
Analysis of risk factors for negative emotions in perioperative period of ovarian cancer patients and their impact on prognosis
Highlight box
Key findings
• Negative emotions can significantly affect the prognosis of ovarian cancer (OC) patients. Clinically, patients should be communicated with and helped to grasp relevant medical knowledge in order to stabilize their emotions in order to improve their prognosis.
What is known and what is new?
• Patients with OC have a higher incidence of negative emotions.
• Negative emotions can lead to higher rates of complications and reduced quality of life in the patients with OC.
What is the implication, and what should change now?
• During the treatment of OC, in addition to paying attention to the patient’s condition, attention should be paid to the patient’s mood and early detection of relevant risk factors. Relevant psychological care should be provided to relieve patients’ anxiety and depression when necessary, so as to improve patients’ prognosis and postoperative quality of life.
Introduction
Ovarian cancer (OC) is a malignant tumor that can occur in any histological part of the ovary, including the epithelium, stroma, or germ cells. Among these, high-grade plasmacytoma arising from ovarian epithelium is the most common type (1). Of gynecologic cancers, OC has the third highest incidence after cervical cancer and uterine corpus cancer, accounting for approximately 4% of all malignant tumors in women (2). According to global cancer statistics, in 2020 there were 310,000 new cases of OC and 200,000 deaths worldwide, and its incidence and mortality rate ranked 8th among female malignancies (3). In China, about 50,000 women are diagnosed with OC every year (4). The occurrence of OC is insidious, and effective screening and early diagnosis measures are lacking. At the time of diagnosis, 75% of OC patients are already at an advanced stage (5). Depending on the type of OC pathology, the 5-year survival rate of Federation International of Gynecology and Obstetrics (FIGO) stage III and IV patients ranges from 23.9% to 37.0% (6). The prognosis of advanced OC is poor, with a 5-year survival rate of less than 20% (7,8). Moreover, it has been shown that even after complete remission with first-line therapy, approximately 70–85% of OC patients will experience recurrence, and the median survival of these patients is 12–24 months (9). In addition, OC is highly invasive and metastatic, readily transporting tumor cells to many organs and tissues throughout the body by blood and lymphatic metastasis, which can lead to various adverse symptoms, including thromboembolism, blood complications, and infections, among others. The complications caused by cancer metastasis or invasion are also the main causes of death in OC patients (10,11). Therefore, OC remains one of the most dangerous gynecologic cancers and a serious threat to women’s lives and health. However, the etiology of OC is still unclear, and its development may be related to age, fertility, blood type, psychological factors, and environment (12).
Current clinical treatment for OC involves surgery supplemented with chemotherapy, and there is an emphasis on comprehensive treatment. However, the disease itself and its treatment process can produce many adverse effects (13). The treatment process for OC is relatively long, and the initial treatment of surgery and chemotherapy can cause serious physical and psychological burdens (14). A study has shown that OC patients suffer from severe long-term fatigue, depression, neuropathy, and sleep disorders after treatment (15). In addition, patients with OC may experience negative emotions during the perioperative period due to their instinctive fear of malignancy or inadequate knowledge of OC itself and the treatment process. Another common reason for patients’ negative emotions is their fear and worry about tumor recurrence, which is common among OC patients (16,17). For OC patients, fear of recurrence or progression raises the high likelihood of cancer recurrence (18). A study by Li et al. (19) showed that depressive symptoms in OC patients prior to diagnosis were associated with decreased OC survival. Another study showed that while many OC survivors reported few unmet physical needs, they recognized a large number of unmet psychological needs (20). These studies indicate that the importance of quality of life and psychological well-being of OC patients is receiving increasing attention. However, there are fewer studies on the impact of negative emotions on patient prognosis in perioperative OC patients. The aim of this study was to compare the clinical characteristics and postoperative complications between patients with and without negative emotions and to elucidate the independent risk factors affecting patients’ negative emotions and prognosis to provide a reference for the early identification of high-risk groups in clinical practice and the adoption of targeted measures to reduce postoperative complications, improve patients’ prognosis, and enhance their postoperative quality of life. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-94/rc).
Methods
Research participants
A total of 258 OC patients admitted to the Affiliated Hospital of Jiangnan University between August 2014 and December 2019 were included in this study.
The inclusion criteria were: (I) patients diagnosed with OC by medical history, symptoms, physical signs, and adjuvant and histopathological examinations, and patients had obvious indications for surgery and chemotherapy; (II) patients and family members signed informed consent, had good compliance, and could actively cooperate with medical and nursing staff for corresponding treatment and care; (III) patients had good understanding and cooperation ability; and (IV) clinical data were complete.
The exclusion criteria were: (I) poor condition of patient or combined with systemic diseases such as serious heart disease, malignant tumors of other organ systems, or could not tolerate the surgery and chemotherapy process; (II) the patient had certain neurological disorders, a history of mental illness, or the patient and family members could not actively cooperate with the relevant care measures; and (III) clinical case information was incomplete (Figure 1).
Binary logistic regression requires a ratio of item number to sample size of 1:5–1:10. Therefore, the sample size of the study population planned for this study was 280 cases. On the basis of the exclusion criteria, 22 cases were excluded, including 8 lost to follow-up, and 258 cases were finally included in the study. The study was approved by the ethics committee of The Affiliated Hospital of Jiangnan University (No. KYLC2014262). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all patients.
General information questionnaire
The general information questionnaire included demographic data [gender, age, body mass index (BMI), address, monthly family income, education level, fertility, and marital status] and clinical data (presence of hypertension, hyperlipidemia, diabetes, pathological type of OC, FIGO staging, maximum tumor diameter, pathological histological type, presence of ascites, presence of lymph node metastasis, residual postoperative lesions, postoperative chemotherapy or not, time to postoperative bowel function recovery, and length of hospitalization, among others).
Negative emotion assessment
A self-rating anxiety scale (SAS) was used to reflect the existence and degree of anxiety, which consisting of 20 items, each rated from 1–4. The scores for each item were summed to obtain a crude score and then multiplied by 1.25 to provide a standard score. The SAS scores of 50–59, 60–69, and ≥70 were defined as mild, moderate, and severe anxiety, respectively. The scale had good reliability and validity, and Cronbach’s α coefficients were above 0.75.
