
Indocyanine green fluorescence guided resection of parathyroid adenoma of the carotid sheath: a case report and review of the literature
Highlight box
Key Findings
• Case of primary hyperparathyroidism cure after resection of ectopic parathyroid adenoma from within the Carotid Sheath.
• Intraoperative ICG-Fluorescence assisted in complication-free and complete resection.
What is known and what is new?
• Diversity of anatomic location is common cause of failed resection of ectopic parathyroid tissue, potentially due to poor intraoperative identification.
• This case demonstrates the benefit of distinguishing parathyroid tissue in resection of carotid sheath parathyroid adenoma.
What is the implication, and what should change now?
• Use of ICG-fluorescence has potential benefit for surgeons in cases of poor pre-operative localization of ectopic parathyroid tissue or in cases of ectopic adenomas in difficult anatomic locations like the carotid sheath.
Introduction
Primary hyperparathyroidism is a common endocrinologic disease with clinical features including renal stones, pancreatitis, gastric ulcers, bone demineralization, and elevated serum calcium levels. Solitary parathyroid adenomas account for approximately 80–89% of cases of primary hyperparathyroidism, and ectopic tissue has been reported in 1–16% of cases (1-3). The small size and broad localization of ectopic parathyroid tissue poses difficulties in both initial diagnosis and resection via parathyroidectomy. There have been several advances in pre-operative testing to identify ectopic glands and current American Academy of Endocrine Surgery (AAES) guidelines recommend a combination of 99-mTc Sestamibi scan, ultrasound, or 4-dimensional computed tomography (CT) scan preoperatively (4). Intraoperative techniques have been developed to assist in minimally-invasive parathyroidectomy including use of parathyroid hormone (PTH) monitoring (5), 99-mTc Sestamibi scintography (6), and near-infrared fluorescence (NIRF) (7) imaging. More recently, indocyanine green (ICG) fluorescence-guidance has become an important adjunct in minimally-invasive parathyroidectomy (8-10).
One anatomical variant that presents a unique challenge in both diagnosis and surgical resection is a parathyroid adenoma within the carotid sheath. In two large reports, which together examined nearly 1,200 parathyroid surgeries performed for primary hyperparathyroidism, only two cases had adenomas involving the carotid sheath (11,12). Despite its rare presentation, there have been multiple cases in which failed primary parathyroid resection has been attributed to ectopic tissue later localized to the carotid sheath (13,14). One of these cases demonstrated an intravagal parathyroid adenoma within the carotid sheath which necessitated careful dissection from the nerve (13). These cases demonstrate the potential need for intra-operative adjuncts like ICG fluorescence in resection of this unique variant. Although groups have described resection of adenomas within the carotid sheath (13,15) previously, use of intraoperative ICG fluorescence imaging has not been demonstrated in these cases. The case below demonstrates the use of ICG fluorescence to guide resection of an ectopic parathyroid gland adenoma located within the left posterior carotid sheath. We present the following case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-589/rc).
Case presentation
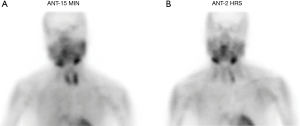
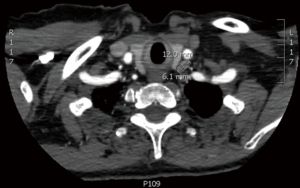
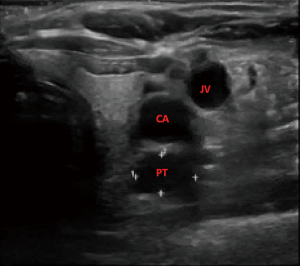
A 75-year-old woman with history of anemia and spinal stenosis presented to her primary care physician for follow-up after a routine post-menopausal bone density scan revealed a DEXA T-score of −2.6. She reported no personal or family history of renal calculi, pathological fractures, or neck surgery. Her biochemical evaluation revealed serum calcium at 11.3 mg/dL (normal range, 8.5–10.6 mg/dL), alkaline phosphatase at 98 U/L (normal range, 35–140 U/L), and PTH at 74 pg/mL (normal range, 15–65 pg/mL), with serum creatinine and 25-hydroxyvitamin D within normal ranges. A diagnosis of primary hyperparathyroidism was made, and localization studies were performed, including 99mTc Sestamibi scintigraphy with single-photon emission computed tomography (SPECT) (Figure 1) and CT scan (SPECT/CT) (Figure 2). This imaging showed a soft tissue lesion measuring 1.2×0.6×2.0 cm present along the inferior margin of the left thyroid lobe, posterior to the carotid artery, suggestive of left superior parathyroid adenoma. Upon presentation and evaluation in surgical oncology clinic, ultrasound of the neck showed a hypoechoic homogenous lesion, measuring 2.0×1.5 cm, located 3 mm deep to the carotid artery (Figure 3).
