
The comparison between young and old patients and the prognostic roles of magnetic resonance imaging-related parameter characteristics in young patients—a retrospective cohort study
Highlight box
Key findings
• Parameters related to breast dynamic enhancement MRI in young breast cancer patients are specific.
What is known and what is new?
• Breast dynamic enhanced MRI is an important examination in the diagnosis and treatment of breast cancer, and it is of great clinical significance for clinicians to evaluate surgical plans;
• An increased proportion of young breast cancer patients presented with non-mass enhancement and a decreased apparent diffusion coefficient, which is associated with clinical features and prognosis.
What is the implication, and what should change now?
• The parameters related to breast dynamic enhancement MRI in young breast cancer patients are specific, which provides a reference for further evaluation of the characteristics of young breast cancer patients.
Introduction
The incidence of breast cancer is increasing year by year, with an incidence rate which currently ranks first among malignant tumors (1). In recent years, studies have found that the age-related incidence of breast cancer is tending to be younger (2,3). Young breast cancer patients refer to breast cancer patients under the age of 40, and the current studies have found that young breast cancer patients have different clinical features, manifested by a higher positive rate of human epidermal growth factor receptor 2 (HER-2), a lower positive rate of estrogen receptor (ER), and a worse prognosis (4-6). Magnetic resonance imaging (MRI) is currently mainly used for staging assessment and preoperative evaluation in breast cancer, which has great advantages in finding small lesions, evaluating the extent of lesions, predicting the efficacy of neoadjuvant chemotherapy and evaluating prognosis (7,8). Due to the specific clinical features of young breast cancer patients, we speculated that parameters related to breast dynamic enhancement MRI may also be specific. However, there is a lack of relevant studies, so we designed this study to investigate the characteristics of dynamic enhancement MRI parameters in young breast cancer patients and their correlation with clinical features. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-84/rc).
Methods
General information
The present study was a retrospective cohort study. A total of 196 breast cancer patients admitted to the People’s Hospital of Zhaoyuan City from January 2017 to December 2017 were retrospectively collected, and the patients were divided into a young breast cancer group (n=56) and a control group (n=140) according to whether the patient’s age was <40 years old. The clinical and pathological features of the 2 groups were compared. The inclusion criteria were as follows: (I) female patients with invasive breast cancer; (II) newly diagnosed patients who did not receive special treatments such as neoadjuvant therapy before surgery; (III) age ≥18 years; (IV) breast dynamic enhancement MRI examination in our hospital, with complete information. The exclusion criteria were as follows: (I) carcinoma in situ or benign mass; (II) inflammatory breast cancer; (III) special types of breast cancer such as metaplastic carcinoma; (IV) combined with other malignant tumors; (V) recurrent breast cancer; (VI) distant metastases. This study was in accordance with the Declaration of Helsinki (revised in 2013), and this retrospective clinical study was approved by The Ethics Committee of People’s Hospital of Zhaoyuan City (No. 202200842). The informed consent requirement was waived for this retrospective study. A flowchart of patient inclusion is shown in Figure 1.
Examination method of breast dynamic enhancement MRI
Examination instrument: Siemens 3.0T MRI (Siemens, Erlangen, Germany); contrast agent: gadolinium acid glucosamine, dose 0.2 mmol/kg (intravenous injection, flow rate: 2.5 mL/s). A total of 6 consecutive acquisitions were performed before and after enhancement.
Data collection
- General information: age, site of onset, smoking history, alcohol history, body mass index (BMI), family history, comorbidities;
- MRI characteristics: mass enhancement features (non-mass enhancement or mass enhancement), early enhancement rate, enhancement platform, apparent diffusion coefficient (ADC);
- Pathological features: lesion size, ER, progesterone receptor (PR), Ki-67 (%), and HER-2 positive rate, lymph node metastasis rate and skin or chest wall invasion rate. We confirmed the presence of lymph node metastasis and skin or chest wall invasion based on the pathology;
- Prognostic factor: rate of recurrence or metastasis at 5 years postoperatively. After the surgery, all patients were followed up by clinical visits at least once a year. The patients received breast ultrasound, chest computed tomography examination, head computed tomography examination, breast MRI and other examinations to observe the rate of recurrence or metastasis at 5 years postoperatively.
Treatment method
After admission, all patients completed relevant examinations, underwent radical mastectomy, and were provided symptomatic supportive treatment such as early functional exercise, prevention of infection, and maintenance of water-electrolyte balance after surgery. Within 1 month after surgery, the patients received chemotherapy, radiotherapy, targeted drug therapy, or endocrine therapy according to the postoperative pathological results.
