
Analysis of risk factors for postoperative bleeding and recurrence after laparoscopic myomectomy in patients with uterine fibroids: a retrospective cohort study
Highlight box
Key findings
• Postoperative bleeding and recurrence after LM can seriously affect the prognosis of patients. Strict observation and nursing, achieve early detection, early diagnosis, early treatment, reduce the risk of bleeding and recurrence after laparoscopic myomectomy.
What is known and what is new?
• There is still a high incidence of the postoperative bleeding and recurrence in patients treated with LM.
• Uterine fibroids’ size, number, and type; age of menarche; and other clinical features can affect postoperative bleeding and recurrence in LM.
What are the implications, and what should change now?
• During the treatment of UFs, targeted preventive measures should be provided, including intraoperative ultrasonography and postoperative treatment with Gn RH-a, reduce the possibility of postoperative bleeding and recurrence, and promote postoperative recovery and early discharge of patients.
Introduction
Uterine fibroids (UFs) are benign monoclonal tumors of the myometrium that occur in three main anatomical locations: submucosal, intramural, and subserous. The tumor is driven by ovarian steroids, estrogen, and progesterone, and is the most common tumor among women worldwide (1). Currently, the prevalence of UFs is increasing in some populations, such as African-American women (2). The reported annual incidence of UFs in Asian and African American women is 1.278% and 3.745%, respectively (3), and the prevalence of UFs is higher in Black women than in White women (4). However, their reported incidence may be underestimated. Many tumors are asymptomatic or mildly symptomatic, so they remain undiagnosed (5). A study in the United States showed that the total annual cost of treatment involving UF was approximately $5.9 to $34.4 billion (6), and data suggests that fibroids not only threaten women’s health but also place a heavy burden placed on the national economy.
It is speculated that fibroids may occur in more than 70% of women of childbearing age (7). Although they do not cause symptoms in most cases, about 25–30% of women with fibroids develop symptoms that are severe enough to necessitate interventional treatment (8). Symptoms of fibroids can be divided into three categories: abnormal uterine bleeding, pelvic pressure and pain, and reproductive dysfunction (9). Among them, heavy menstrual bleeding is the main symptom of UFs, which can lead to anemia and even life-threatening conditions. The occurrence of such symptoms can reduce the quality of life of patients. The treatment of UFs includes surgical treatment and conventional drug therapy. Surgical treatment includes hysterectomy, myomectomy, hysteroscopy-assisted myomectomy, and laparoscopic uterine artery block. Hysterectomy includes negative hysterectomy, transabdominal hysterectomy, and laparoscopic hysterectomy. Myomectomy includes transabdominal myomectomy, laparoscopic myomectomy (LM), and cathartic myomectomy (10). Traditional open myomectomy is a common clinical treatment for UFs, which can completely remove fibroids under direct vision and improve the clinical symptoms of patients. However, it has the characteristics of a large incision, long duration, high intraoperative bleeding, and severe postoperative pain. A study has shown that complications after open myomectomy have been increasing in the last decade (11).
A meta-analysis of six randomized controlled trials involving 576 patients showed that LM resulted in less intraoperative bleeding, less postoperative pain, faster postoperative recovery, and fewer overall complications than open myomectomy (12). LM is performed by placing a laparoscope in the abdominal cavity and performing myomectomy under direct visualization, which can effectively remove the lesion and prevent damage to the surrounding organ tissues, as the location of the myoma and its adjacent tissues can be observed during the surgical treatment. The advantages of laparoscopic surgery over open surgery are significant in terms of postoperative recovery time and incision size and meet the concept of aesthetics and minimal invasiveness (13). However, some complications, such as postoperative bleeding, wound infection, urinary retention, adnexitis, abdominal adhesions, and recurrence of fibroids, are inevitable after LM, which has become a particular area of concern. It has been reported in the literature that the probability of bleeding after LM is about 2% (14), and the cumulative recurrence rate 8 years after surgery is 76.2% (15), indicating that the recurrence rate is still relatively high. However, there are few studies on the risk factors of bleeding and recurrence after LM. Therefore, the present study aimed to analyze the clinical characteristics of patients with UFs treated by LM and the independent risk factors for postoperative bleeding and fibroid recurrence, to identify the relevant risk factors as early as possible and provide a reference basis for reducing the occurrence of postoperative complications, improving the prognosis of patients, and enhancing their quality of life. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-92/rc).
Methods
Research participants
A total of 621 patients with UFs treated by LM at Fenyang Hospital of Shanxi Province from April 2018 to June 2021 were included in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Fenyang Hospital of Shanxi Province (No. KYLC2018117). Informed consent was obtained from all patients.
Inclusion criteria: (I) patients diagnosed with UFs by pelvic ultrasound, bimanual gynecological examination, and postoperative pathological examination; (II) patients with complete clinical data; (III) patients who met the indications for myomectomy treatment; (IV) patients who signed the informed consent; and (V) patients with normal coagulation function.
Exclusion criteria: (I) patients with other concurrent cervical diseases; (II) patients with contraindications to surgery; (III) patients with malignant diseases; (IV) patients with hematologic diseases; (V) patients with cognitive impairment; and (VI) patients with UFs in pregnancy (Figure 1).