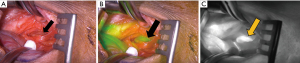
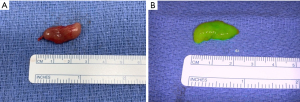
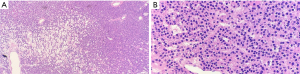
Parathyroidectomy was performed using a minimally invasive approach through a transverse cervical incision. After exploration of the left posterior neck and verification of the integrity of the left recurrent laryngeal nerve, careful dissection of the left carotid sheath was conducted. Adequate exposure revealed an enlarged parathyroid gland, which appeared as a moderately distinguished soft tissue mass deep to the left common carotid artery at the level of the Level III Cervical Lymph nodes (Figure 4A). The vagus nerve was visually identified within the carotid sheath and confirmed to be uninvolved with the adenoma and therefore did not necessitate dissection. To confirm location and hyperperfusion of the gland, 1 mL of ICG (2.5 mg/mL of ICG dissolved in sterile water) was administered as an intravenous bolus by the anesthesiologist. The Stryker SPY-PHI (SPY - Portable Handheld Imaging) system (Stryker Corporation, Kalamazoo, MI) was used to visualize the fluorescing parathyroid tissue (Figure 4B,4C) (Video 1). A HARMONIC FOCUS®+ Shears (Ethicon Endo-Surgery, Cincinnati, OH, USA) were then used to ligate the soft tissue and vascular pedicle adherent to the parathyroid and resect the adenoma within 60 seconds of ICG administration. The SPY-PHI system was then used to visualize the gross specimen on back-table and to ensure no fluorescing parathyroid tissue remained in the operative field (Video 1). Intraoperative, 10-minute post-resection PTH levels normalized to 27 pg/mL (normal range, 15–65 pg/mL) and calcium in the recovery room was 10.5 mg/dL (normal range, 8.5–10.6 mg/dL). The frozen section histopathology identified hypercellular parathyroid tissue consistent with parathyroid adenoma and final pathology report confirmed an 800 mg, 2.4×1.1×0.7 cm gross specimen (Figures 5,6). Following surgery, the patient was discharged home. The patient had an unremarkable post-operative recovery including a normal physical exam and normal speech at her 2-week follow-up appointment. She maintained a calcium level of 10.1 mg/dL (normal range, 8.5–10.6 mg/dL) six months after surgery.
The procedure performed in this study was in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The anatomic variation of ectopic parathyroid tissue is broad, including but not limited to the thymus (19–37%), retro-esophageal space (8–34%), thyroid gland (3–14%), and mediastinum (6–19%) (2,3). The embryological origins of the superior and inferior parathyroid glands are from the fourth and third pharyngeal arches respectively, with the inferior parathyroids travelling a greater distance and therefore having higher rates of ectopia (3). The superior parathyroid glands tend to descend posteriorly, and ectopic superior glands are more commonly found in posterior locations, including the vagus nerve and carotid sheath. Inferior parathyroid glands tend to descend more anteriorly, along the thyrothymic tract, and ectopic inferior glands are more commonly found anteriorly, including the superior (anterior) mediastinum and aortopulmonary window (3,16). We suspect the ectopic gland in this patient was most likely a superior parathyroid adenoma, although without identification of a normally located gland (either superior or inferior) it is difficult to be certain.
The carotid sheath, home to the carotid artery, jugular vein, and vagus nerve, with the sympathetic trunk posteriorly and ansa cervicalis anteriorly, is a critical anatomic structure that has the potential to house ectopic parathyroid tissue (2,3). Iatrogenic disruption of these structures can have critical hemodynamic or neurologic implications, and any procedure involving the carotid sheath necessitates high precision and preservation of surrounding structures.