Statistical analysis
The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used to complete the data analysis, and the difference was considered statistically significant when P<0.05 (two-tailed). The BMI and other measurement data of the 2 groups were expressed by mean ± standard deviation, and the independent sample t-test was used to analyze the differences of the measurement data between the 2 groups. The counting data of the 2 groups were expressed by n (%), and the chi-square test was used to analyze the difference of the counting data between the 2 groups. The receiver operating characteristic (ROC) curve was used to analyze the predictive value of ADC on recurrence or metastasis in breast cancer patients at 5 years after surgery.
Results
Comparison of clinical features and parameters related to breast dynamic enhancement MRI in the 2 groups
Compared with the control group, the ADC of the young breast cancer group was significantly reduced (0.84±0.13 vs. 0.93±0.14 ×10−3 mm2/s, P<0.001); the proportion of patients with non-mass enhancement was significantly increased in the young breast cancer group (25.00% vs. 8.57%, P=0.002); the maximum tumor diameter was increased in the young breast cancer group (3.14±1.07 vs. 2.26±1.07 cm, P<0.001); the rate of recurrence or metastasis was increased at 5 years after surgery in the young breast cancer group (16.07% vs. 5.00%, P=0.011) (Table 1 and Figure 2).
Table 1
| Group | Young breast cancer group (n=56) | Control group (n=140) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 31.48±5.31 | 60.19±11.85 | 17.429 | <0.001 |
| Site of onset | 0.207 | 0.649 | ||
| Left | 30 (53.57) | 80 (57.14) | ||
| Right | 26 (46.43) | 60 (42.86) | ||
| History of smoking | 6 (10.71) | 14 (10.00) | 0.022 | 0.881 |
| History of alcoholism | 4 (7.14) | 9 (6.43) | 0.033 | 0.856 |
| Body mass index (kg/m2) | 24.04±2.37 | 24.14±2.31 | 0.253 | 0.800 |
| Family history | 3 (5.36) | 6 (4.29) | 0.105 | 0.746 |
| Hypertensive disease | 4 (7.14) | 9 (6.43) | 0.033 | 0.856 |
| Diabetes | 3 (5.36) | 8 (5.71) | 0.010 | 0.922 |
| Apparent diffusion coefficient (×10−3 mm2/s) | 0.84±0.13 | 0.93±0.14 | 4.266 | <0.001 |
| Mass enhancement features | 9.383 | 0.002 | ||
| Non-mass enhancement | 14 (25.00) | 12 (8.57) | ||
| Mass enhancement | 42 (75.00) | 128 (91.43) | ||
| Early enhancement rate (%) | 0.662 | 0.416 | ||
| >120 | 30 (53.57) | 66 (47.14) | ||
| ≤120 | 26 (46.43) | 74 (52.86) | ||
| Enhancement platform | 0.002 | 0.964 | ||
| Outflow type | 31 (55.36) | 77 (55.00) | ||
| Platform type | 25 (44.64) | 63 (45.00) | ||
| Inflow type | 0 (0.00) | 0 (0.00) | ||
| Maximum tumor diameter (cm) | 3.14±1.07 | 2.26±1.07 | 5.180 | <0.001 |
| Positive HER-2 rate | 17 (30.36) | 27 (19.29) | 2.186 | 0.093 |
| Positive Ki-67 rate | 51 (91.07) | 125 (89.29) | 0.139 | 0.709 |
| Positive ER rate | 48 (85.71) | 131 (93.57) | 3.117 | 0.077 |
| Positive PR rate | 50 (89.29) | 128 (91.43) | 0.220 | 0.639 |
| Lymph node metastases | 22 (39.29) | 41 (29.29) | 1.834 | 0.176 |
| Invasion of the skin or chest wall | 3 (5.36) | 7 (5.00) | 0.011 | 0.198 |
| Rate of recurrence or metastasis at 5 years postoperatively | 9 (16.07) | 7 (5.00) | 6.540 | 0.011 |
Data are presented as mean ± standard deviation or n (%). MRI, magnetic resonance imaging; HER-2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
Correlation analysis between ADC and age, maximum tumor diameter
The ADC was significantly positively correlated with age (r=0.226, P=0.001), and negatively correlated with the maximum tumor diameter (r=−0.199, P=0.005) (Table 2).
