
Microsurgery in oncoplastic breast reconstruction
Introduction
Breast conserving surgery has demonstrated survival benefits when compared to mastectomy in recent population-based studies (1). Oncoplastic breast surgery has become increasingly accepted as a multidisciplinary team-based approach to optimizing oncologic and reconstructive outcomes after breast-conserving surgery (BCS) (2). Studies have demonstrated fewer positive margins (3), lower re-excision rates (4), decreased complications (5) and improved patient satisfaction (6) compared to standard BCS. Oncoplastic breast surgery has therefore preserved the oncologic benefits of breast conserving therapy while minimizing the negative aesthetic sequelae associated with radiation therapy.
The goals of on oncoplastic breast reconstruction include the restoration of breast shape and contour while optimizing breast symmetry through aesthetically-placed incisions. This is achieved either through glandular rearrangement, volume displacement techniques, or using volume replacement with regional or remote tissue to minimize deadspace and restore contour. Oncoplastic reconstruction is typically indicated for Level 2 BCS procedures, or when 15–20% of the breast volume is to be removed (7).
Several factors are taken into consideration when deciding on the appropriate reconstructive technique including tumor size, breast size and patient desired breast size, tumor-to-breast ratio and tumor location. Many algorithms have been described to help guide patients and surgeons to the appropriate displacement and replacement techniques based on these factors (8). Volume displacement procedures typically capitalize on larger breast size and/or ptosis to rearrange the breast parenchyma and redrape the skin through mastopexy and reduction-type procedures. On the other hand, volume replacement techniques are preferred in patients with inadequate residual breast tissue to restore shape and contour or in smaller-breasted patients that wish to preserve breast size. The most common volume replacement techniques utilize regional tissue based on chest wall perforators including the intercostal vessels, thoracodorsal and lateral thoracic vessel and internal mammary perforators (9-16). In certain select cases, free tissue transfer for volume replacement also serves as an excellent technique to restore shape and contour.
Microvascular oncoplastic reconstruction
Microvascular partial breast reconstruction was first described by Rizzuto et al. in 2004 in which a superficial inferior epigastric artery (SIEA) flap was used to reconstruct a partial mastectomy defect in a delayed fashion after completion of radiation (17). Since then, multiple small series have utilized both abdominal and non-abdominal donor sites for oncoplastic breast reconstruction in the immediate, delayed-immediate and delayed settings (18-20).
While not as common as pedicled techniques, free tissue transfer for oncoplastic volume replacement offers unique advantages in appropriately indicated cases including flexibility in flap design and volume, limited disruption of the anatomic borders of the breast and avoidance of chest wall and back scars. Microvascular oncoplastic reconstruction also has unique considerations compared to traditional volume replacement techniques including incision planning, recipient vessel access and flap choice with preservation of options for future autologous breast reconstruction. Understanding the appropriate indications, available techniques, donor site morbidity and common challenges for these procedures can help optimize the use of free tissue transfer in the oncoplastic algorithm.
Preoperative considerations
Patient selection
Volume replacement oncoplastic procedures have traditionally been indicated for patients with small-to-medium sized breasts and planned resections of greater than 50% of breast tissue (7). However, patients with smaller breasts and smaller tumor-to-breast resection ratios can also benefit from volume replacement, as opposed to simple glandular rearrangement (21), if they wish to preserve breast size. Volume replacement with both pedicled and free tissue transfer is feasible in these patients, and several defect and patient-specific factors help guide decision-making.
Pedicled chest wall perforator flaps are particularly well-suited for lateral and central tumors when rotated or transposed from the lateral chest wall (9,22,23). Medial and superior defects can also be reached with anterior intercostal and internal mammary perforator-based flaps (10,24); however, these locations can be more challenging to address with pedicled options. Infero- and supero-medial defects are particularly well-suited for partial reconstruction with free flaps (18,20) given the proximity to internal mammary perforators for recipient vessels.
Estimated defect size should also be considered in relation to the available donor tissue volume. Patients with lower body mass index (BMI) and a paucity of regional chest wall tissue may be better suited for free tissue transfer for volume restoration. Remote donor sites should also be assessed for adequate volume, especially if preservation of future total autologous breast reconstruction sites is attempted. Finally, concealed remote donor sites such as the lower abdomen and medial thigh can be considered in patients who do not desire additional back or chest wall scars (8).