Though carotid sheath involvement is rare (11,12), identification and resection of parathyroid adenomas within the sheath has been demonstrated to be especially difficult. Groups have previously described cases in which failed primary surgery for hyperparathyroidism has been attributed to adenomas later localized to the carotid sheath (13-15,17). Ojha et al. described identification of a carotid sheath parathyroid adenoma between the carotid artery and jugular vein via Tc-99m Sestamibi after failed primary resection (17). After SPECT/CT and ultrasound failed to reveal obvious pathology and a subsequent negative neck exploration, Chopra et al. identified a 4 cm parathyroid adenoma on contrast-enhanced CT posterior to the common carotid artery which was later resected successfully (15). Ng et al. described a case of a failed bilateral neck exploration including resection of three pathologically normal parathyroid glands with postoperative Sestamibi scan showing no evidence of parathyroid adenoma. However, in that case, 4-dimensional CT of the neck later identified a lesion and surgeons found a parathyroid adenoma adherent to the vagus nerve 3 cm above the carotid bifurcation and subsequently used a careful “nerve-sparing approach” with lymph node excision to resect the lesion (13). The adenoma in the present case was visually confirmed to be independent from the vagus nerve and therefore no dissection of ectopic tissue from the nerve was needed. Sanders et al. described a case of failed primary resection in a left hemithyroidectomy and bilateral explorative dissection. Later, dual-phase parathyroid scintigraphy confirmed the presence of residual adenomatous tissue and SPECT/CT localized this tissue within the left carotid sheath, and it was subsequently resected (14). These cases help demonstrate the diagnostic complexity and surgical difficulty that this anatomic presentation creates for clinicians; however, to the best of our knowledge, no one has previously described the use of intraoperative surgical adjuncts, such as ICG fluorescence, to aide successful primary resection of carotid sheath parathyroid adenomas.
The use of ICG fluorescence imaging has been shown to benefit surgical accuracy and therapeutic success during parathyroidectomy. In cervical surgeries, this technique was initially utilized to help preserve the parathyroid glands during thyroidectomy (8,18). Sound et al. reported the use of ICG in three patients undergoing re-operative neck surgery for primary hyperparathyroidism with success in all three cases (19). Zaidi et al. further demonstrated the utility of ICG fluorescence imaging specifically for patients undergoing initial surgery for primary hyperparathyroidism, demonstrating that 92.9% of glands visible to the naked eye demonstrated ICG uptake and that all 33 patients in the study had been biochemically cured of their hyperparathyroidism after parathyroidectomy (10). Given that ICG fluorescence imaging is a safe and adaptable modality for multiple procedures, groups have refined the technique with more standardized protocols for use, including guidelines for optimal administration dosing and timing (20). The power of ICG fluorescence-guided parathyroidectomy was importantly demonstrated by DeLong et al. when 18 of 54 patients in the study had adenomas which failed to localize on pre-operative Sestamibi scan. All 18 of these patients subsequently had an adenoma fluoresce on ICG imaging intra-operatively, allowing for resection (9). This illustrates the utility of ICG fluorescence, specifically in cases of difficult anatomical localization, because it is capable of identifying adenomas which were not located pre- or sometimes even intra-operatively. In the present case report, ICG was helpful although not critical for resection of the ectopic parathyroid; but could aid in other cases of parathyroid adenomas within the carotid sheath in redo parathyroid surgery.
The case which we present above specifically illustrates the utility of multi-modal imaging both prior to and during parathyroidectomy. While SPECT/CT and ultrasound aided in adenoma identification, the use of ICG fluorescence allowed for targeted intraoperative visualization and successful resection of the adenoma. This case builds upon previous studies using ICG for parathyroid fluorescence (9,10,19) by highlighting its utility in a rare anatomic location where clear identification and careful resection of the pathologic tissue was paramount. Despite the anatomic and operative challenges of this patient’s case, multi-modal imaging including ICG fluorescence-guidance allowed for careful surgical approach, complication free resection, and uneventful postoperative recovery. This case serves as important evidence that familiarity with this intraoperative modality is important for surgeons, as fluorescence guidance has widespread clinical utility both in parathyroidectomies and other procedures including but not limited to thyroidectomy (8) and adrenalectomy (21).
In conclusion, ectopic parathyroid adenomas are a known cause of failed parathyroidectomy, and this is particularly true for adenomas localized to the carotid sheath. This case describes a patient with primary hyperparathyroidism due to a rare parathyroid adenoma within the carotid sheath, deep to the carotid artery, in which the patient was cured utilizing ICG fluorescence-guided parathyroidectomy. Although ectopic parathyroid tissue involving the carotid sheath is rare, this location should be considered and explored with a variety of imaging modalities to prevent failed primary resection or intraoperative complications including injury to critical surrounding structures. Use of intraoperative ICG fluorescence should be considered particularly in cases where pre-operative localization involves critical anatomical structures like the carotid sheath, or when pre-operative imaging is indeterminant or negative.