Table 2
| Age (years) | Apparent diffusion coefficient (×10−3 mm2/s) | Maximum tumor diameter (cm) | ||||||
|---|---|---|---|---|---|---|---|---|
| r value | P value | r value | P value | r value | P value | |||
| Age (years) | – | – | 0.226 | 0.001 | −0.312 | <0.001 | ||
| Apparent diffusion coefficient (×10−3 mm2/s) | 0.226 | 0.001 | – | – | −0.199 | 0.005 | ||
| Maximum tumor diameter (cm) | −0.312 | <0.001 | −0.199 | 0.005 | – | – | ||
ADC, apparent diffusion coefficient.
Predictive value of ADC on the absence of lymph node metastasis in young breast cancer patients
The ADC was found to be valuable in predicting the absence of lymph node metastasis in young breast cancer patients, and the area under the curve (AUC) was 0.817 [95% confidence interval (CI): 0.702–0.932, P<0.001] (Figure 3).
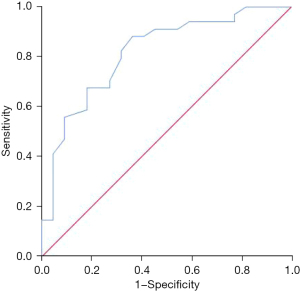
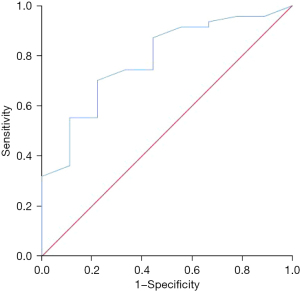
Predictive value of ADC on the absence of recurrence or metastasis in young breast cancer patients
The ADC was shown to be valuable in predicting the absence of recurrence or metastasis at 5 years postoperatively, and the AUC was 0.784 (95% CI: 0.630–0.937, P=0.007) (Figure 4).
Correlation analysis of mass enhancement characteristics and lymph node metastasis, recurrence or metastasis in young breast cancer patients
Lymph node metastasis and recurrence or metastasis at 5 years postoperatively were significantly increased in the young breast cancer patients with non-mass enhancement (P<0.05) (Table 3).
Table 3
| Group | Non-mass enhancement (n=14) | Mass enhancement (n=42) | χ2 value | P value |
|---|---|---|---|---|
| Lymph node metastases | 4.891 | 0.027 | ||
| Yes | 9 (64.29) | 13 (30.95) | ||
| No | 5 (35.71) | 29 (69.05) | ||
| Recurrence or metastasis at 5 years postoperatively | 5.340 | 0.021 | ||
| Yes | 5 (35.71) | 4 (9.52) | ||
| No | 9 (64.29) | 38 (90.48) | ||
Discussion
Since the clinical features of young breast cancer patients are different from those of other patients, we speculated that parameters related to breast dynamic enhancement MRI may also be specific in young breast cancer patients. To confirm this hypothesis, we designed this study and showed that younger breast cancer patients had a lower ADC and a higher proportion of patients with non-mass enhancement compared to other patients.
ADC is an indicator of water molecule movement in the tissue, which can reflect the density of tumor cell arrangement, cell matrix, and cell membrane integrity. When the tumor cell density is high, the movement of water molecules is restricted, which is manifested by a decrease in the ADC (9,10). The lower the ADC, the higher the tumor cell density in breast cancer patients, and the more likely they are to have lymph node metastasis, postoperative recurrence, or metastasis (11,12). Studies on patients with other malignancies have also confirmed that the ADC were associated with lymph node metastasis (13-15). In the present study, the ADC had a high predictive value for the absence of lymph node metastasis, and the AUC was 0.817 (95% CI: 0.702–0.932, P<0.001). Another study on breast cancer patients also confirmed that a decrease in the ADC was associated with postoperative recurrence or metastasis (16), supporting the present study. This study showed that the ADC was valuable in predicting the absence of recurrence or metastasis in the young breast cancer patients, with an AUC of 0.784 (95% CI: 0.630–0.937, P=0.007). Studies on patients with other malignancies have also shown that the ADC was associated with recurrence or metastasis (17-19). It can be seen from the above that the ADC of young breast cancer patients was reduced, which indicated that the tumor cell density of young breast cancer patients was high, and it was related to the poor prognosis of young breast cancer patients. In addition, this study also showed that the ADC of breast cancer patients was negatively correlated with the maximum tumor diameter, which indicated that the lower the ADC, the larger the tumor. This had also been confirmed by a study in patients with renal cancer (20). A previous study also showed that ADC value was valuable in predicting the pathologic complete response in breast cancer receiving the neoadjuvant chemotherapy (21).