A self-rating depression scale (SDS) was used to reflect the existence and degree of depression, also consisting of 20 items. An SDS score >52 was used as the evaluation criterion. An SDS score of 53–62, 63–72, and >72 were defined as mild, moderate, and severe depression, respectively. The SDS had good reliability and validity, and Cronbach’s α coefficients were above 0.75. Whether the patient has anxiety or depression or both, it is defined as co-negative emotion. All patients were assessed by SAS and SDS 1 day before discharge.
Postoperative recurrence
Postoperative recurrence was defined as any 2 of the following occurring after 6 months of complete remission with treatment: elevated tumor marker levels, mass detected by computed tomography (CT)/magnetic resonance imaging (MRI), mass on physical examination, unexplained bowel obstruction, and the presence of pleural effusion or ascites. The last follow-up occurred in December 2022.
Ovarian function assessment
The ovarian cross-sectional area, resistance index (RI), peak systolic blood flow velocity (PSV), and end-diastolic blood flow velocity (EDV) were measured by transvaginal color Doppler ultrasound before surgery and 3 months after surgery. The last follow-up occurred in March 2020.
Prognostic assessment of Functional Assessment of Cancer Therapy-Ovarian (FACT-O)
The FACT-O (21) consists of a core scale for all cancer patients (the Functional Assessment of Cancer Therapy-General, FACT-G V4.0) and an additional 12-item module for OC. The assessment contains 39 items in 5 domains, including physical status (7 items), social/family status (7 items), emotional status (6 items), functional status (7 items), and an OC-specific module (12 items). The quality-of-life questionnaire was administered to patients during follow-up assessments at two different stages of treatment, 1 month postoperatively and 6 months postoperatively. This scale has been used worldwide and has good reliability, validity, and response.
Each item was scored on a five-point scale from 0 to 4 according to the degree of disease impact, i.e., “not at all, some, average, quite a lot, very much”. The scores of the corresponding items were summed, with high scores indicating high quality of life and low scores indicating low quality of life. Some items in the middle were inverse items, and positive conversion was performed when calculating the scores of these items. The last follow-up visit occurred in June 2020.
Statistical analysis
The results of each scale were entered into a computer for score conversion, and statistical analysis was performed using SPSS 26 (IBM Corp., Armonk, NY, USA). Measurement data are expressed as mean and standard deviation, and count data are expressed as frequencies and percentages. Statistical analysis between the two groups (with and without negative emotions) was performed using t-test and chi-square test, and independent risk factors for assessing patients’ prognosis were analyzed using binary logistics regression. P<0.05 was considered statistically significant.
Results
Baseline data
Baseline data of the patients are shown in Table 1. A total of 258 patients with OC were included in this study. There were significant differences in SAS and SDS scores between the two groups in terms of age, marital status, presence of diabetes, lymph node metastasis, postoperative chemotherapy, and time to postoperative bowel function recovery in patients with or without combined negative emotions (P<0.05). For age, the 124 patients (48.1%) with age <40 had a mean SAS score of 45.36±6.65 and a mean SDS score of 46.00±7.29, while the 134 patients (51.9%) with age ≥40 had a mean SAS score of 43.89±5.30 and a mean SDS score of 43.73±6.11. The 54 cases (20.9%) with combined diabetes had a mean SAS score of 46.35±4.91 and mean SDS score of 46.91±7.16, while the 204 cases (79.8%) without combined diabetes had a mean SAS score of 44.13±6.21 and mean SDS score of 44.27±6.59. For marital status, the 20 patients (7.8%) who did not have a spouse had a mean SAS score of 47.50±5.71 and a mean SDS score of 49.90±6.46, both of which were significantly higher than those for patients with a spouse (P<0.05). A total of 66 patients (25.6%) had lymph node metastasis, with a mean SAS score of 47.33±6.63 and a mean SDS score of 47.27±7.08, while the 192 patients (74.4%) who did not have lymph node metastasis had a mean SAS score of 43.66±5.51 and a mean SDS score of 43.98±6.49. The 109 patients (42.2%) who received chemotherapy after surgery had a mean SAS score of 45.28±5.83 and a mean SDS score of 46.30±7.01, both of which were significantly higher than for those who did not receive chemotherapy (P<0.05). A total of 96 patients (37.2%) whose postoperative bowel function recovery time was ≥24 hours had a mean SAS score of 45.19±5.73 and a mean SDS score of 46.06±7.19, which were significantly higher than those who had passed gas within 24 hours of surgery (P<0.05). For combined hyperlipidemia, there was a significant difference between the two groups in terms of SDS score only (P<0.05). A total of 31 patients (12.0%) had combined hyperlipidemia and their mean SDS score was 42.13±6.58, while the 227 patients (88.0%) who did not have combined hyperlipidemia had a mean SDS score of 45.19±6.75. For SAS scores, there were significant differences between the two groups in terms of the presence of children, educational level, monthly household income, and length of hospitalization (P<0.05). Among the patients, 17 (6.6%) had no children, and their mean SAS score was 48.82±6.47, while 241 patients (93.4%) had children, and their mean SAS score was 44.30±5.89, which was significantly lower than the score of those without children. The 86 patients (33.3%) with high school or higher education had a mean SAS score of 46.13±6.34, and the 172 patients (66.7%) with high school or lower education had a mean SAS score of 43.83±5.72. The mean SAS score was 45.41±5.83 for the 135 patients (52.3%) with monthly family income <5,000 and 43.70±6.12 for the 123 patients (47.7%) with monthly family income ≥5,000. A total of 113 patients (43.8%) were hospitalized for ≥10 days, and their mean SAS score was 45.51±6.47, while 145 patients (56.2%) were hospitalized for <10 days, and their mean SAS score was 43.88±5.56.