This is a retrospective study, and based on the results of the previous survey and clinical experience of our group, the incidence of postoperative bleeding after laparoscopic treatment of uterine fibroids is approximately 9.0%, and the probability of postoperative recurrence is approximately 5.0%. This incidence was brought into the software PASS 15 for sample size estimation, which yielded a total sample size of 239 cases, and considering a data attrition rate of 10%, the minimum sample size was calculated to be 263 cases. The general rule of logistic regression requires the ratio of the item number to sample size to be 1:5–1:10. Therefore, the sample size of the study population planned for this study was 650 patients. The actual attrition and loss were 29 patients, with a final count of 621 patients.
General information questionnaire
The general information questionnaire included demographic data [age, body mass index (BMI), age of menarche] and clinical data [e.g., number of fibroids, size of fibroids, uterine size, maximum fibroid type, maximum fibroid site, type of pathology, history of abdominal surgery, preoperative delivery, preoperative activated partial thromboplastin time (APTT) level, preoperative prothrombin time (PT) level, preoperative hemoglobin (Hb) level, preoperative C-reactive protein (CRP) level, surgery time, hospitalization time, intraoperative bleeding, intraoperative ultrasound or not, postoperative gonadotropin-releasing hormone agonist (Gn RH-a) therapy or not, and postoperative complications].
Postoperative complications
Although LM has been widely performed in clinical work, its postoperative complications such as postoperative bleeding, recurrence, infection, subcutaneous emphysema, abdominal adhesions, urinary retention, adnexitis, and incisional bleeding are still inevitable. Among them, combined postoperative bleeding is a common complication, which is often classified as postoperative complications such as abdominal bleeding, vaginal bleeding, and bleeding from the abdominal puncture hole.
Postoperative recurrence
The criteria for determining recurrence after myomectomy were: ultrasound suggestive of normal at 3 months postoperatively, but ultrasound suggestive of fibroids at 6 months postoperatively was considered as recurrence. All enrolled patients were followed up for 1 year by telephone, internet or outpatient to determine the presence or absence of recurrence of fibroids. The last follow-up occurred in June 2022.
Pay a visit
Postoperative follow-up was initiated for 1 year by telephone, short message service (SMS) and outpatient review. Patients visited the hospital every 3 months for routine gynecological examination, ultrasound and tumor marker testing. If there is any discomfort during the period, patients should visit the hospital at any time. The follow-up end point is 1 year after the patient’s recurrence or discharge from the hospital, and the follow-up deadline is June 2022.
Statistical analysis
The results of each scale were entered into the computer for score conversion, and statistical analysis was performed using SPSS 26 (IBM SPSS, USA), with measured data expressed as the mean and standard deviation and count data expressed as a frequency and percentage. Statistical analysis between the groups was performed using the t-test, analysis of variance (ANOVA), and chi-square test, and the influential factors for postoperative bleeding and recurrence were analyzed by binary logistic regression. A two-sided P<0.05 was considered statistically significant.
Results
Baseline data
The baseline characteristics of the patients are shown in Table 1. A total of 621 patients who underwent LM were included in this study, of whom 28 (4.51%) patients had postoperative bleeding and 44 (7.09%) patients had postoperative recurrent fibroids. The mean BMI was 24.13±3.28 kg/m2 in patients with recurrence and 22.59±2.38 kg/m2 in those without recurrence, with a statistically significant difference between these groups (P<0.05). Among patients with postoperative bleeding, a total of 12 patients (42.9%) had ≤2 fibroids and 16 patients (57.1%) had >2 fibroids. Among the patients with recurrent fibroids after surgery, a total of 19 patients (43.2%) had ≤2 fibroids and 25 patients (56.8%) had >2 fibroids. A total of 10 patients (35.7%) with postoperative bleeding had fibroids ≤5 cm in size and 18 patients (64.3%) had fibroids >5 cm in size. Also, 19 patients (43.2%) with postoperative recurrence had fibroids ≤5 cm in size and 25 patients (56.8%) had fibroids >5 cm in size.
Table 1
| Item | Postoperative bleeding | Uterine fibroid recurrence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | t/χ2 | P | Yes | No | t/χ2 | P | ||
| Age (years), mean ± SD | 44.57±10.56 | 44.70±10.63 | 0.065 | 0.948 | 42.41±11.38 | 44.87±10.55 | 1.485 | 0.138 | |
| BMI (kg/m2), mean ± SD | 22.47±2.41 | 22.71±2.49 | 0.511 | 0.610 | 24.13±3.28 | 22.59±2.38 | −3.037 | 0.004 | |
| Age of menarche (years), mean ± SD | 13.39±2.23 | 13.08±2.02 | −0.791 | 0.429 | 12.30±2.08 | 13.16±2.01 | 2.733 | 0.006 | |