According to the morphological characteristics of breast dynamic enhancement MRI enhancement, breast lesions can be divided into punctiform enhancement, mass enhancement, and non-mass enhancement. Punctiform enhancement lesions refer to enhancement lesion less than 5 mm, most of which are benign lesions, so punctiform enhancement is rare in patients with invasive breast cancer. The mass enhancement is a space-occupying lesion, which was most commonly seen in patients with invasive breast cancer. Non-mass enhancement can be benign or malignant. Patients with non-mass enhancement lesions are scattered and more extensive, making them more prone to recurrence and metastasis (22,23). The present study showed that non-mass enhancement was characterized by a significantly increased rate of lymph node metastasis, recurrence, or metastasis at 5 years postoperatively in young breast cancer patients compared with mass enhancement breast cancer (P<0.05).
A study which explored the characteristics of breast dynamic enhanced MRI in young breast cancer patients found that young breast cancer patients have more extensive lesions and are associated with poor prognosis (24). Another study showed that young patients with triple-negative breast cancer had different MRI characteristics compared with other young breast cancer patients (25). This illustrated the unique characteristics of breast dynamic enhancement nuclear magnetic resonance (NMR) characteristics in young breast cancer patients. Studying the dynamic enhancement MRI characteristics of young breast cancer patients is conducive to further revealing the lesion characteristics of young breast cancer patients, which is helpful for fine management of young breast cancer patients. But further studies are still needed to confirm the clinical values.
Shortcomings
This was a retrospectively clinical study that included a relative limited number of young breast cancer patients, which was likely to cause some deviations in the results. Therefore, the results needed to be further confirmed by large sample multi-center clinical trials. Moreover, only 16 patients in the present study suffered from postoperative recurrence or metastasis, Therefore, COX regression analysis cannot be carried out.
Conclusions
The research on the prognosis and related biological indicators of different diseases is the focus of current research (26-30). The parameters of breast dynamic enhancement MRI in young breast cancer patients are specific, which is manifested by an increased proportion of patients with non-mass enhancement, a decrease in the ADC, and is related to the clinical features and prognosis of patients. This provides a reference for further evaluation of the characteristics of young breast cancer patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-84/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-84/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-84/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-84/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was in accordance with the Declaration of Helsinki (revised in 2013), and this retrospective clinical study was approved by The Ethics Committee of People’s Hospital of Zhaoyuan City (No. 202200842). The informed consent requirement was waived for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022;72:524-41. [Crossref] [PubMed]
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022;66:15-23. [Crossref] [PubMed]
- Wang W, Tian B, Xu X, et al. Clinical features and prognostic factors of breast cancer in young women: a retrospective single-center study. Arch Gynecol Obstet 2023;307:957-68. [Crossref] [PubMed]
- Eren SK, Arslan A, Çalışkan EÇ, et al. Comparison of clinical features and the impact of reproductive factors on by age at diagnosis young and elderly breast cancer patients in the middle Anatolian region of Turkey. Eur Rev Med Pharmacol Sci 2022;26:2227-37. [PubMed]
- Birnbaum Z, Jones G, Diaz G, et al. Association of socioeconomic status with the clinical management and outcomes in young patients (≤35 years) diagnosed with breast cancer: A retrospective analysis. Ann Med Surg (Lond) 2022;82:104524. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- You C, Xiao Q, Zhu X, et al. The clinicopathological and MRI features of patients with BRCA1/2 mutations in familial breast cancer. Gland Surg 2021;10:262-72. [Crossref] [PubMed]
- Wang Z, Ren GY, Yin Q, et al. Correlation of magnetic resonance imaging quantitative parameters and apparent diffusion coefficient value with pathological breast cancer. World J Clin Cases 2022;10:7333-40. [Crossref] [PubMed]
