIR fluorescence-guided tumor surgery: new strategies for the use of indocyanine green. Int J Nanomedicine 2019;14:7823-38. [Crossref] [PubMed]
- Boni L, David G, Mangano A, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 2015;29:2046-55. [Crossref] [PubMed]
- Uccella S, Nero C, Vizza E, et al. Sentinel-node biopsy in early-stage ovarian cancer: preliminary results of a prospective multicentre study (SELLY). Am J Obstet Gynecol 2019;221:324.e1-324.e10. [Crossref] [PubMed]
- Buda A, Papadia A, Zapardiel I, et al. From conventional radiotracer Tc-99(m) with blue dye to indocyanine green fluorescence: a comparison of methods towards optimization of sentinel lymph node mapping in early stage cervical cancer for a laparoscopic approach. Ann Surg Oncol 2016;23:2959-65. [Crossref] [PubMed]
- Currie AC, Brigic A, Thomas-Gibson S, et al. A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur J Surg Oncol 2017;43:2044-51. [Crossref] [PubMed]
- Kitai T, Inomoto T, Miwa M, et al. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 2005;12:211-5. [Crossref] [PubMed]
- Murawa D, Hirche C, Dresel S, et al. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg 2009;96:1289-94. [Crossref] [PubMed]
- Guo J, Yang H, Wang S, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol 2017;15:196. [Crossref] [PubMed]
- Sugie T, Kassim KA, Takeuchi M, et al. A novel method for sentinel lymph node biopsy by indocyanine green fluorescence technique in breast cancer. Cancers (Basel) 2010;2:713-20. [Crossref] [PubMed]
- Guo W, Zhang L, Ji J, et al. Evaluation of the benefit of using blue dye in addition to indocyanine green fluorescence for sentinel lymph node biopsy in patients with breast cancer. World J Surg Oncol 2014;12:290. [Crossref] [PubMed]
- Ogasawara Y, Ikeda H, Takahashi M, et al. Evaluation of breast lymphatic pathways with indocyanine green fluorescence imaging in patients with breast cancer. World J Surg 2008;32:1924-9. [Crossref] [PubMed]
- Thongvitokomarn S, Polchai N. Indocyanine green fluorescence versus blue dye or radioisotope regarding detection rate of sentinel lymph node biopsy and nodes removed in breast cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev 2020;21:1187-95. [Crossref] [PubMed]
- Tong M, Guo W, Gao W. Use of fluorescence imaging in combination with patent blue dye versus patent blue dye alone in sentinel lymph node biopsy in breast cancer. J Breast Cancer 2014;17:250-5. [Crossref] [PubMed]
- Agrawal SK, Hashlamoun I, Karki B, et al. Diagnostic performance of indocyanine green plus methylene blue versus radioisotope plus methylene blue dye method for sentinel lymph node biopsy in node-negative early breast cancer. JCO Glob Oncol 2020;6:1225-31. [Crossref] [PubMed]
- Yuan L, Qi X, Zhang Y, et al. Comparison of sentinel lymph node detection performances using blue dye in conjunction with indocyanine green or radioisotope in breast cancer patients: a prospective single-center randomized study. Cancer Biol Med 2018;15:452-60. [Crossref] [PubMed]
- Dixon JM, Grewar J, Twelves D, et al. Factors affecting the number of sentinel lymph nodes removed in patients having surgery for breast cancer. Breast Cancer Res Treat 2020;184:335-43. [Crossref] [PubMed]
- Ban EJ, Lee JS, Koo JS, et al. How many sentinel lymph nodes are enough for accurate axillary staging in T1-2 breast cancer? J Breast Cancer 2011;14:296-300. [Crossref] [PubMed]
- Zhang C, Li Y, Wang X, et al. Clinical study of combined application of indocyanine green and methylene blue for sentinel lymph node biopsy in breast cancer. Medicine (Baltimore) 2021;100:e25365. [Crossref] [PubMed]
- van der Vorst JR, Schaafsma BE, Verbeek FP, et al. Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99(m) technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann Surg Oncol 2012;19:4104-11. [Crossref] [PubMed]
- Zhang P, Luo Y, Deng J, et al. Endoscopic axillary lymphadenectomy combined with laparoscopically harvested pedicled omentum for immediate breast reconstruction. Surg Endosc 2015;29:1376-83. [Crossref] [PubMed]
- Sorrentino L, Sartani A, Pietropaolo G, et al. A novel indocyanine green fluorescence-guided video-assisted technique for sentinel node biopsy in breast cancer. World J Surg 2018;42:2815-24. [Crossref] [PubMed]
- Hirche C, Murawa D, Mohr Z, et al. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat 2010;121:373-8. [Crossref] [PubMed]
- Xiong H, Chen Z, Xu L, et al. Contrast of mastoscopic and conventional axillary lymph node dissection of patients with breast cancer: meta-analysis. Cancer Control 2020;27:1073274820932987. [Crossref] [PubMed]
- Goldberg JI, Riedel ER, Morrow M, et al. Morbidity of sentinel node biopsy: relationship between number of excised lymph nodes and patient perceptions of lymphedema. Ann Surg Oncol 2011;18:2866-72. [Crossref] [PubMed]
- Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol 2010;102:111-8. [Crossref] [PubMed]
(English Language Editor: J. Gray)



