
Detection value of endoscopic ultrasound-guided 19G fine-needle wet-heparinized suction for pancreatic solid tumors: a randomized controlled trial
Highlight box
Key findings
• Wet-heparinized suction in conjunction with macroscopic on-site evaluation improved the quality and aspiration efficiency of tissue biopsy for pancreatic solid tumors.
What is known, and what is new?
• EUS-FNA has some defects, such as an incomplete histological structure of the pancreatic biopsy specimen and blood coagulation. Wet-heparinized suction could improve the histological integrity of pancreatic biopsy specimens and reduce blood contamination.
• Macroscopic on-site evaluation can improve FNA efficiency.
What is the implication, and what should change now?
• Wet-heparinized suction can improve the quality and efficiency of FNA for pancreatic biopsy specimens in conjunction with macroscopic on-site evaluation, thus reducing surgical complications.
Introduction
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is an eminent minimally invasive technique for diagnosing gastrointestinal tumors and potentially of great value in diagnosing and treating pancreatic solid tumors (1,2). However, conventional EUS-FNA has some defects, such as an incomplete histological structure of the obtained pancreatic biopsy tissues and blood coagulation, that compromise the quality of the specimen (3). There are currently multiple approaches to improve the quality of biopsy samples, such as negative micro-pressure and fan-shaped puncture, but these methods are subject to the operator’s experience and skill (4-6). Wet suction can improve the biopsy specimen integrity but fails to effectively reduce blood contamination (3,7). Heparin can prevent blood coagulation by reducing the adhesion between the biopsy tissue strips and the wall of the needle tube, thus improving the structural integrity of the specimen, increasing the sample quality, and reducing blood contamination (8). Although its safety for aspiration has been fully validated, whether EUS-FNA combined with wet heparin is helpful for the detection of pancreatic solid tumors remains to be proven. Therefore, this study aimed to perform EUS-FNA using wet-heparinized suction for patients with pancreatic solid tumors to assess the effect of heparin on improving the structural integrity of the biopsied tissue, increasing specimen quality, and reducing blood contamination (9). We used a 19G fine needle for the puncture and applied macroscopic on-site evaluation (MOSE) (10) for the specimen evaluation. MOSE could be helpful in the diagnosis of pancreatic malignancies. It is poorly studied how to improve the diagnostic efficiency of MOSE in pancreatic cancer diagnosis by improving the quality of the samples. Therefore, further exploration of this issue would be necessary. We have proposed for the first time the use of wet-heparinized suction in processing the puncture path, in combination with MOSE. We hope this would improve the diagnostic efficiency of MOSE for pancreatic cancer by improving the quality of the samples.
A larger puncture needle could obtain as many specimens as possible to facilitate the subsequent diagnosis. We aimed to assess the effectiveness of this method in improving biopsy specimen quality in pancreatic solid tumors and guiding optimum aspiration strategies. We present the following article in accordance with the STARD and CONSORT reporting checklists (available at https://gs.amegroups.com/article/view/10.21037/gs-22-742/rc).
Methods
Study participants
A total of 52 patients with pancreatic solid tumors admitted to the Wuhan Fourth Hospital between August 2019 and April 2021 and who agreed to receive EUS-FNA were enrolled in the current study. All patients were given detailed information about the two aspiration methods and potential complications and were assigned to two groups using a randomized number table. There were 27 patients assigned to the experimental group (heparin-based aspiration, heparin group) and 25 to the control group (conventional wet-suction group). Clinical data were collected, including patient age, gender, type of tumor, and puncture site. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Wuhan Fourth Hospital (ID: KY 2019-022-01). Participants signed informed consent.
Inclusion criteria
- Patients who were aged over 18.
- The presence of a solid space-occupying lesion in the pancreas confirmed by imaging and laboratory testing.
- A suspected diagnosis of solid pancreatic tumor.
Exclusion criteria
- Concomitant with coagulation dysfunction: international normalized ratio (INR) >1.5 or blood platelet count <8×104/mm3.
- Unable to cooperate with the procedure.
- Solid lesions with cystic changes in the pancreas.
Operating equipment and operator
Ultrasound mainframe: HI VISION Avius. Ultrasonic endoscope: EG-3870UTK. Fine needle: 19G SonoTipProContr puncture needle (GUS-33-21-019, Germany, Medi-Globe GmbH). All procedures were conducted by one highly qualified endoscopist who had performed more than 500 EUS-FNA procedures.