Table 1
| Item | N (%) | SAS | SDS | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | t | P | Mean ± SD | t | P | |||
| Age (years) | 1.977 | 0.049 | 2.716 | 0.007 | ||||
| <40 | 124 (48.1) | 45.36±6.65 | 46.00±7.29 | |||||
| ≥40 | 134 (51.9) | 43.89±5.30 | 43.73±6.11 | |||||
| BMI (kg/m2) | 0.058 | 0.954 | 1.590 | 0.113 | ||||
| <24 | 136 (52.7) | 44.62±6.38 | 45.46±6.92 | |||||
| ≥24 | 122 (47.3) | 44.57±5.63 | 44.11±6.60 | |||||
| Have hypertension or not | 0.055 | 0.956 | 0.006 | 0.995 | ||||
| Yes | 88 (34.1) | 44.57±5.84 | 44.82±6.81 | |||||
| No | 170 (65.9) | 44.61±6.13 | 44.82±6.80 | |||||
| Have hyperlipidemia or not | 0.970 | 0.333 | 2.377 | 0.018 | ||||
| Yes | 31 (12.0) | 43.61±7.38 | 42.13±6.58 | |||||
| No | 227 (88.0) | 44.73±5.82 | 45.19±6.75 | |||||
| Have diabetes or not | −2.432 | 0.016 | −2.568 | 0.011 | ||||
| Yes | 54 (20.9) | 46.35±4.91 | 46.91±7.16 | |||||
| No | 204 (79.8) | 44.13±6.21 | 44.27±6.59 | |||||
| Fertile or not | 3.043 | 0.003 | 1.223 | 0.223 | ||||
| Have children | 241 (93.4) | 44.30±5.89 | 44.68±6.76 | |||||
| No children | 17 (6.6) | 48.82±6.47 | 46.76±7.01 | |||||
| Marital status | 2.263 | 0.024 | 3.563 | 0.000 | ||||
| Have spouse | 238 (92.2) | 44.35±5.99 | 44.39±6.65 | |||||
| No spouse | 20 (7.8) | 47.50±5.71 | 49.90±6.46 | |||||
| Education level | −2.930 | 0.004 | −1.195 | 0.233 | ||||
| High school and below | 172 (66.7) | 43.83±5.72 | 44.47±6.67 | |||||
| High school or above | 86 (33.3) | 46.13±6.34 | 45.53±6.99 | |||||
| Address | −0.177 | 0.859 | 0.423 | 0.673 | ||||
| Countryside | 135 (52.3) | 44.53±5.84 | 44.99±7.16 | |||||
| City | 123 (47.7) | 44.67±6.24 | 44.63±6.37 | |||||
| Monthly household income (yuan) | 2.305 | 0.022 | 1.289 | 0.199 | ||||
| <5,000 | 135 (52.3) | 45.41±5.83 | 45.34±7.06 | |||||
| ≥5,000 | 123 (47.7) | 43.70±6.12 | 44.25±6.45 | |||||
| Types of ovarian cancer | −0.451 | 0.653 | 0.869 | 0.386 | ||||
| Plasmacytoid | 108 (41.9) | 44.80±5.77 | 44.39±6.21 | |||||
| Non-plasmacytoid | 150 (58.1) | 44.45±6.21 | 45.13±7.18 | |||||
| FIGO staging | 0.727 | 0.468 | −0.228 | 0.820 | ||||
| I–II | 81 (31.4) | 45.00±5.37 | 44.68±6.82 | |||||
| III–IV | 177 (68.6) | 44.41±6.30 | 44.89±6.79 | |||||
| Maximum diameter of tumor | −0.103 | 0.918 | −0.972 | 0.332 | ||||
| ≥3 cm | 44 (17.1) | 44.68±5.46 | 45.73±6.78 | |||||
| <3 cm | 214 (82.9) | 44.58±6.14 | 44.64±6.79 | |||||
| Pathological histological type | 0.476 | 0.634 | 0.966 | 0.335 | ||||
| Low and medium differentiation | 175 (67.8) | 44.72±5.25 | 45.10±7.14 | |||||
| High differentiation | 83 (32.2) | 44.34±7.41 | 44.23±5.98 | |||||
| With ascites or not | −0.265 | 0.791 | −0.934 | 0.351 | ||||
| Yes | 56 (21.7) | 44.79±5.57 | 45.57±6.96 | |||||
| No | 202 (78.3) | 44.54±6.23 | 44.61±6.74 | |||||
| Lymph node metastasis | −4.433 | 0.000 | −3.474 | 0.001 | ||||
| Yes | 66 (25.6) | 47.33±6.63 | 47.27±7.08 | |||||
| No | 192 (74.4) | 43.66±5.51 | 43.98±6.49 | |||||
| Residual postoperative lesion | −0.750 | 0.454 | −1.215 | 0.225 | ||||
| ≥1 cm | 91 (35.3) | 44.98±5.96 | 45.52±5.69 | |||||
| <1 cm | 167 (64.7) | 44.39±6.06 | 44.44±6.83 | |||||
| Postoperative chemotherapy or not | −1.573 | 0.117 | −3.046 | 0.003 | ||||
| Yes | 109 (42.2) | 45.28±5.83 | 46.30±7.01 | |||||
| No | 149 (57.8) | 44.09±6.13 | 43.74±6.43 | |||||
| Time to bowel function recovery | −1.214 | 0.226 | −2.279 | 0.023 | ||||
| ≥24 h | 96 (37.2) | 45.19±5.73 | 46.06±7.19 | |||||
| <24 h | 162 (62.8) | 44.25±6.18 | 44.09±6.45 | |||||
| Length of hospitalization | −2.174 | 0.031 | −1.861 | 0.064 | ||||
| ≥10 d | 113 (43.8) | 45.51±6.47 | 45.71±7.38 | |||||
| <10 d | 145 (56.2) | 43.88±5.56 | 44.13±6.22 | |||||
SAS, self-rating anxiety scale; SDS, self-rating depression scale; SD, standard deviation; BMI, body mass index; FIGO, Federation International of Gynecology and Obstetrics.