Acknowledgments
Many thanks to the patient for generously authorizing us to share her rare case.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-589/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-589/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-589/coif). MB is a consultant for Stryker, Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The procedure performed in this study was in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roy M, Mazeh H, Chen H, et al. Incidence and localization of ectopic parathyroid adenomas in previously unexplored patients. World J Surg 2013;37:102-6. [Crossref] [PubMed]
- Gunasekaran S, Wallace H, Snowden C, et al. Parathyroid ectopia: development of a surgical algorithm based on operative findings. J Laryngol Otol 2015;129:1115-20. [Crossref] [PubMed]
- Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg 2006;191:418-23. [Crossref] [PubMed]
- Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg 2016;151:959-68. [Crossref] [PubMed]
- Chen H, Pruhs Z, Starling JR, et al. Intraoperative parathyroid hormone testing improves cure rates in patients undergoing minimally invasive parathyroidectomy. Surgery 2005;138:583-7; discussion 587-90. [Crossref] [PubMed]
- Usmani S, Khan HA, al Mohannadi S, et al. Minimally invasive radionuclide-guided parathyroidectomy using 99mTc-sestamibi in patients with primary hyperparathyroidism: a single-institution experience. Med Princ Pract 2009;18:373-7. [Crossref] [PubMed]
- McWade MA, Paras C, White LM, et al. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013;154:1371-7; discussion 1377. [Crossref] [PubMed]
- Fanaropoulou NM, Chorti A, Markakis M, et al. The use of Indocyanine green in endocrine surgery of the neck: A systematic review. Medicine (Baltimore) 2019;98:e14765. [Crossref] [PubMed]
- DeLong JC, Ward EP, Lwin TM, et al. Indocyanine green fluorescence-guided parathyroidectomy for primary hyperparathyroidism. Surgery 2018;163:388-92. [Crossref] [PubMed]
- Zaidi N, Bucak E, Okoh A, et al. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J Surg Oncol 2016;113:771-4. [Crossref] [PubMed]
- Lee JC, Mazeh H, Serpell J, et al. Adenomas of cervical maldescended parathyroid glands: pearls and pitfalls. ANZ J Surg 2015;85:957-61. [Crossref] [PubMed]
- Rioja P, Mateu G, Lorente-Poch L, et al. Undescended parathyroid adenomas as cause of persistent hyperparathyroidism. Gland Surg 2015;4:295-300. [PubMed]
- Ng J, Roche P, Weir J, et al. Ectopic parathyroid adenoma adherent to vagus nerve at internal carotid artery. Otolaryngol Case Rep 2020;16:100197. [Crossref]
- Sanders CD, Kirkland JD, Wolin EA. Ectopic Parathyroid Adenoma in the Carotid Sheath. J Nucl Med Technol 2016;44:201-2. [Crossref] [PubMed]
- Chopra A, Bansal R, Sharma N, et al. Parathyroid Adenoma within the Carotid Sheath. Acta Endocrinol (Buchar) 2020;16:497-500. [Crossref] [PubMed]
- Wang C. The anatomic basis of parathyroid surgery. Ann Surg 1976;183:271-5. [Crossref] [PubMed]
- Ojha B, Liu HG, Mountz JM. Large carotid sheath parathyroid adenoma localized by Tc-99m sestamibi. Clin Nucl Med 2001;26:27-8. [Crossref] [PubMed]
- Zaidi N, Bucak E, Yazici P, et al. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 2016;113:775-8. [Crossref] [PubMed]
- Sound S, Okoh A, Yigitbas H, et al. Utility of Indocyanine Green Fluorescence Imaging for Intraoperative Localization in Reoperative Parathyroid Surgery. Surg Innov 2019;26:774-9. [Crossref] [PubMed]
- Matson J, Lwin TM, Bouvet M. Rapid intraoperative perfusion assessment of parathyroid adenomas with ICG using a wide-field portable hand-held fluorescence imaging system. Am J Surg 2022;223:686-93. [Crossref] [PubMed]
- DeLong JC, Chakedis JM, Hosseini A, et al. Indocyanine green (ICG) fluorescence-guided laparoscopic adrenalectomy. J Surg Oncol 2015;112:650-3. [Crossref] [PubMed]