Puncture process
Conventional wet suction
EUS was used to locate the puncture target, and the puncture was conducted without a needle core. Saline was injected into the needle cavity before each puncture with 10 mL negative pressure. Punctures were performed three times, lifting and thrusting 40–50 times each.
Wet-heparinized suction
The pre-puncture preparation was the same as the conventional wet suction, without a needle core, and 100 U/mL of heparin saline was injected into the needle cavity. The tail of the needle was connected to a negative pressure syringe (containing 5 mL of heparin solution at the same concentration with 10 mL of negative pressure). The puncture method was the same as the routine puncture, and the above operation was repeated before each puncture. Liquid-based cytology was performed after collecting the flushing fluid. The remainder of the process was the same as the conventional method.
Specimen collection and processing
Collection
(I) A needle core was used to push the strip tissue specimen onto a transparent plate with a diameter of 10 cm (10% neutral formalin-fixed solution). The plate was shaken intermittently, and the quality of the specimen was observed by the naked eye. (II) A 10 mL syringe was vacuumized, and the residual hemorrhagic tissues in the needle cavity were extracted onto the slide to make cell smears (3–6 smears at a time). (III) The hemorrhagic tissues within the negative pressure syringe and the flushing fluid of the needle were collected.
Processing
(I) Paraffin sections were made for hematoxylin-eosin (HE) and immunohistochemical staining. (II) The cell smears were air-dried for Pap staining. (III) The hemorrhagic tissues and flushing fluid of the needle were collected for liquid-based cytological examination (centrifugation and membrane negative pressure suction).
Data measurement and evaluation criteria
The measured value was accurate to 1 mm.
The total length of the biopsy tissue strip was measured (the length of the needle core used to push out the tissue strip completely from the needle cavity). The total length of the white part in the tissue strip (white tissue core) was measured using a metric ruler. The length of the white tissue core obtained by the first, second, and third punctures was measured respectively.
Pathological result assessment criteria
Positive: benign, malignant, or atypical cells were identified in the pancreatic tissues; negative: no benign, malignant, or atypical cells were observed in the pancreatic tissues.
Specimen integrity evaluation criteria
Great: continuous tissue strips with minimal breakages (<30% of the total length); good: partially continuous tissue strips with partial breakages (<60% of the total length); poor: discontinuous tissue strips with multiple breakages (>60% of the total length).
Specimen pathological quality assessment
Good: sufficient tissues were obtained for pathological diagnosis, and enough tissues for immunohistochemical staining if necessary; poor: insufficient tissues were obtained for pathological diagnosis. The quality was assessed by a pathologist.
Evaluation criteria of blood contamination
Great: no red blood cells/monolayer red blood cells were observed, with no accumulation; good: red blood cell accumulation was less than one high-power field; qualified: red blood cell accumulation was over one high-power field.
The pathological results were diagnosed by two senior pathologists.
Follow-up
Routine blood examination, serum amylase, and other indexes and clinical symptoms and signs were monitored within 48 hours of puncture to identify any complications (intrapancreatic hemorrhage, elevated serum amylase, gastrointestinal bleeding, or gastrointestinal perforation). In-hospital observation or telephone follow-up after discharge was continued for one week.
Statistical analysis
The statistical analysis was performed using SPSS (v 18.0, IBM.com). A normality test was conducted for the quantitative data using the Shapiro-Wilk test. Normally distributed quantitative data were expressed as the mean ± standard deviation (SD), and the independent-sample t-test was performed for comparisons between the groups. Non-normally distributed quantitative data were expressed as M (P25, P75), and the Wilcoxon rank-sum test was performed for comparisons between the groups. The qualitative data are expressed by the number of cases. Chi-square test was used for comparison between groups, and correction for continuity chi-square test was used when 25% of the cell frequencies were lower than 5. The Fisher exact probability method was used when the theoretical frequency was less than 5. Measurement data were expressed by percentage, and the Wilcoxon rank-sum test was applied for ranked data. A linear correlation analysis was used for normally distributed data. Receiver operating characteristic (ROC) curve was used to reflect the detection value of EUS-FNA combined with wet heparin for pancreatic solid tumors. The area under the curve (AUC) was compared to identify the optimal cut-off value. A two-sided P value less than 0.05 indicated a statistically significant difference.