Complications in patients with or without negative emotions
Analysis of the results by chi-square test showed that urinary tract infection, irregular bleeding, and pressure ulcers were significantly different (P<0.05) between patients with combined negative emotions and those without negative emotions. Among the patients without combined negative emotions, 5 (38.5%) had urinary tract infections, 7 (36.8%) had irregular postoperative bleeding, and 5 (50.0%) had pressure sores. Among patients with combined negative emotions, 8 (61.5%) developed urinary tract infections, 12 (63.2%) had irregular postoperative bleeding, and 5 (50.0%) had pressure sores (Table 2).
Table 2
| Item | Subcutaneous emphysema | Urinary tract infection | Deep vein thrombosis | Irregular bleeding | Pressure sores | Intestinal adhesions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||||||
| Without negative emotions | 1 (14.3) | 42 (16.7) | 5 (38.5) | 38 (15.5) | 3 (25.0) | 40 (16.3) | 7 (36.8) | 36 (15.1) | 5 (50.0) | 38 (15.3) | 4 (22.2) | 39 (16.3) | |||||
| With negative emotions | 6 (85.7) | 209 (83.3) | 8 (61.5) | 207 (84.5) | 9 (75.0) | 206 (83.7) | 12 (63.2) | 203 (84.9) | 5 (50.0) | 210 (84.7) | 14 (77.8) | 201 (83.8) | |||||
| χ2 | 0.029 | 4.682 | 0.629 | 6.011 | 8.323 | 0.430 | |||||||||||
| P | 0.864 | 0.030 | 0.428 | 0.014 | 0.004 | 0.512 | |||||||||||
Data are shown as n (%).
Binary logistic regression analysis of patients’ negative emotion
Binary logistic regression analysis showed that age, monthly household income, education level, being fertile or not, whether lymph nodes were metastatic, whether postoperative chemotherapy was given, the time to postoperative bowel function recovery, and whether postoperative complications such as irregular bleeding and pressure sores occurred were independent risk factors for patients’ negative emotions (Table 3, Figure 2).
Table 3
| Related factor | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| Age (years) | −1.000 | 0.426 | 5.513 | 0.019 | 0.368 | 0.848 | 0.160 |
| Have diabetes or not | 0.354 | 0.473 | 0.559 | 0.455 | 1.425 | 3.603 | 0.563 |
| Have hyperlipidemia or not | 0.459 | 0.589 | 0.606 | 0.436 | 1.582 | 5.019 | 0.499 |
| Monthly household income (yuan) | −1.159 | 0.432 | 7.189 | 0.007 | 0.314 | 0.732 | 0.135 |
| Education level | 1.059 | 0.406 | 6.794 | 0.009 | 2.885 | 6.399 | 1.301 |
| Fertile or not | −1.495 | 0.671 | 4.969 | 0.026 | 0.224 | 0.835 | 0.060 |
| Have spouse or not | −0.154 | 0.655 | 0.055 | 0.814 | 0.857 | 3.096 | 0.237 |
| Lymph node metastasis | 0.902 | 0.412 | 4.800 | 0.028 | 2.465 | 5.525 | 1.100 |
| Postoperative chemotherapy | 0.839 | 0.417 | 4.054 | 0.044 | 2.314 | 5.238 | 1.023 |
| Time to bowel function recovery | 0.809 | 0.409 | 3.912 | 0.048 | 2.245 | 5.001 | 1.007 |
| Length of hospitalization | 0.424 | 0.419 | 1.022 | 0.312 | 1.528 | 3.473 | 0.672 |
| Urinary tract infection | 0.867 | 0.786 | 1.218 | 0.270 | 2.380 | 11.098 | 0.510 |
| Irregular bleeding | 1.388 | 0.629 | 4.872 | 0.027 | 4.008 | 13.754 | 1.168 |
| Pressure sores | 2.163 | 0.770 | 7.893 | 0.005 | 8.699 | 39.348 | 1.923 |
SE, standard error; OR, odds ratio; CI, confidence interval.
Comparison of ovarian function between the two groups of patients before and after surgery
The results of t-test analysis showed that there was no significant difference between the two groups of patients with and without combined negative emotions in all assessments of preoperative ovarian function. At 3 months postoperatively, there was a significant difference in PSV, RI, and EDV (P<0.05) between the two groups. Among patients with combined negative emotions, mean PSV was 17.57±0.76 cm/s, mean RI was 0.58±0.06, and mean EDV was 7.56±0.58 cm/s. Among patients without combined negative emotions, mean PSV was 17.28±0.66 cm/s, mean RI was 0.61±0.07, and mean EDV was 7.32±0.45 cm/s (Table 4).
Table 4
| Item | Time | With negative emotions | Without negative emotions | t | P |
|---|---|---|---|---|---|
| Ovarian cross-sectional area (cm2) | Preoperative | 4.16±0.54 | 4.20±0.53 | 0.533 | 0.595 |
| 3 months postoperative | 3.84±0.55 | 3.82±0.53 | −0.266 | 0.790 | |
| PSV (cm/s) | Preoperative | 16.34±0.53 | 16.39±0.60 | 0.493 | 0.622 |
| 3 months postoperative | 17.57±0.76 | 17.28±0.66 | −2.593 | 0.010 | |
| RI | Preoperative | 0.69±0.06 | 0.68±0.06 | −1.018 | 0.309 |
| 3 months postoperative | 0.58±0.06 | 0.61±0.07 | 2.758 | 0.006 | |
| EDV (cm/s) | Preoperative | 7.04±0.41 | 7.01±0.42 | −0.521 | 0.603 |
| 3 months postoperative | 7.56±0.58 | 7.32±0.45 | −3.125 | 0.002 |
Data are shown as mean ± SD. PSV, peak systolic blood flow velocity; RI, resistance index; EDV, end-diastolic blood flow velocity; SD, standard deviation.
FACT-O of patients in two groups after surgery in 1 and 6 months
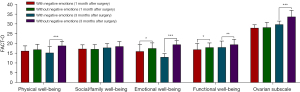
T-test analysis showed statistically significant differences between the two groups in the 3 dimensions of emotional well-being, functional well-being, and FACT-O at 1 month postoperatively (P<0.05). Among the patients with combined negative emotions, the score for emotional well-being was 16.09±3.50, functional well-being was 16.98±3.11, and FACT-O was 94.70±6.61. Meanwhile, among the patients without combined negative emotions, the score for emotional well-being was 17.61±2.76, functional well-being was 18.05±2.31, and FACT-O was 98.09±6.04. Patients with combined negative emotions had significantly lower scores in all 3 areas compared with those without combined negative emotions.