| Number of uterine fibroids, N (%) | |||||||||
| ≤2 | 12 (42.9) | 384 (64.8) | 5.549 | 0.018 | 19 (43.2) | 377 (65.3) | 8.686 | 0.003 | |
| >2 | 16 (57.1) | 209 (35.2) | 25 (56.8) | 200 (34.7) | |||||
| Size of uterine fibroids (cm), N (%) | |||||||||
| ≤5 | 10 (35.7) | 425 (71.7) | 16.475 | 0.000 | 19 (43.2) | 416 (72.1) | 16.292 | 0.000 | |
| >5 | 18 (64.3) | 168 (28.3) | 25 (56.8) | 161 (27.9) | |||||
| Uterine size, N (%) | |||||||||
| ≤ size of 14 weeks’ gestation | 16 (57.1) | 367 (61.9) | 0.255 | 0.614 | 20 (45.5) | 363 (62.9) | 5.271 | 0.022 | |
| > size of 14 weeks’ gestation | 12 (42.9) | 226 (38.1) | 24 (54.5) | 214 (37.1) | |||||
| Maximum uterine fibroid type, N (%) | |||||||||
| Interstitial fibroids | 23 (82.1) | 258 (43.5) | 16.110 | 0.000 | 31 (70.5) | 250 (43.3) | 12.143 | 0.000 | |
| Subserosal fibroids or submucosal fibroid | 5 (17.9) | 335 (56.5) | 13 (29.5) | 327 (56.7) | |||||
| Maximum uterine fibroid position, N (%) | |||||||||
| Anterior wall of the uterus | 7 (25.0) | 166 (28.0) | 2.856 | 0.414 | 16 (36.4) | 157 (27.2) | 2.657 | 0.448 | |
| Posterior wall of the uterus | 10 (35.7) | 153 (25.8) | 9 (20.5) | 154 (26.7) | |||||
| Lateral wall of the uterus | 3 (10.7) | 130 (21.9) | 7 (15.9) | 126 (21.8) | |||||
| Bottom of the uterus | 8 (28.6) | 144 (24.3) | 12 (27.3) | 140 (24.3) | |||||
| Type of pathology, N (%) | |||||||||
| General type | 11 (39.3) | 416 (70.2) | 11.859 | 0.001 | 21 (47.7) | 406 (70.4) | 9.752 | 0.002 | |
| Cell-rich type | 17 (60.7) | 177 (29.8) | 23 (52.3) | 171 (29.6) | |||||
| History of abdominal surgery, N (%) | |||||||||
| Yes | 11 (39.3) | 106 (17.9) | 8.016 | 0.005 | 7 (15.9) | 110 (19.1) | 0.266 | 0.606 | |
| No | 17 (60.7) | 487 (82.1) | 37 (84.1) | 467 (80.9) | |||||
| Preoperative delivery, N (%) | |||||||||
| <2 | 20 (71.4) | 482 (81.3) | 1.676 | 0.195 | 29 (65.9) | 473 (82.0) | 6.813 | 0.009 | |
| ≥2 | 8 (28.6) | 111 (18.7) | 15 (34.1) | 104 (18.0) | |||||
SD, standard deviation; BMI, body mass index.
Chi-square test analysis showed that the number and size of fibroids were significantly different based on whether the patient had bleeding or recurrence postoperatively (P<0.05). Among the patients with combined recurrence, 20 patients (45.5%) had a uterine size ≤14 gestational weeks, 24 patients (54.5%) had a uterine size >14 gestational weeks, 29 patients (65.9%) had <2 preoperative deliveries, and 15 patients (34.1%) had ≥2 deliveries. The chi-square analysis results showed that there was a significant difference between uterine size and the number of preoperative deliveries in terms of whether recurrence occurred after surgery (P<0.05). Among patients with postoperative bleeding, a total of 23 (82.1%) of the largest fibroids were interstitial fibroids, 5 (17.9%) were subserosal fibroids or submucosal fibroid, 11 (39.3%) patients exhibited a normal type of fibroid pathology, and 17 (60.7%) were a cell-rich type. Meanwhile, among patients who experienced postoperative recurrence, a total of 31 (70.5%) patients had interstitial fibroids, 13 (29.5%) patients had subserosal fibroids or submucosal fibroid, 21 (47.7%) patients had common fibroids, and 23 (52.3%) patients had cell-rich fibroids.
Both fibroid type and pathological type exhibited significant differences in terms of whether bleeding and recurrence occurred postoperatively (P<0.05). Among patients who developed postoperative bleeding, 11 (39.3%) had a history of abdominal surgery and 17 (60.7%) did not; meanwhile, among patients without postoperative bleeding, 106 (17.9%) had a history of abdominal surgery and 487 (82.1%) did not. The chi-square test showed that postoperative bleeding was statistically different in terms of the presence or absence of a history of abdominal surgery (P<0.05).
Perioperative and follow-up clinical data of the included patients
A total of 7 patients (1.13%) had postoperative bleeding requiring blood transfusion, and their mean preoperative APTT and PT levels were 33.00±4.04 and 13.86±32.9 s, respectively. The preoperative Hb of these seven patients was ≤110 g/L and mean surgery time was 112.57±5.71 min, and the mean intraoperative bleeding was 150.86±9.58 mL. A total of 21 patients (3.81%) who had postoperative bleeding without blood transfusion had a mean preoperative APTT of 27.81±5.47 s and a mean preoperative PT of 14.23±3.53 s. Among these patients, 16 (76.19%) had a preoperative Hb ≤110 g/L, a mean surgery time of 107.90±7.31 min, and a mean intraoperative bleeding volume of bleeding of 123.62±4.83 mL. Moreover, a total of 593 patients (95.49%) did not have postoperative bleeding patients; their mean preoperative APTT and PT levels were 25.21±7.78 and 12.59±2.68 s, respectively. Among these patients, 273 (46.04%) had a preoperative Hb >110 g/L, a mean surgery time of 103.00±11.27 min, and a mean intraoperative bleeding volume of 122.62±4.52 mL. The chi-square results showed significant differences in preoperative APTT, preoperative PT, preoperative Hb, operative time, and intraoperative bleeding in patients with or without postoperative bleeding (P<0.05).