Results
Patient characteristics
A total of 48 patients were included, with 22 in the conventional group and 26 in the heparin group. Three cases were excluded because they were unable to cooperate with the procedure, and one case contained cystic lesions (Figure 1) (Table 1).
Table 1
| Characteristics | Heparin group | Conventional group | Statistic | P value |
|---|---|---|---|---|
| Age (year) (mean ± SD) | 60.50±10.44 | 59.55±12.04 | t =0.294 | 0.770 |
| Gender | t =0.099 | 0.753 | ||
| Male | 13 | 12 | ||
| Female | 13 | 10 | ||
| Tumor site | ||||
| Pancreatic head | 5 | 4 | ||
| Pancreatic body | 12 | 10 | ||
| Pancreatic tail | 9 | 8 | ||
| The longest diameter (mm) (mean ± SD) | 17.35±3.50 | 17.95±3.34 | t =−0.613 | 0.543 |
| Pathological type | ||||
| PDAC | 25 | 22 | ||
| PET | 1 | 0 | ||
| Site of puncture | ||||
| Stomach | 23 | 18 | ||
| Duodenum | 3 | 4 |
SD, standard deviation; PDAC, pancreatic ductal adenocarcinoma; PET, pancreatic endocrine tumor.
There were 13 males and 13 females in the heparin group (mean age: 60.50±10.44) and 12 males and 10 females in the conventional group (mean age: 59.55±12.04) (t =0.294, P=0.770). No statistically significant difference was observed in the solid tumor volumes between the two groups (t =−0.613, P=0.543). A total of seven patients received duodenum puncture, and pancreatic ductal adenocarcinoma accounted for most of the diagnosed cases (47/48, the remaining case was diagnosed with pancreatic endocrine tumor). Most of the tumors occurred in the body and tail of the pancreas (39/48).
Comparison of puncture results
The heparin group had a longer total length of tissue strips (P<0.05) and a longer total length of white tissue core (P<0.05) than the conventional group (Figure 2). The total length of white tissue core in the heparin group was greater in the first puncture than in the second or third puncture (P<0.05). The heparin group had a significantly longer total white tissue core length from the first puncture compared with the conventional group (W =545, P<0.05) (Table 2). There was a positive correlation between the total length of white tissue core and the total length of tissue strips in the two groups (conventional wet-suction group: r =0.470, P<0.05; heparin group: r =0.433, P<0.05). The heparin group had milder erythrocyte contamination in the paraffin sections (χ2=6.506, P<0.05) (Table 3).
Table 2
| Tissue strips and White tissue core | Heparin group | Conventional group | Statistic | P |
|---|---|---|---|---|
| Total length of tissue strips (mm) (M, P25, P75) | 469.50 (458.50, 529.25) | 379 (335.75, 418.75) | W =535 | <0.05 |
| Total length of white tissue core (mm) (mean ± SD) | 62.23±9.92 | 50.55±12.05 | t =3.690 | <0.05 |
| Total length of white tissue core (mm) (first puncture) (M, P25, P75) | 34.50 (28.50, 42.25) | 17 (14.25, 20) | W =545 | <0.05 |
| Total length of white tissue core (second puncture) (mm) (mean ± SD) | 16.31±4.66 | 21.00±5.63 | t =3.159 | <0.05 |
| Total length of white tissue core (third puncture) (mm) (M, P25, P75) | 11 (9, 13) | 11.50 (10, 14.75) | W =257.500 | >0.05 |
M, median; P25, lower quartile; P75, upper quartile.
Table 3
| Specimen quality and BCPS | Heparin group | Conventional group | Statistic | P |
|---|---|---|---|---|
| Specimen integrity | ||||
| Great | 3.30 | 1.27 | W =181 | <0.05 |
| Good | 36.85 | 26.82 | W =186 | <0.05 |
| Poor | 59.85 | 71.91 | W =409.500 | <0.05 |
| SPQ | χ2=3.884 | <0.05 | ||
| Good | 18 | 9 | ||
| Poor | 8 | 13 | ||
| BCPS | χ2=6.506 | <0.05 | ||
| Great | 8 | 4 | ||
| Good | 13 | 6 | ||
| Qualified | 5 | 12 |
BCPS, blood contamination in paraffin section; SPQ, specimen pathological quality.