The differences between the two groups were statistically significant (P<0.05) in the 5 dimensions of physical well-being, emotional well-being, functional well-being, ovarian subscale, and FACT-O at 6 months postoperatively. Among the patients with combined negative emotions, the score for physical well-being was 15.35±3.18, emotional well-being was 13.14±1.79, functional well-being was 18.14±3.16, ovarian subscale was 29.81±1.72, and FACT-O score was 94.40±5.12. In patients without combined negative emotions, the score for physical well-being was 18.94±2.21, emotional well-being was 19.54±2.05, functional well-being was 19.53±2.64, ovarian subscale was 33.74±3.25, and FACT-O was 110.38±5.69. Patients with combined negative emotions had significantly lower scores in all 5 areas compared with those without combined negative emotions (Table 5, Figure 3).
Table 5
| Item | Physical well-being | Social/family well-being | Emotional well-being | Functional well-being | Ovarian subscale | FACT-O | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-m after surgery | 6-m after surgery | 1-m after surgery | 6-m after surgery | 1-m after surgery | 6-m after surgery | 1-m after surgery | 6-m after surgery | 1-m after surgery | 6-m after surgery | 1-m after surgery | 6-m after surgery | ||||||
| With negative emotions | 16.28±2.58 | 15.35±3.18 | 17.33±2.06 | 17.95±2.06 | 16.09±3.50 | 13.14±1.79 | 16.98±3.11 | 18.14±3.16 | 28.02±1.61 | 29.81±1.72 | 94.70±6.61 | 94.40±5.12 | |||||
| Without negative emotions | 16.97±2.68 | 18.94±2.21 | 17.19±2.32 | 18.62±2.43 | 17.61±2.76 | 19.54±2.05 | 18.05±2.31 | 19.53±2.64 | 28.28±2.54 | 33.74±3.25 | 98.09±6.04 | 110.38±5.69 | |||||
| t | 1.546 | 7.064 | −0.367 | 1.678 | 2.689 | 19.054 | 2.143 | 3.057 | 0.851 | 11.446 | 3.312 | 17.081 | |||||
| P | 0.123 | 0.000 | 0.714 | 0.094 | 0.010 | 0.000 | 0.037 | 0.002 | 0.397 | 0.000 | 0.001 | 0.000 | |||||
Data are shown as mean ± SD. FACT-O, Functional Assessment of Cancer Therapy-Ovarian; m, months; SD, standard deviation.
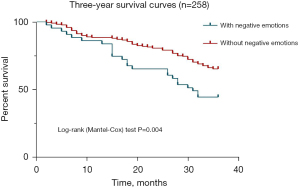
Survival rate and recurrence rate 1–3 years after surgery in two groups
The results of chi-square test analysis showed that there was a significant difference between the two groups of patients in the recurrence rate at 3 years postoperatively and the survival rate at 2 and 3 years postoperatively (P<0.05). The survival rate of patients in the group with negative emotions was 65.1% 2 years after surgery and 44.2% 3 years after surgery, and the survival rate of patients in the group without negative emotions was 80.9% 2 years after surgery and 65.1% 3 years after surgery. The survival rates of patients in the group with negative emotions were significantly lower than those in the group without negative emotions at 2 and 3 years postoperatively. The recurrence rate at 3 years after surgery was 72.1% in the group with negative emotions, while it was 54.9% in the group without negative emotions. The recurrence rate at 3 years after surgery was significantly higher in the group with negative emotions compared with the group without negative emotions (Table 6, Figure 4).
Table 6
| Item | 1 year after surgery | 2 years after surgery | 3 years after surgery | |||||
|---|---|---|---|---|---|---|---|---|
| Survival rate | Recurrence rate | Survival rate | Recurrence rate | Survival rate | Recurrence rate | |||
| With negative emotions | 86.0% | 37.2% | 65.1% | 51.2% | 44.2% | 72.1% | ||
| Without negative emotions | 88.8% | 26.0% | 80.9% | 42.3% | 65.1% | 54.9% | ||
| χ2 | 0.272 | 2.219 | 5.273 | 1.137 | 6.638 | 4.350 | ||
| P | 0.602 | 0.136 | 0.022 | 0.286 | 0.010 | 0.037 | ||
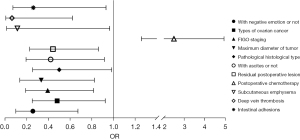
Binary logistic regression analysis of patients’ prognosis
Negative emotions, pathological type, FIGO stage, maximum tumor diameter, histological type, with or without ascites, postoperative lesion residual size, whether postoperative chemotherapy was given, and whether postoperative complications such as subcutaneous emphysema, deep vein thrombosis, and intestinal adhesions occurred were independent risk factors for patients’ prognosis (Table 7, Figure 5).