- Mori N, Inoue C, Tamura H, et al. Apparent diffusion coefficient and intravoxel incoherent motion-diffusion kurtosis model parameters in invasive breast cancer: Correlation with the histological parameters of whole-slide imaging. Magn Reson Imaging 2022;90:53-60. [Crossref] [PubMed]
- De Cataldo C, Bruno F, Palumbo P, et al. Apparent diffusion coefficient magnetic resonance imaging (ADC-MRI) in the axillary breast cancer lymph node metastasis detection: a narrative review. Gland Surg 2020;9:2225-34. [Crossref] [PubMed]
- Luo N, Su D, Jin G, et al. Apparent diffusion coefficient ratio between axillary lymph node with primary tumor to detect nodal metastasis in breast cancer patients. J Magn Reson Imaging 2013;38:824-8. [Crossref] [PubMed]
- Belfiore MP, Nardone V, D'Onofrio I, et al. Diffusion-weighted imaging and apparent diffusion coefficient mapping of head and neck lymph node metastasis: a systematic review. Explor Target Antitumor Ther 2022;3:734-45. [Crossref] [PubMed]
- Li C, Yin J. Radiomics Based on T2-Weighted Imaging and Apparent Diffusion Coefficient Images for Preoperative Evaluation of Lymph Node Metastasis in Rectal Cancer Patients. Front Oncol 2021;11:671354. [Crossref] [PubMed]
- Zhou Y, Zhou G, Gao X, et al. Apparent diffusion coefficient value of mass-forming intrahepatic cholangiocarcinoma: a potential imaging biomarker for prediction of lymph node metastasis. Abdom Radiol (NY) 2020;45:3109-18. [Crossref] [PubMed]
- Thakur SB, Durando M, Milans S, et al. Apparent diffusion coefficient in estrogen receptor-positive and lymph node-negative invasive breast cancers at 3.0T DW-MRI: A potential predictor for an oncotype Dx test recurrence score. J Magn Reson Imaging 2018;47:401-9. [Crossref] [PubMed]
- Wang L, Feng B, Wang S, et al. Diagnostic value of whole-tumor apparent diffusion coefficient map radiomics analysis in predicting early recurrence of solitary hepatocellular carcinoma ≤ 5 cm. Abdom Radiol (NY) 2022;47:3290-300. [Crossref] [PubMed]
- Oh HC, Hong CK, Yoo J, et al. The role of apparent diffusion coefficient as a predictive factor for tumor recurrence in patients with cerebellopontine angle epidermoid tumor. Neurosurg Rev 2022;45:1383-92. [Crossref] [PubMed]
- Mytsyk Y, Borzhiyevskyy A, Dutka I, et al. Local recurrence of renal cell carcinoma after partial nephrectomy: applicability of the apparent diffusion coefficient of MRI as an imaging marker - a multicentre study. Pol J Radiol 2022;87:e325-32. [Crossref] [PubMed]
- Maruyama M, Yoshizako T, Uchida K, et al. Comparison of utility of tumor size and apparent diffusion coefficient for differentiation of low- and high-grade clear-cell renal cell carcinoma. Acta Radiol 2015;56:250-6. [Crossref] [PubMed]
- Liang X, Chen X, Yang Z, et al. Early prediction of pathological complete response to neoadjuvant chemotherapy combining DCE-MRI and apparent diffusion coefficient values in breast Cancer. BMC Cancer 2022;22:1250. [Crossref] [PubMed]
- Zhao Q, Xie T, Fu C, et al. Differentiation between idiopathic granulomatous mastitis and invasive breast carcinoma, both presenting with non-mass enhancement without rim-enhanced masses: The value of whole-lesion histogram and texture analysis using apparent diffusion coefficient. Eur J Radiol 2020;123:108782. [Crossref] [PubMed]
- Avendano D, Marino MA, Leithner D, et al. Limited role of DWI with apparent diffusion coefficient mapping in breast lesions presenting as non-mass enhancement on dynamic contrast-enhanced MRI. Breast Cancer Res 2019;21:136. [Crossref] [PubMed]
- Bitencourt AGV, Eugênio DSG, Souza JA, et al. Prognostic significance of preoperative MRI findings in young patients with breast cancer. Sci Rep 2019;9:3106. [Crossref] [PubMed]
- Li Q, Dormer J, Daryani P, et al. Radiomics Analysis of MRI for Predicting Molecular Subtypes of Breast Cancer in Young Women. Proc SPIE Int Soc Opt Eng 2019;10950:1095044.
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Qiu Y, Chen H, Dai Y, et al. Nontherapeutic Risk Factors of Different Grouped Stage IIIC Breast Cancer Patients' Mortality: A Study of the US Surveillance, Epidemiology, and End Results Database. Breast J 2022;2022:6705052. [Crossref] [PubMed]
- Qiu Y, Chen Y, Zhu L, et al. Differences of Clinicopathological Features between Metaplastic Breast Carcinoma and Nonspecific Invasive Breast Carcinoma and Prognostic Profile of Metaplastic Breast Carcinoma. Breast J 2022;2022:2500594. [Crossref] [PubMed]
- Chen H, Meng X, Hao X, et al. Correlation Analysis of Pathological Features and Axillary Lymph Node Metastasis in Patients with Invasive Breast Cancer. J Immunol Res 2022;2022:7150304. [Crossref] [PubMed]
- Chen Y, Si H, Bao B, et al. Integrated analysis of intestinal microbiota and host gene expression in colorectal cancer patients. 2022;71:
10.1099/jmm.0.001596 .
(English Language Editor: J. Jones)