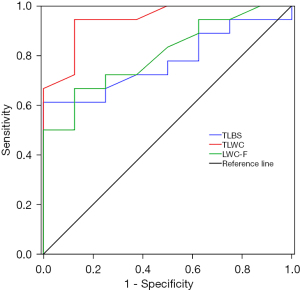
The heparin group had higher-quality specimens compared with the conventional group, and the difference was statistically significant (P<0.05). Taking the “good” pathological specimen quality as the gold standard for diagnostic analysis, the optimal cut-off value of the specimen total length in the heparin group was 477.50 mm, the sensitivity was 61.10%, the specificity was 100%, and the Youden index was 0.611 [AUC =0.774; 95% confidence interval (CI): 0.597–0.951; P<0.005]. The optimal cut-off value of white tissue core total length in the heparin group was 55.50 mm, the sensitivity was 94.40%, the specificity was 87.50%, and the Youden index was 0.819 (AUC =0.944; 95% CI: 0.857–1.000; P<0.05). The optimal cut-off value of the white tissue core length in the first puncture was 34.50 mm in the heparin group, the sensitivity was 66.70%, the specificity was 87.50%, and the Youden index was 0.542 (AUC =0.806; 95% CI: 0.637–0.974; P<0.05) (Figure 3).
Complications
Postoperative hyperamylasemia occurred in five patients, three of whom were in the heparin group (two received intra-stomach puncture, and one received duodenum puncture). The two patients from the conventional group both received intra-stomach punctures. They all recovered after receiving conservative medical treatment. There was no difference in the incidence of complications between the two groups (χ2=0.077, P>0.05) or among the different puncture sites (χ2=0.132, P>0.05).
Discussion
EUS-guided biopsy is the preferred method for diagnosing pancreatic solid tumors (11).
Wet fine-needle puncture is an improved puncture technique. Sisman et al. (12) reported that the wet tubing wall forms a water film to prevent adhesion between the tissues and the tubing wall, which increases the amount of tissue obtained. We selected the 19G puncture needle for wet suction in this study because a puncture needle with a thicker diameter ensures a maximum amount of specimen can be obtained to facilitate the pathological and immunohistochemical diagnosis (13). Although it is generally believed that the performance of thicker puncture needles is unsatisfactory in some specific locations, such as the duodenum (14), a recent study by de Nucci et al. (15) has confirmed the feasibility and accuracy of using 19G puncture needles to obtain samples from the duodenum.
Heparin (16) has previously been used in percutaneous liver puncture to increase the specimen yield by reducing blood coagulation during the puncture process. Subsequent studies (8,12) have adopted heparin for EUS-guided fine-needle liver biopsy and discovered that heparin prevented the adhesion of tissue strips to the needle tubing wall because of blood coagulation, thus increasing the amount of obtained specimen. A recent study has explored the merits of wet-heparinized pancreas biopsy (17), but there is currently no data available to explore its effect on the quality of pancreatic specimens. Our study found that the heparin group had longer pancreatic tissue strips than the conventional group (P<0.05), which indicated that the coagulative activity of heparin increased the sample yield in the pancreatic biopsies.
Attempts have been made for the assessment of biopsy pancreatic cancer specimens under the guidance of EUS. Rapid On-site Evaluation (ROSE) could perform real-time cytological assessment for the biopsy specimens, and could determine the number of times for FNA (18). Even so, controversies exist among the current studies about using ROSE to improve the diagnostic performance of EUS-FNA (19). Despite the practicability of ROSE, its operation still needs the assistance of pathologists. Some researchers have proposed MOSE as an alternative that could also improve the diagnostic performance of EUS-FNA (20,21).