Table 7
| Related factor | B | SE | Wald | P | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| With negative emotion or not | −1.361 | 0.492 | 7.655 | 0.006 | 0.256 | 0.672 | 0.098 |
| Age (years) | −0.182 | 0.333 | 0.298 | 0.585 | 0.834 | 1.601 | 0.434 |
| BMI (kg/m2) | 0.534 | 0.334 | 2.548 | 0.110 | 1.705 | 3.285 | 0.885 |
| Have hypertension or not | 0.227 | 0.343 | 0.440 | 0.507 | 1.255 | 2.456 | 0.641 |
| Have hyperlipidemia or not | 0.849 | 0.547 | 2.405 | 0.121 | 2.338 | 6..836 | 0.799 |
| Have diabetes or not | 0.169 | 0.419 | 0.163 | 0.686 | 1.184 | 2.691 | 0.521 |
| Fertile or not | −1.024 | 0.698 | 2.152 | 0.142 | 0.359 | 1.411 | 0.091 |
| Have spouse or not | 0.961 | 0.597 | 2.588 | 0.108 | 2.614 | 8.432 | 0.811 |
| Education level | −0.025 | 0.340 | 0.006 | 0.941 | 0.975 | 1.899 | 0.501 |
| Address | 0.137 | 0.318 | 0.186 | 0.667 | 1.147 | 2.138 | 0.615 |
| Monthly household income (yuan) | 0.162 | 0.331 | 0.241 | 0.623 | 1.176 | 2.249 | 0.615 |
| Types of ovarian cancer | −0.733 | 0.333 | 4.833 | 0.028 | 0.480 | 0.924 | 0.250 |
| FIGO staging | −0.935 | 0.373 | 6.278 | 0.012 | 0.393 | 0.816 | 0.189 |
| Maximum diameter of tumor | −1.100 | 0.462 | 5.679 | 0.017 | 0.333 | 0.823 | 0.135 |
| Pathological histological type | −0.696 | 0.346 | 4.037 | 0.045 | 0.499 | 0.983 | 0.253 |
| With ascites or not | −0.867 | 0.398 | 4.760 | 0.029 | 0.420 | 0.916 | 0.193 |
| Lymph node metastasis | −0.241 | 0.375 | 0.414 | 0.520 | 0.786 | 1.638 | 0.377 |
| Residual postoperative lesion | −0.818 | 0.341 | 5.752 | 0.016 | 0.441 | 0.861 | 0.226 |
| Postoperative chemotherapy | 0.914 | 0.349 | 6.846 | 0.009 | 2.494 | 4.947 | 1.258 |
| Time to bowel function recovery | 0.570 | 0.355 | 2.584 | 0.108 | 1.769 | 3.545 | 0.882 |
| Length of hospitalization | 0.440 | 0.358 | 1.508 | 0.220 | 1.553 | 3.135 | 0.769 |
| Subcutaneous emphysema | −2.182 | 1.095 | 3.970 | 0.046 | 0.113 | 0.965 | 0.013 |
| Urinary tract infection | −0.542 | 0.830 | 0.426 | 0.514 | 0.582 | 2.961 | 0.114 |
| Deep vein thrombosis | −2.772 | 1.171 | 5.604 | 0.018 | 0.063 | 0.621 | 0.006 |
| Irregular bleeding | −0.676 | 0.688 | 0.966 | 0.326 | 0.509 | 1.958 | 0.132 |
| Pressure sores | 0.248 | 0.866 | 0.082 | 0.775 | 1.281 | 6.999 | 0.235 |
| Intestinal adhesions | −1.348 | 0.651 | 4.281 | 0.039 | 0.260 | 0.931 | 0.072 |
BMI, body mass index; FIGO, Federation International of Gynecology and Obstetrics; SE, standard error; OR, odds ratio; CI, confidence interval.
Discussion
According to statistics, there were 295,414 new cancer cases and 184,799 cancer deaths in the United States in 2018 (22). Worldwide, 314,000 OC cases and 207,300 deaths were reported in 2020, with incidence and death standardized rates of 6.6/100,000 and 4.2/100,000, respectively (23). After the occurrence of OC, a large amount of abdominal fluid appears, causing symptoms such as abdominal distension and decreased appetite, and if effective treatment measures are not taken in time, it can lead to symptoms such as wasting, anemia, and multiorgan failure, and even endanger patients’ lives (24). The general 5-year survival rate of early-stage OC is about 70–80% (25). However, in advanced stage (stage III/IV) OC, the 5-year overall survival (OS) rate is less than 30% (26). Surgery is currently an important clinical treatment approach for OC, and it can rapidly remove tumor tissues and inhibit disease progression (27). However, patients suffer from multiple stress factors, including pain caused by the disease itself, trauma of surgery and chemotherapy, and high treatment costs, which often lead to negative emotions such as anxiety, depression, and even fear, seriously affecting their treatment outcome and quality of life. Therefore, medical professionals are paying more and more attention to patients’ mental health issues.
The results of this study showed that age, monthly family income, education level, being fertile or not, whether lymph nodes were metastasized, whether chemotherapy was given postoperatively, the time to postoperative bowel function recovery, and whether postoperative complications such as irregular bleeding and pressure sores occurred were independent risk factors for the development of negative emotions in patients. Influenced by our traditional thinking, family members give more importance to genetically-related children. The pressure to have children, the absence of sexual organs, and the fear of high mortality often lead to patients becoming vulnerable to psychological problems. In addition, progesterone and estrogen in the female body can directly affect a woman’s mood by influencing neurotransmitter transmission and neuroendocrine function. The removal of ovaries causes a large change in estrogen and progesterone in women’s bodies, which makes them more prone to negative emotions such as anxiety. Family income directly reflects the economic status of the family. Patients may feel that they are burdening their families and loved ones and may have no source of income during surgery and recuperation, and thus their psychological stress is higher, and the probability of depression is also higher. The lymphatic system is the main diffusion pathway of gynecologic malignancies (28), and patients who have lymph node metastasis often think their prognosis is hopeless, have low treatment motivation, and have a weak desire to live. Postoperative chemotherapy will kill normal cells as well as cancer cells, which will have a certain negative impact on the patient’s body and cause many adverse reactions, thus affecting the patient’s psychology, and some patients become depressed because they cannot tolerate the adverse reactions brought on by treatment. The long recovery time of intestinal function after surgery and the occurrence of complications will result in further physical and mental suffering and lead patients to doubt the effect of treatment, which will increase the psychological burden of patients. Therefore, in clinical work, while actively treating patients, we should also pay attention to their emotional needs, encourage them, actively provide psychological comfort and emotional support, ensure the efficacy of treatment based on the lowest possible treatment costs for low-income patients, and in accordance with the patient and their family’s condition, provide personalized treatment plans to reduce the psychological and economic burden on the patient and their family. At the same time, the patient’s indicators should be monitored at all times, and timely measures should be taken to prevent complications to improve the patient’s prognosis.