A total of 44 patients (7.09%) had postoperative recurrence, with a mean preoperative CRP level of 6.63±3.23 mg/L. Among these patients, 14 (31.82%) used intraoperative ultrasonography, and 23 (52.27%) used postoperative Gn RH-a (gonadotropin-releasing hormone agonist) treatment. A total of 577 patients (92.91%) did not experience recurrence, with mean preoperative CRP levels of 4.87±3.02 mg/L. Among these patients, 334 (57.89%) underwent ultrasonography intraoperatively and 190 patients (32.93%) received Gn RH-a postoperatively. The chi-square results showed significant differences in the preoperative CRP, intraoperative use of ultrasonography, and postoperative treatment with Gn RH-a in patients with or without postoperative recurrence (P<0.05), as shown in Table 2.
Table 2
| Postoperative bleeding | Uterine fibroid recurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood transfusion | No blood transfusion | Without postoperative bleeding | χ2/F | P | Yes | No | χ2/t | P | ||
| N (%) | 7 (1.13) | 21 (3.81) | 593 (95.49) | 44 (7.09) | 577 (92.91) | |||||
| Perioperative APTT (s), mean ± SD | 33.00±4.04 | 27.81±5.47 | 25.21±7.78 | 4.624 | 0.01 | 24.98±7.82 | 25.42±7.74 | 0.133 | 0.715 | |
| Perioperative PT (s), mean ± SD | 13.86±32.9 | 14.23±3.53 | 12.59±2.68 | 4.83 | 0.008 | 12.82±3.21 | 12.65±2.70 | 0.148 | 0.701 | |
| Perioperative Hb ≥110 (g/L), n (%) | 9.791 | 0.007 | 0.285 | 0.593 | ||||||
| Yes | 0 (0.00) | 5 (23.81) | 273 (46.04) | 18 (40.91) | 260 (45.06) | |||||
| No | 7 (100.00) | 16 (76.19) | 320 (53.96) | 26 (59.09) | 317 (54.94) | |||||
| Perioperative CRP (mg/L), mean ± SD | 3.58±2.46 | 4.00±2.32 | 5.05±3.09 | 1.955 | 0.142 | 6.63±3.23 | 4.87±3.02 | 13.757 | 0 | |
| Surgery time (min), mean ± SD | 112.57±5.71 | 107.90±7.31 | 103.00±11.27 | 4.447 | 0.012 | 101.07±11.90 | 103.44±11.13 | 1.837 | 0.176 | |
| Hospitalization time (d), mean ± SD | 5.00±1.92 | 5.81±1.89 | 5.47±2.23 | 0.399 | 0.671 | 6.09±2.21 | 5.43±2.21 | 3.625 | 0.057 | |
| Intraoperative bleeding volume (mL), mean ± SD | 150.86±9.58 | 123.62±4.83 | 122.62±4.52 | 130.419 | 0 | 123.55±4.61 | 122.93±5.542 | 0.523 | 0.47 | |
| Intraoperative ultrasonography was applied or not, n (%) | 0.695 | 0.706 | 11.277 | 0.001 | ||||||
| Yes | 5 (71.43) | 12 (57.14) | 331 (55.82) | 14 (31.82) | 334 (57.89) | |||||
| No | 2 (28.57) | 9 (42.86) | 262 (44.18) | 30 (68.18) | 243 (42.11) | |||||
| Postoperative treated with Gn RH-a or not, n (%) | 4.838 | 0.089 | 6.788 | 0.009 | ||||||
| Yes | 0 (0.00) | 5 (23.81) | 208 (35.08) | 23 (52.27) | 190 (32.93) | |||||
| No | 7 (100.00) | 16 (76.19) | 385 (64.92) | 21 (47.73) | 387 (67.07) | |||||
APTT, activated partial thromboplastin time; SD, standard deviation; PT, prothrombin time; Hb, hemoglobin; CRP, C-reactive protein; Gn RH-a, gonadotropin-releasing hormone agonist.
Complications
Among patients who had postoperative bleeding requiring blood transfusion, 3 (42.86%) had a postoperative infection and 2 (28.57%) had postoperative abdominal adhesions; meanwhile, among those who had postoperative bleeding without blood transfusion, 2 (9.52%) had a postoperative infection and 1 (4.76%) had postoperative abdominal adhesions. Among patients who did not experience postoperative bleeding, 19 (3.20%) had a postoperative infection and 12 (2.02%) had postoperative abdominal adhesions. The chi-square test showed that postoperative infection and abdominal adhesions were significantly different in the above three groups (P<0.05).
Among those with postoperative recurrence, 5 (11.36%) had a postoperative infection and 4 (9.09%) had postoperative adnexitis; meanwhile, among patients with uncomplicated recurrence, 19 (3.29%) had a postoperative infection and 10 (1.73%) had postoperative adnexitis. The chi-square test showed that postoperative infection and adnexitis were significantly different in the two groups (P<0.05) (Table 3).