We also used MOSE, which can quantitatively evaluate the red and white parts of the puncture tissues, in which the white part (white tissue core) is often purer pancreatic lesion tissue (22). The total length of white tissue core in the heparin group was longer than in the control group (P<0.05), suggesting that using heparin may increase the amount of white tissue core collected. Meanwhile, the specimen integrity in the heparin group was better than in the control group, which is essential for accurate pathological diagnosis. Kaneko et al. measured the length of the white tissue core by manual cutting and linear arrangement (23). However, this method potentially results in a large amount of tissue loss and compromises the integrity of the tissue strip, so we chose not to cut the specimen to perform direct measurement in an effort to guarantee histological integrity. The heparin group had more “great” and “good” specimen integrity categories than the control group (P<0.05). In summary, wet-heparinized suction improved the extraction of pancreatic white tissue core more effectively than the conventional wet-suction method (19). With the deepening exploration by recent studies, the quality of the EUS biopsy specimens has been significantly improved. The pathological diagnosis of the specimens has shifted from cytology to histology, and is now changing to genetics. Several studies perform Next Generation Sequencing (NGS) for endoscopic ultrasound-guided tissue acquisition (EUS-TA), use genetic analyses to guide the clinical diagnosis, and construct clinical models to guide the treatment (24,25). These make it possible to conduct the Precision Medicine.
Diagnostic analysis of the heparin group showed that the total length of the biopsy specimen, total length of white tissue core, and the white tissue core length in the first puncture were associated with “good” pathological quality. A comparison of the AUC of the three variables indicated that the total length of white tissue core had the largest area under the curve If the total length of white tissue core obtained by wet-heparinized suction reached 55.50 mm (sensitivity 94.40%, specificity 87.50%), the biopsy specimen was considered great quality. We found that in most cases, the total length of white tissue core reached above the optimal cut-off value after the second puncture, indicating that wet-heparinized suction might help to reduce the number of punctures needed, which in turn could reduce the incidence of related complications.
Severe fibrosis in the pancreatic peritumoral tissues can cause the white tissue strips to mingle with segmental fibrotic tissues (10,17,23), leading to bias in the MOSE. Therefore, additional laboratory diagnosis is essential. There were 45 cases that were directly diagnosed in this study via paraffin section, and the remaining three cases (two in the conventional group and one in the heparin group) were subsequently diagnosed by cytological smear and immunohistochemistry.
Liquid-based cytology is an effective complement to FNA, with the ability to collect and concentrate more tumor cells and reduce blood contamination. Sekita-Hatakeyama et al. (26) performed NGS for liquid-based cytological specimens to assess the mutations of KRAS, TP53, CDKN2A, SMAD4, and PIK3CA, which could improve the diagnostic efficiency of FNA for pancreatic tumors. The PDAC protein found by Souche et al. (27) in liquid-based cytological specimens could be a potential biomarker for pancreatic tumors.
The cytological images from the paraffin sections of the diagnosed cases were all positive, which suggests that laboratory examination might be more sensitive in positive cases (28). There was very little erythrocyte contamination in the heparin group (P<0.05), supporting the previous speculation that heparin might reduce the hemagglutination in the puncture tissue strip. Heparin processing of the puncture specimens did not interfere with the cytological or immunohistochemical detection, which is consistent with the conclusion made by Diehl (29).
Hyperamylasemia occurred in five patients but returned to normal after 1–2 days of medical treatment. The remaining patients had no related complications. There was no significant difference in the incidence of complications between the groups or among the different puncture sites (P<0.05). The follow-up was extended to March 2023 for all the participants. There were 5 participants (2 in the heparin group and 3 in the control group) who had lost to follow-up. Among the remaining 43 patients, only 2 are still alive after receiving surgery and conventional treatments, and the other patients had died. Their survival time ranged from 3 months to 5 months. All of these patients reported no relevant adverse events after receiving the biopsy. This demonstrates that heparin is safe for pancreatic puncture (30,31).
In this study, we made the following observations: (I) heparin can increase the total length of pancreatic white tissue core specimens, improve histological integrity, reduce blood contamination, and facilitate pathological diagnosis. (II) The use of MOSE for the total length of pancreatic white tissue core and specimen integrity evaluation is an important reference index for wet-heparinized suction strategies for pancreatic solid tumors. It has certain merits in reducing the number of unnecessary punctures and the risk of complications. (III) Cytological and immunohistochemical detection of the puncture specimens is an essential adjunct and supplement for pathological diagnosis after FNA and provides evidential support for the follow-up treatment of pancreatic tumors. (IV) The use of heparin is safe and feasible in pancreatic EUS-FNA.
The main limitation of this study was the small number of cases included, in the future, randomized controlled trials with larger sample sizes will be needed. Additionally, the efficacy of using different puncture needle sizes and tubing diameters when performing EUS-FNA remains to be elucidated.