The results of this study also found that the development of negative emotions could seriously affect the prognosis of patients. Negative emotions were an independent influencing factor on patient prognosis. In this study, RI decreased and PSV and EDV increased in both groups at 3 months after surgery, but RI in patients with combined negative emotions was lower than those in the group without negative emotions, and PSV and EDV were both higher than those in the group without negative emotions. Because the tumor tissue is in a high metabolic state during the development of OC, neovascularization is an important pathological feature of OC and the pathological basis for cancer cell invasion and abnormal proliferation. Usually, new tumor blood vessels are characterized by high permeability of the wall, few smooth muscle structures, and numerous arteriovenous short circuits, so the blood flow parameters of malignant tumors are characterized by high flow velocity and low resistance (29). Decreased blood flow resistance and increased blood flow velocity imply the deterioration of OC, and this result indicates that the recovery of ovarian function after surgery is significantly worse in patients presenting negative emotions than in those without negative emotions. In addition, the survival rate at 2 and 3 years after surgery was significantly lower in patients with negative emotions than in patients without negative emotions, and the recurrence rate at 3 years after surgery was significantly higher than in patients without negative emotions. These results suggested that patients with combined negative emotions have a worse prognosis.
Furthermore, the results of this study found that, in addition to negative emotions, pathological type, FIGO stage, maximum tumor diameter, histological type, with or without ascites, and size of residual postoperative lesions were also independent risk factors affecting the prognosis of OC patients. Each pathological type has its own unique tumor biology affecting the prognosis of the disease. Among all OCs, high-grade plasmacytoma is the most common subtype and is responsible for 70–80% of all OC-related deaths (30). Lan et al. (31) found that low-grade plasmacytoma, high-grade plasmacytoma, and carcinosarcoma are usually at advanced stages at diagnosis and have much higher lymph node and distant metastases compared to other tissue types. Regardless of stage, high-grade plasmacytoma has the worst prognosis. Median OS and median progression-free survival (PFS) of OC patients with relevant clinicopathologic factors have been studied in a large number of multifactorial analyses, confirming that FIGO stage is a very meaningful prognostic factor in OC, and patients with early stage are significantly better off than those with advanced stage in terms of prognosis (32). Patients with early FIGO stage can have more complete resection of the lesion during surgery, and because the residual lesion is relatively small, it is more sensitive to chemotherapy, and thus there is a lower risk of recurrence and metastasis. Therefore, these patients have a good prognosis. Patients with advanced FIGO stage have more widespread tumor cells, thus complete resection is more difficult to achieve, and drug resistance in chemotherapy is more likely, leading to a worse prognosis (33). Previous study has found that histological grading is an important factor affecting prognosis (34). Tissue with low differentiation and higher stage indicates higher tumor progression, which can lead to extensive tumor spread and implantation, making surgery more complicated, and as it is difficult to completely resect the lesion, cancer cells are more likely to invade blood vessels and lymphatic tissues and deepen infiltration, leading to tumor metastasis and high risk of recurrence after surgery (35,36). Ascites is the accumulation of excess fluid in the peritoneal cavity, and as a microenvironment for tumor inflammation and immunosuppression, it contains various cytokines, chemokines, and growth factors which can increase tumor aggressiveness and decrease the ability of drug metabolism, including tumor cell shedding (37,38). In addition, it has been found that malignant ascites can provide a microenvironment for tumor cells to promote inflammation and carcinogenesis, increasing the risk of postoperative recurrence (39). For the prognostic impact of postoperative residual lesions, Tseng et al. (40) analyzed clinical data and found residual lesions to be an independent risk factor for prognosis. Postoperative residual size >1 cm leads to residual cancer cells that remain unavoidable and also increases the risk of recurrence (41), thus shortening survival.
In conclusion, there are many factors that affect the appearance of negative emotions in OC patients, and combined negative emotions can seriously affect patients’ prognosis. Therefore, clinical personnel should identify relevant risk factors in a timely manner and actively provide psychological guidance to improve treatment compliance, thereby reducing complications and improving postoperative quality of life for patients.
Due to limited time and manpower, the sample size of this study was small, and a larger sample size is necessary for an in-depth study in the future to provide a reference basis for improving the prognosis of OC patients.
Conclusions
In the perioperative period of OC, patients are prone to anxiety, depression, and other psychological disorders, which seriously affect the treatment effect. Therefore, in clinical work, patients’ negative emotions should be predicted as early as possible, and active communication with patients and timely psychological counseling should be provided. Improve surgical accuracy and reduce the complication rate.