Table 3
| Postoperative bleeding | Uterine fibroid recurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood transfusion | No blood transfusion | With no postoperative bleeding | χ2 | P | Yes | No | χ2 | P | ||
| Infection, n (%) | 31.152 | 0 | 7.167 | 0.007 | ||||||
| Yes | 3 (42.86) | 2 (9.52) | 19 (3.20) | 5 (11.36) | 19 (3.29) | |||||
| No | 4 (57.14) | 19 (90.48) | 574 (96.80) | 39 (88.64) | 558 (96.71) | |||||
| Subcutaneous emphysema, n (%) | 1.758 | 0.415 | 0.225 | 0.635 | ||||||
| Yes | 0 (0.00) | 1 (4.76) | 8 (1.35) | 1 (2.27) | 8 (1.39) | |||||
| No | 7 (100.00) | 20 (95.24) | 585 (98.65) | 43 (97.73) | 569 (98.61) | |||||
| Abdominal adhesions, n (%) | 21.194 | 0 | 0.004 | 0.949 | ||||||
| Yes | 2 (28.57) | 1 (4.76) | 12 (2.02) | 1 (2.27) | 14 (2.43) | |||||
| No | 5 (71.43) | 20 (95.24) | 581 (97.98) | 43 (97.73) | 563 (97.57) | |||||
| Urinary retention, n (%) | 0.59 | 0.745 | 0.731 | 0.392 | ||||||
| Yes | 0 (0.00) | 1 (4.76) | 15 (2.53) | 2 (4.55) | 14 (2.43) | |||||
| No | 7 (100.00) | 20 (95.24) | 578 (97.47) | 42 (95.45) | 563 (97.57) | |||||
| Adnexitis, n (%) | 0.771 | 0.68 | 10.044 | 0.002 | ||||||
| Yes | 0 (0.00) | 1 (4.76) | 13 (2.19) | 4 (9.09) | 10 (1.73) | |||||
| No | 7 (100.00) | 20 (95.24) | 580 (97.81) | 40 (90.91) | 567 (97.57) | |||||
| Incisional bleeding, n (%) | 1.97 | 0.373 | 0.033 | 0.856 | ||||||
| Yes | 0 (0.00) | 2 (9.52) | 23 (3.88) | 2 (4.55) | 23 (3.99) | |||||
| No | 7 (100.00) | 19 (90.48) | 570 (96.12) | 42 (95.45) | 554 (96.01) | |||||
Risk factors of postoperative bleeding and recurrence analyzed by binary logistic regression models
Binary logistic regression analysis showed that fibroid size, maximum fibroid type, pathological type, preoperative PT level, preoperative Hb level, surgery time, intraoperative bleeding volume, and postoperative infection were independent risk factors for postoperative bleeding (Table 4, Figure 2).
Table 4
| Item | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| Number of uterine fibroids | 0.464 | 0.540 | 0.739 | 0.390 | 1.591 | 4.586 | 0.552 |
| Size of uterine fibroids (cm) | 1.705 | 0.571 | 8.907 | 0.003 | 5.502 | 16.857 | 1.796 |
| Maximum uterine fibroid type | −1.227 | 0.619 | 3.923 | 0.048 | 0.293 | 0.987 | 0.087 |
| Type of pathology | 1.301 | 0.525 | 6.151 | 0.013 | 3.673 | 10.270 | 1.314 |
| History of abdominal surgery | −0.978 | 0.565 | 2.993 | 0.084 | 0.376 | 1.139 | 0.124 |
| Perioperative APTT | 0.054 | 0.039 | 1.913 | 0.167 | 1.055 | 1.139 | 0.978 |
| Perioperative PT | 0.293 | 0.098 | 8.888 | 0.003 | 1.340 | 1.625 | 1.106 |
| Perioperative Hb | −1.283 | 0.611 | 4.412 | 0.036 | 0.227 | 0.918 | 0.084 |
| Perioperative CRP | −0.132 | 0.104 | 1.608 | 0.205 | 0.876 | 1.075 | 0.714 |
| Surgery time | 0.064 | 0.028 | 5.212 | 0.022 | 1.066 | 1.126 | 1.009 |
| Hospitalization time | 0.077 | 0.121 | 0.406 | 0.524 | 1.080 | 1.371 | 0.852 |
| Intraoperative bleeding volume | 0.135 | 0.050 | 7.388 | 0.007 | 1.145 | 1.262 | 1.038 |
| Intraoperative ultrasonography | 0.103 | 0.530 | 0.038 | 0.846 | 1.109 | 3.132 | 0.392 |
| Postoperative treatment with Gn RH-a | −0.830 | 0.634 | 1.715 | 0.190 | 0.436 | 1.510 | 0.126 |
| Infection | 2.256 | 0.873 | 6.675 | 0.010 | 9.540 | 52.807 | 1.724 |
| Subcutaneous emphysema | 2.129 | 1.223 | 3.030 | 0.082 | 8.407 | 92.433 | 0.765 |
| Abdominal adhesions | 1.998 | 1.192 | 2.811 | 0.094 | 7.373 | 76.211 | 0.713 |
| Urinary retention | −0.595 | 1.659 | 0.129 | 0.720 | 0.552 | 14.247 | 0.021 |
| Adnexitis | −0.925 | 1.696 | 0.298 | 0.585 | 0.396 | 11.012 | 0.014 |
| Incisional bleeding | 1.813 | 0.978 | 3.436 | 0.064 | 6.127 | 41.650 | 0.901 |
SE, standard error; OR, odds ratio; CI, confidence interval; APTT, activated partial thromboplastin time; PT, prothrombin time; Hb, hemoglobin; CRP, C-reactive protein; Gn RH-a, gonadotropin-releasing hormone agonist.
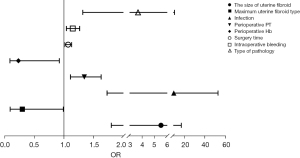
BMI, age of menarche, fibroid size, fibroid number, maximum fibroid type, pathological type, preoperative delivery, preoperative CRP level, intraoperative ultrasonography, postoperative GH-a treatment, and postoperative infection were independent risk factors for recurrence (Table 5, Figure 3).