Conclusions
This study has demonstrated that wet-heparinized suction improved the quality of biopsy specimens obtained by 19G fine-needle aspiration for pancreatic solid tumors, with considerable safety. MOSE of biopsy specimens is helpful for improving puncture efficiency and reducing the risk of related complications.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD and CONSORT reporting checklists. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-742/rc
Trial Protocol: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-742/tp
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-742/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-742/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-742/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Wuhan Fourth Hospital (ID: KY 2019-022-01). Participants signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jeon TY, Moon SH, Kim JH, et al. Diagnostic Performance of EUS-Guided Sampling in Indeterminate Radiological Diagnosis of Pancreatic Disease and Intra-Abdominal Lymphadenopathy. J Clin Med 2021;10:3850. [Crossref] [PubMed]
- He MJ, Chen TY, Liu XY, et al. Utility of endoscopic ultrasound-guided fine-needle aspiration in pancreatic cancer patients who failed to obtain a pathological diagnosis in surgical exploration. Gland Surg 2022;11:426-31. [Crossref] [PubMed]
- Wang Y, Wang RH, Ding Z, et al. Wet- versus dry-suction techniques for endoscopic ultrasound-guided fine-needle aspiration of solid lesions: a multicenter randomized controlled trial. Endoscopy 2020;52:995-1003. [Crossref] [PubMed]
- Del Vecchio Blanco G, Palmieri G, Giannarelli D, et al. Factors influencing diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic and biliary tumors. Scand J Gastroenterol 2021;56:498-504. [Crossref] [PubMed]
- Chen TY, Cao JW, Jin C, et al. Comparison of specimen quality among the standard suction, slow-pull, and wet suction techniques for EUS-FNA: A multicenter, prospective, randomized controlled trial. Endosc Ultrasound 2022;11:393-400. [Crossref] [PubMed]
- Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy 2017;49:989-1006. [Crossref] [PubMed]
- Chen D, Ren Y, Chen S, et al. The Wet Suction Technique Enhances the Diagnostic Efficacy and Aspirate Quality of EUS-FNA for Solid Lesions: A Multicenter Retrospective Study in China. J Clin Gastroenterol 2023;57:417-22. [Crossref] [PubMed]
- Mok SRS, Diehl DL, Johal AS, et al. A prospective pilot comparison of wet and dry heparinized suction for EUS-guided liver biopsy (with videos). Gastrointest Endosc 2018;88:919-25. [Crossref] [PubMed]
- Diehl DL, Mok SRS, Khara HS, et al. Heparin priming of EUS-FNA needles does not adversely affect tissue cytology or immunohistochemical staining. Endosc Int Open 2018;6:E356-62. [Crossref] [PubMed]
- Mohan BP, Madhu D, Reddy N, et al. Diagnostic accuracy of EUS-guided fine-needle biopsy sampling by macroscopic on-site evaluation: a systematic review and meta-analysis. Gastrointest Endosc 2022;96:909-17.e11. [Crossref] [PubMed]
- Capurso G, Archibugi L, Petrone MC, et al. Slow-pull compared to suction technique for EUS-guided sampling of pancreatic solid lesions: a meta-analysis of randomized controlled trials. Endosc Int Open 2020;8:E636-43. [Crossref] [PubMed]
- Sisman G, Barbur E, Saka D, et al. Endoscopic ultrasound-guided liver biopsy using a 20-gauge fine needle biopsy needle with the wet-heparinized suction technique. Eur J Gastroenterol Hepatol 2020;32:1470-4. [Crossref] [PubMed]
- Patel HK, Saxena R, Rush N, et al. A Comparative Study of 22G versus 19G Needles for EUS-Guided Biopsies for Parenchymal Liver Disease: Are Thinner Needles Better? Dig Dis Sci 2021;66:238-46. [Crossref] [PubMed]
- Ginès A, Fusaroli P, Sendino O, et al. Performance of a new flexible 19 G EUS needle in pancreatic solid lesions located in the head and uncinate process: A prospective multicenter study. Endosc Int Open 2021;9:E1269-75. [Crossref] [PubMed]