Acknowledgments
Funding: This study was supported by Scientific Research Project of Maternal and Child Health of Wuxi Health Committee (No. FYKY201905).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-94/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-94/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-94/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-94/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all patients. The study was approved by the ethics committee of The Affiliated Hospital of Jiangnan University (No. KYLC2014262).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- O’Shea AS. Clinical Staging of Ovarian Cancer. Methods Mol Biol 2022;2424:3-10. [Crossref] [PubMed]
- Younes N, Zayed H. Genetic epidemiology of ovarian cancer in the 22 Arab countries: A systematic review. Gene 2019;684:154-64. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783-91. [Crossref] [PubMed]
- Sambasivan S. Epithelial ovarian cancer: Review article. Cancer Treat Res Commun 2022;33:100629. [Crossref] [PubMed]
- Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95:S161-92. [Crossref] [PubMed]
- Gu ZH, Qiu T, Yang SH, et al. A Study on the Psychological Factors Affecting the Quality of Life Among Ovarian Cancer Patients in China. Cancer Manag Res 2020;12:905-12. [Crossref] [PubMed]
- James NE, Woodman M, Ribeiro JR. Prognostic immunologic signatures in epithelial ovarian cancer. Oncogene 2022;41:1389-96. [Crossref] [PubMed]
- Chuang YT, Chang CL. Extending platinum-free interval in partially platinum-sensitive recurrent ovarian cancer by a non-platinum regimen: its possible clinical significance. Taiwan J Obstet Gynecol 2012;51:336-41. [Crossref] [PubMed]
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin 2019;69:280-304. [Crossref] [PubMed]
- Filippova OT, Kim SW, Cowan RA, et al. Hematologic changes after splenectomy for ovarian cancer debulking surgery, and association with infection and venous thromboembolism. Int J Gynecol Cancer 2020;30:1183-8. [Crossref] [PubMed]
- Yang Y, Qi S, Shi C, et al. Identification of metastasis and prognosis-associated genes for serous ovarian cancer. Biosci Rep 2020;40:BSR20194324. [Crossref] [PubMed]
- Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines Cancer-related fatigue. J Natl Compr Canc Netw 2010;8:904-31. [Crossref] [PubMed]
- Cesario SK, Nelson LS, Broxson A, et al. Sword of Damocles cutting through the life stages of women with ovarian cancer. Oncol Nurs Forum 2010;37:609-17. [Crossref] [PubMed]
- Joly F, Ahmed-Lecheheb D, Kalbacher E, et al. Long-term fatigue and quality of life among epithelial ovarian cancer survivors: a GINECO case/control VIVROVAIRE I study. Ann Oncol 2019;30:845-52. [Crossref] [PubMed]
- Shinn EH, Taylor CL, Kilgore K, et al. Associations with worry about dying and hopelessness in ambulatory ovarian cancer patients. Palliat Support Care 2009;7:299-306. [Crossref] [PubMed]
- Wenzel LB, Donnelly JP, Fowler JM, et al. Resilience, reflection, and residual stress in ovarian cancer survivorship: a gynecologic oncology group study. Psychooncology 2002;11:142-53. [Crossref] [PubMed]
- Ozga M, Aghajanian C, Myers-Virtue S, et al. A systematic review of ovarian cancer and fear of recurrence. Palliat Support Care 2015;13:1771-80. [Crossref] [PubMed]
- Li YZ, Qin X, Liu FH, et al. Prediagnosis Depression Rather Than Anxiety Symptoms Is Associated with Decreased Ovarian Cancer Survival: Findings from the Ovarian Cancer Follow-Up Study (OOPS). J Clin Med 2022;11:7394. [Crossref] [PubMed]
- Matulonis UA, Kornblith A, Lee H, et al. Long-term adjustment of early-stage ovarian cancer survivors. Int J Gynecol Cancer 2008;18:1183-93. [Crossref] [PubMed]
- Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol 2001;19:1809-17. [Crossref] [PubMed]
- Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2020;70:313. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Lavie O, Chetrit A, Novikov I, et al. Fifteen-year survival of invasive epithelial ovarian cancer in women with BRCA1/2 mutations - the National Israeli Study of Ovarian Cancer. Gynecol Oncol 2019;153:320-5. [Crossref] [PubMed]
- Braga EA, Loginov VI, Burdennyi AM, et al. Five Hypermethylated MicroRNA Genes as Potential Markers of Ovarian Cancer. Bull Exp Biol Med 2018;164:351-5. [Crossref] [PubMed]
- Zong L, Zhou Y, Zhang M, et al. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol Immunother 2020;69:33-42. [Crossref] [PubMed]
- Chambers LM, Yao M, Morton M, et al. Patterns of recurrence in women with advanced and recurrent epithelial ovarian cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Gynecol Oncol 2021;161:389-95. [Crossref] [PubMed]
- Ercelep O, Ozcelik M, Gumus M. Association of lymphadenectomy and survival in epithelial ovarian cancer. Curr Probl Cancer 2019;43:151-9. [Crossref] [PubMed]
- Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur J Cancer 2014;50:2463-77. [Crossref] [PubMed]
- Launonen IM, Lyytikäinen N, Casado J, et al. Single-cell tumor-immune microenvironment of BRCA1/2 mutated high-grade serous ovarian cancer. Nat Commun 2022;13:835. [Crossref] [PubMed]
- Lan A, Yang G. Clinicopathological parameters and survival of invasive epithelial ovarian cancer by histotype and disease stage. Future Oncol 2019;15:2029-39. [Crossref] [PubMed]
- Fu Y, Wang X, Pan Z, et al. Clinical outcomes and prognostic factors of patients with epithelial ovarian cancer subjected to first-line treatment: a retrospective study of 251 cases. Front Med 2014;8:91-5. [Crossref] [PubMed]
- Ge L, Li N, Yuan GW, et al. Nedaplatin and paclitaxel compared with carboplatin and paclitaxel for patients with platinum-sensitive recurrent ovarian cancer. Am J Cancer Res 2018;8:1074-82. [Crossref] [PubMed]
- Muraji M, Sudo T, Iwasaki S, et al. Histopathology predicts clinical outcome in advanced epithelial ovarian cancer patients treated with neoadjuvant chemotherapy and debulking surgery. Gynecol Oncol 2013;131:531-4. [Crossref] [PubMed]
- Ducie J, Dao F, Considine M, et al. Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat Commun 2017;8:990. [Crossref] [PubMed]
- Guo N, Peng Z. Does serum CA125 have clinical value for follow-up monitoring of postoperative patients with epithelial ovarian cancer? Results of a 12-year study. J Ovarian Res 2017;10:14. [Crossref] [PubMed]
- Penet MF, Krishnamachary B, Wildes FB, et al. Ascites Volumes and the Ovarian Cancer Microenvironment. Front Oncol 2018;8:595. [Crossref] [PubMed]
- Lane D, Matte I, Garde-Granger P, et al. Ascites IL-10 Promotes Ovarian Cancer Cell Migration. Cancer Microenviron 2018;11:115-24. [Crossref] [PubMed]
- Mikuła-Pietrasik J, Uruski P, Szubert S, et al. Malignant ascites determine the transmesothelial invasion of ovarian cancer cells. Int J Biochem Cell Biol 2017;92:6-13. [Crossref] [PubMed]
- Tseng JH, Cowan RA, Afonso AM, et al. Perioperative epidural use and survival outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer. Gynecol Oncol 2018;151:287-93. [Crossref] [PubMed]
- Fan XM, Zhang J, Niu SH, et al. Secondary cytoreductive surgery in recurrent epithelial ovarian cancer: A prognostic analysis with 103 cases. Int J Surg 2017;38:61-6. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)