Table 5
| Item | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| BMI (kg/m2) | 0.237 | 0.073 | 10.684 | 0.001 | 1.268 | 1.461 | 1.100 |
| Age of menarche (years) | −0.248 | 0.100 | 6.167 | 0.013 | 0.780 | 0.949 | 0.642 |
| Number of uterine fibroids | 0.867 | 0.406 | 4.556 | 0.033 | 2.381 | 5.280 | 1.073 |
| Size of uterine fibroids (cm) | 1.508 | 0.412 | 13.384 | 0.000 | 4.519 | 10.139 | 2.014 |
| Uterine size | 0.670 | 0.400 | 2.809 | 0.094 | 1.955 | 4.282 | 0.893 |
| Maximum uterine fibroid type | −1.474 | 0.441 | 11.187 | 0.001 | 0.229 | 0.543 | 0.096 |
| Type of pathology | 1.086 | 0.408 | 7.097 | 0.008 | 2.963 | 6.588 | 1.333 |
| Preoperative delivery | 1.341 | 0.444 | 9.101 | 0.003 | 3.822 | 9.133 | 1.599 |
| Perioperative APTT | −0.016 | 0.029 | 0.313 | 0.576 | 0.984 | 1.042 | 0.929 |
| Perioperative PT | −0.054 | 0.065 | 0.695 | 0.404 | 0.947 | 1.076 | 0.834 |
| Perioperative Hb | −0.216 | 0.411 | 0.276 | 0.599 | 0.806 | 1.803 | 0.360 |
| Perioperative CRP | 0.150 | 0.054 | 7.840 | 0.005 | 1.162 | 1.291 | 1.046 |
| Surgery time | −0.024 | 0.020 | 1.467 | 0.226 | 0.976 | 1.015 | 0.939 |
| Hospitalization time | 0.100 | 0.079 | 1.583 | 0.208 | 1.105 | 1.290 | 0.946 |
| Intraoperative bleeding volume | −0.004 | 0.033 | 0.013 | 0.910 | 0.996 | 1.063 | 0.934 |
| Intraoperative ultrasonography | −1.307 | 0.428 | 9.338 | 0.002 | 0.271 | 0.626 | 0.117 |
| Postoperative treatment with Gn RH-a | 0.878 | 0.402 | 4.784 | 0.029 | 2.407 | 5.287 | 1.096 |
| Infection | 2.002 | 0.713 | 7.877 | 0.005 | 7.402 | 29.954 | 1.829 |
| Subcutaneous emphysema | 1.663 | 1.243 | 1.788 | 0.181 | 5.273 | 60.306 | 0.461 |
| Abdominal adhesions | 0.567 | 1.342 | 0.178 | 0.673 | 1.763 | 24.489 | 0.127 |
| Urinary retention | 1.406 | 0.902 | 2.429 | 0.119 | 4.079 | 23.898 | 0.696 |
| Adnexitis | 1.516 | 0.841 | 3.248 | 0.072 | 4.555 | 23.698 | 0.876 |
| Incisional bleeding | −0.483 | 0.950 | 0.258 | 0.611 | 0.617 | 0.096 | 3.975 |
SE, standard error; OR, odds ratio; CI, confidence interval; BMI, body mass index; APTT, activated partial thromboplastin time; PT, prothrombin time; Hb, hemoglobin; CRP, C-reactive protein; Gn RH-a, gonadotropin-releasing hormone agonist.
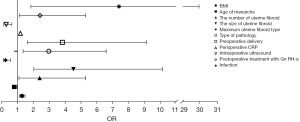
Discussion
UFs are the most common benign tumors of the reproductive system in women, and their prevalence is as high as 20–30% in fertile women (16), with an incidence of 40% in women at the age of 35 years that can approach 70–80% by the age of menopause (17). Despite their high prevalence, up to 70% of fibroids are asymptomatic and thus do not require frequent follow-up or medical intervention (8). The differences in the sizes and locations of fibroids can lead to different clinical symptoms, with 15–30% of patients experiencing severe symptoms (18), such as abnormal uterine bleeding, dysmenorrhea, urinary frequency or constipation, sexual dysfunction, and infertility, which impair their physical and mental health (19). Although UFs are benign tumors, there is also a possibility of malignant transformation, and the malignancy rate of patients with UFs is about 0.5% in China and about 0.13–1.0% in foreign countries. Therefore, when diagnosing patients, doctors should provide targeted treatment according to the size, location, clinical symptoms, and examination results of patients (15). At present, the treatment measures for UFs are mainly drug and surgical treatments; although drug treatment is effective, the chances of fibroid recurrence after stopping are high and clinical symptoms appear repeatedly (20). Surgery is widely used in clinical treatment, and mainly involves myomectomy or hysterectomy, including myomectomy through hysteroscopy, myomectomy through open or laparoscopy, uterine artery embolization, and interventions to induce thermal ablation of UFs under radiological or ultrasound guidance (21). Among them, LM has been widely used in clinical practice owing to its small abdominal wall incision, aesthetics, minimal trauma, and faster postoperative recovery (22,23). However, regardless of the treatment method, complications such as postoperative bleeding, recurrence or complications of urological diseases, and even adverse effects on fertility may still occur after treatment (24).