- de Nucci G, Petrone MC, Imperatore N, et al. Feasibility and Accuracy of Transduodenal Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Solid Lesions Using a 19-Gauge Flexible Needle: A Multicenter Study. Clin Endosc 2021;54:229-35. [Crossref] [PubMed]
- Saraireh H, Abdelfattah T, Hassouneh R, et al. "Wet Heparin" and "Wet Saline" EUS-Guided Liver Biopsy Techniques Both Provide High Rates of Specimen Adequacy for Benign Parenchymal Liver Disease. Dig Dis Sci 2022;67:5256-61. [Crossref] [PubMed]
- Antkowiak R, Bialecki J, Chabowski M, et al. Treatment of Microcirculatory Disturbances in Acute Pancreatitis: Where Are We Now? Pancreas 2022;51:415-21. [Crossref] [PubMed]
- de Moura DTH, McCarty TR, Jirapinyo P, et al. Evaluation of endoscopic ultrasound fine-needle aspiration versus fine-needle biopsy and impact of rapid on-site evaluation for pancreatic masses. Endosc Int Open 2020;8:E738-47. [Crossref] [PubMed]
- Crinò SF, Di Mitri R, Nguyen NQ, et al. Endoscopic Ultrasound-guided Fine-needle Biopsy With or Without Rapid On-site Evaluation for Diagnosis of Solid Pancreatic Lesions: A Randomized Controlled Non-Inferiority Trial. Gastroenterology 2021;161:899-909.e5. [Crossref] [PubMed]
- Mangiavillano B, Crinò SF, Facciorusso A, et al. Endoscopic ultrasound-guided fine-needle biopsy with or without macroscopic on-site evaluation: a randomized controlled noninferiority trial. Endoscopy 2023;55:129-37. [Crossref] [PubMed]
- So H, Seo DW, Hwang JS, et al. Macroscopic on-site evaluation after EUS-guided fine needle biopsy may replace rapid on-site evaluation. Endosc Ultrasound 2021;10:111-5. [Crossref] [PubMed]
- Ishiwatari H, Sato J, Fujie S, et al. Gross visual inspection by endosonographers during endoscopic ultrasound-guided fine needle aspiration. Pancreatology 2019;19:191-5. [Crossref] [PubMed]
- Kaneko J, Ishiwatari H, Sasaki K, et al. Macroscopic on-site evaluation of biopsy specimens for accurate pathological diagnosis during EUS-guided fine needle biopsy using 22-G Franseen needle. Endosc Ultrasound 2020;9:385-91. [Crossref] [PubMed]
- Ashida R, Kitano M. Endoscopic ultrasound-guided tissue acquisition for pancreatic ductal adenocarcinoma in the era of precision medicine. Dig Endosc 2022;34:1329-39. [Crossref] [PubMed]
- Tong T, Zhang C, Li J, et al. Preclinical models derived from endoscopic ultrasound-guided tissue acquisition for individualized treatment of pancreatic ductal adenocarcinoma. Front Med (Lausanne) 2023;9:934974. [Crossref] [PubMed]
- Sekita-Hatakeyama Y, Fujii T, Nishikawa T, et al. Evaluation and diagnostic value of next-generation sequencing analysis of residual liquid-based cytology specimens of pancreatic masses. Cancer Cytopathol 2022;130:202-14. [Crossref] [PubMed]
- Souche R, Tosato G, Rivière B, et al. Detection of soluble biomarkers of pancreatic cancer in endoscopic ultrasound-guided fine-needle aspiration samples. Endoscopy 2022;54:503-8. [Crossref] [PubMed]
- Whittle MC, Hingorani SR. Fibroblasts in Pancreatic Ductal Adenocarcinoma: Biological Mechanisms and Therapeutic Targets. Gastroenterology 2019;156:2085-96. [Crossref] [PubMed]
- Diehl DL. Top tips regarding EUS-guided liver biopsy. Gastrointest Endosc 2022;95:368-71. [Crossref] [PubMed]
- Du C, Chai N, Linghu E. The diagnostic value of EUS-guided fine-needle aspiration/biopsy for solid pancreatic lesions: contrast-enhanced versus conventional EUS. Gastrointest Endosc 2021;94:200-1. [Crossref] [PubMed]
- Stigliano S, Crescenzi A, Taffon C, et al. Role of fluorescence confocal microscopy for rapid evaluation of EUS fine-needle biopsy sampling in pancreatic solid lesions. Gastrointest Endosc 2021;94:562-8.e1. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)