The results of the present study showed that a total of 28 patients (4.51%) experienced bleeding after undergoing LM and 44 cases (7.09%) had recurrence. Among them, the number, size, type, and pathological type of UFs were significantly different in terms of the occurrence of postoperative bleeding and recurrence. Binary logistic regression analysis showed that fibroid size, maximum fibroid type, preoperative PT, preoperative Hb, surgery time, and intraoperative bleeding volume were independent risk factors for postoperative bleeding. Interstitial fibroids are located within the myometrial wall of the uterus, deeply located, surrounded by the myometrium, and have a rich blood supply, which not only increases the possibility of residual fibroids but also increases the risk of postoperative bleeding (25). In addition, multiple fibroids or large single fibroids with deep cavities may not close completely if the sutures are not tight enough, which may result in bleeding on the wound surface. Moreover, the opening of the blood sinus due to insufficient contraction of the uterine incision and bleeding secondary to incomplete hemostasis after the blood vessel injury during surgery are all causes of postoperative bleeding.
Numerous factors have been shown to influence recurrence after myomectomy, such as incomplete removal of the lesion at the time of surgery, the presence of remnants, the postoperative regrowth of fibroids in the sex hormone environment, or the continuation of pathogenic factors after surgery (26). In this study, binary logistic regression analysis showed that age of menarche <13 years, number of fibroids ≥2, size of fibroids ≥5 cm, interstitial fibroids, and type of pathology were independent risk factors for fibroid recurrence. A previous study (27) found that the number of fibroids removed intraoperatively was not significantly associated with postoperative recurrence, but the number of fibroids revealed by preoperative vaginal ultrasound was associated with postoperative recurrence, and the postoperative recurrence rate was significantly lower in patients with preoperative vaginal ultrasound suggestive of one to two fibroids than in those with more than three fibroids. In our study, the postoperative recurrence rate was higher in patients with ≥2 fibroids than in those with <2 fibroids, probably because the risk of recurrence increases as the number of fibroids rises, which is due to the tendency to overlook small fibroids, especially those in the interstitial space, during the LM procedure (28). For women with early menarche, the regulatory neuroendocrine mechanism of sexual maturation is activated earlier, and compared to those with late menarche, their estrogen peaks earlier, their menstrual periods are more frequent, and their hormone secretion levels are higher. Since fibroids are more influenced by hormones, early menarche is an independent risk factor for recurrence after LM in patients with UFs. Patients with an early age of menarche should be urged to follow-up regularly after surgery to ensure early detection of potential recurrence risk and pharmacological interventions, such as mifepristone and short-acting oral contraceptives, to improve prognosis and avoid readmission and surgical treatment. Preoperative CRP and postoperative infection are also independent risk factors for recurrence, which may be due to the long-term inflammatory cell infiltration in patients with UFs, which can intensify the proliferation of spindle-shaped smooth muscle cells and fibrous connective tissue, increase the expression of sex hormone receptors, and disrupt the normal uterine physiology, thus increasing the risk of recurrence (29). Finally, the relevant literature also reported that the number and pathological type of fibroids are associated with UF recurrence (28,30), which is consistent with the results of our study.
Also, our results showed that the intraoperative application of ultrasonography and postoperative treatment with Gn RH-a were protective factors for recurrence. Clinically, pre-treatment with Gn RH-a before surgery can be used for patients with anemia or excessive fibroid volumes to correct anemia or reduce fibroid volume, thus reducing the occurrence of surgical complications (28). However, other studies have recently shown that treatment with Gn RH-a before fibroid resection leads to an increased risk of recurrence in patients, which may be because Gn RH-a reduces the volume of preoperative fibroids and affects the identification of small fibroids during surgery (31,32) and leads to incomplete intraoperative fibroid resection. This suggests that we should be more meticulous in preoperative and intraoperative color Doppler ultrasonography, strictly control the surgical indications, and select an appropriate surgical plan accordingly to improve the safety and effectiveness of surgery, to reduce the possibility of postoperative recurrence (33). Therefore, in the clinical treatment of UF, preoperative examination and clinical data analysis should be performed to fully understand the size, type, and number of fibroids and the perioperative period indicators, focusing on people with high-risk factors, to reduce the occurrence of complications such as postoperative bleeding and recurrence, thereby improving the quality of life and prognosis of patients.
Finally, the main drawback of this study was the relatively short follow-up period due to limited time and manpower. Thus, it is recommended that future studies involve longer follow-up periods.
Conclusions
At present, there is still a high probability of postoperative bleeding and recurrence after LM for UF. Clinical work should pay close attention to clinical features. Adequate preoperative examination to improve surgical precision, and strengthen postoperative care and education, thus reducing the probability of postoperative bleeding and recurrence in patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-92/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-92/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-92/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-92/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Fenyang Hospital of Shanxi Province (No. KYLC2018117). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang Q, Ciebiera M, Bariani MV, et al. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr Rev 2022;43:678-719. [Crossref] [PubMed]
- Lee M, Chung YJ, Kim HK, et al. Estimated Prevalence and Incidence of Uterine Leiomyoma, and Its Treatment Trend in South Korean Women for 12 years: A National Population-Based Study. J Womens Health (Larchmt) 2021;30:1038-46. [Crossref] [PubMed]
- Sheng B, Song Y, Liu Y, et al. Association between vitamin D and uterine fibroids: a study protocol of an open-label, randomised controlled trial. BMJ Open 2020;10:e038709. [Crossref] [PubMed]
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt) 2013;22:807-16. [Crossref] [PubMed]
- Al-Hendy A, Myers ER, Stewart E. Uterine Fibroids: Burden and Unmet Medical Need. Semin Reprod Med 2017;35:473-80. [Crossref] [PubMed]
- Arip M, Yap VL, Rajagopal M, et al. Evidence-Based Management of Uterine Fibroids With Botanical Drugs-A Review. Front Pharmacol 2022;13:878407. [Crossref] [PubMed]
- Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124:1501-12. [Crossref] [PubMed]
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043. [Crossref] [PubMed]
- Syed YY. Relugolix/Estradiol/Norethisterone (Norethindrone) Acetate: A Review in Symptomatic Uterine Fibroids. Drugs 2022;82:1549-56. [Crossref] [PubMed]
- Fritton K, Borahay MA. New and Emerging Therapies for Uterine Fibroids. Semin Reprod Med 2017;35:549-59. [Crossref] [PubMed]
- Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol 2014;21:486-91. [Crossref] [PubMed]
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy--a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol 2009;145:14-21. [Crossref] [PubMed]
- Yu S, Bhagavath B, Shobeiri SA, et al. Clinical and Patient Reported Outcomes of Pre- and Postsurgical Treatment of Symptomatic Uterine Leiomyomas: A 12-Month Follow-up Review of TRUST, a Surgical Randomized Clinical Trial Comparing Laparoscopic Radiofrequency Ablation and Myomectomy. J Minim Invasive Gynecol 2022;29:726-37. [Crossref] [PubMed]
- Schiffman M, Lamparello N. Uterine-Artery Embolization or Myomectomy for Uterine Fibroids. N Engl J Med 2020;383:2186-7. [PubMed]
- Kotani Y, Tobiume T, Fujishima R, et al. Recurrence of uterine myoma after myomectomy: Open myomectomy versus laparoscopic myomectomy. J Obstet Gynaecol Res 2018;44:298-302. [Crossref] [PubMed]
- Ciarmela P, Delli Carpini G, Greco S, et al. Uterine fibroid vascularization: from morphological evidence to clinical implications. Reprod Biomed Online 2022;44:281-94. [Crossref] [PubMed]
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med 2015;372:1646-55. [Crossref] [PubMed]
- Salas A, Beltrán-Flores S, Évora C, et al. Stem Cell Growth and Differentiation in Organ Culture: New Insights for Uterine Fibroid Treatment. Biomedicines 2022;10:1542. [Crossref] [PubMed]
- Ghant MS, Sengoba KS, Recht H, et al. Beyond the physical: a qualitative assessment of the burden of symptomatic uterine fibroids on women's emotional and psychosocial health. J Psychosom Res 2015;78:499-503. [Crossref] [PubMed]
- Xuan J, Deng G, Liu R, et al. Analysis of medication data of women with uterine fibroids based on data mining technology. J Infect Public Health 2020;13:1513-6. [Crossref] [PubMed]
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update 2016;22:665-86. [Crossref] [PubMed]
- Chen R, Su Z, Yang L, et al. The effects and costs of laparoscopic versus abdominal myomectomy in patients with uterine fibroids: a systematic review and meta-analysis. BMC Surg 2020;20:55. [Crossref] [PubMed]
- Takmaz Ö, Gündoğan S, Özbaşlı E, et al. Laparoscopic assisted robotic myomectomy of a huge myoma; Does robotic surgery change the borders in minimally invasive gynecology? J Turk Ger Gynecol Assoc 2019;20:211-2. [Crossref] [PubMed]
- Berujon E, Thubert T, Fauvet R, et al. Impact of uterine fibroid surgery on lower urinary tract symptoms. J Gynecol Obstet Hum Reprod 2022;51:102355. [Crossref] [PubMed]
- Liang Y, Ren Y, Wan Z, et al. Clinical evaluation of improved MyoSure hysteroscopic tissue removal system for the resection of type II submucosal myomas. Medicine (Baltimore) 2017;96:e9363. [Crossref] [PubMed]
- Sanada S, Ushijima K, Yanai H, et al. A critical review of "uterine leiomyoma" with subsequent recurrence or metastasis: A multicenter study of 62 cases. J Obstet Gynaecol Res 2022;48:3242-51. [Crossref] [PubMed]
- Kim DH, Kim ML, Song T, et al. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol 2014;177:57-60. [Crossref] [PubMed]
- Radosa MP, Owsianowski Z, Mothes A, et al. Long-term risk of fibroid recurrence after laparoscopic myomectomy. Eur J Obstet Gynecol Reprod Biol 2014;180:35-9. [Crossref] [PubMed]
- Levy G, Hill MJ, Beall S, et al. Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances. J Assist Reprod Genet 2012;29:703-12. [Crossref] [PubMed]
- Wright KN, Laufer MR. Leiomyomas in adolescents. Fertil Steril 2011;95:2434.e15-7. [Crossref] [PubMed]
- Shin DG, Yoo HJ, Lee YA, et al. Recurrence factors and reproductive outcomes of laparoscopic myomectomy and minilaparotomic myomectomy for uterine leiomyomas. Obstet Gynecol Sci 2017;60:193-9. [Crossref] [PubMed]
- Şükür YE, Kankaya D, Ateş C, et al. Clinical and histopathologic predictors of reoperation due to recurrence of leiomyoma after laparotomic myomectomy. Int J Gynaecol Obstet 2015;129:75-8. [Crossref] [PubMed]
- Sacco Casamassima MG, Gause C, Goldstein SD, et al. Patient Satisfaction After Minimally Invasive Repair of Pectus Excavatum in Adults: Long-Term Results of Nuss Procedure in Adults. Ann Thorac Surg 2016;101:1338-45. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