Timing
Oncoplastic breast reconstruction is most commonly performed at the time of BCS. Volume displacement procedures have demonstrated significantly higher rates of complications when performed in a delayed fashion after completion of radiation therapy (25). Parenchymal fibrosis, skin contracture, scarring, and tissue damage impede wound healing and also present challenges for reconstructive planning. Delayed oncoplastic reconstruction with free tissue transfer, however, has not demonstrated higher rates of wound healing complications in small series (19). Comparable outcomes to immediate reconstruction are likely secondary to use of non-radiated healthy tissue from remote sites, similar to delayed outcomes with pedicled flaps (25). However, reconstruction prior to radiation may be desired to prevent the sequelae associated with radiation fibrosis and the likely need for skin replacement in true delayed procedures.
Confirmation of negative tumor margins is critical particularly in cases with higher concern for adequate margins, such as patients with ductal carcinoma in situ (DCIS). In these higher-risk cases, a delayed-immediate approach (26) can be utilized to allow for margin confirmation, but reconstruction prior to radiation. Delayed-immediate reconstruction should still be performed in an expedient manner to avoid delaying radiation greater than 12 weeks and increasing risk of recurrence (27).
Preoperative planning
Preoperative planning for microvascular oncoplastic reconstruction requires a thorough understanding of both the extirpative procedure at the recipient site and the available options for donor tissue. Estimated tumor size, focality and location, as well as discussion of the planned resection with the breast surgeon help guide free flap planning. Patient tolerance of additional breast and remote donor site scars as well as desired breast size also play an important role in deciding on the appropriate reconstructive option.
Incision planning should be discussed preoperatively with both the patient and the breast surgeon to arrive at the optimal design. In the immediate setting, variations of incisions for aesthetic breast surgery are typically attempted including partial periareolar, vertical and inframammary fold (IMF) incisions. These incisions must allow for appropriate access for the extirpative procedure but also for recipient vessel access and microsurgery. IMF (Figure 1) and vertical incisions typically allow for access to the medial internal mammary perforators whereas variations of vertical and IMF incisions can provide access to the lateral chest wall vessels (Figure 2). When planning to use recipients along the lateral chest wall, axillary incisions used for sentinel node biopsies can also provide excellent access, though require tunnel the pedicle. Superior pole tumors may require more visible transverse incisions on the breast mound depending on tumor location (18). In delayed cases, existing incisions with or without the use counter-incisions can be utilized to access the lumpectomy defect as well as recipient vessels.
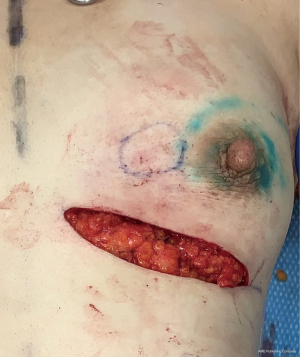
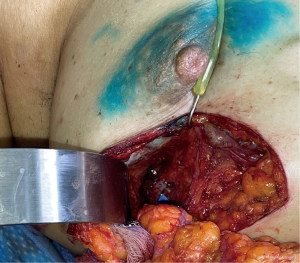
Multiple different types of free flaps have been described for partial breast reconstruction (Table 1). The largest series of microvascular partial breast reconstruction preferentially utilized the abdominal donor site based on the SIEA system, and less frequently deep inferior epigastric artery perforator (DIEP) flaps (20) when the SIEA was intraoperatively deemed to be insufficient (3). A unique consideration in microvascular oncoplastic reconstruction, however, is the preservation of potential donor site for future total breast reconstruction. The SIEA and DIEP flap designs by Spiegel et al. necessitate umbilical transposition and were acknowledged to “burn the bridge” for future abdominally-based breast reconstruction. The authors therefore recommended against this procedure in higher risks patients.
Table 1
| Flap |
| DIEP* |
| Gracilis (transverse, diagonal) |
| Omentum** |
| PAP |
| SIEA† |
| SCIA† |
| TDAP |
*, sacrifices abdominal donor site for future total autologous reconstruction; **, does not allow for cutaneous reconstruction; †, low and narrow flap design can still preserve the abdominal donor site for future total autologous reconstruction. DIEP, deep inferior epigastric artery perforator; PAP, profunda artery perforator; SIEA, superficial inferior epigastric artery; SCIA, superficial circumflex iliac artery; TDAP, thoracodorsal artery perforator.
Alternatively, non-abdominal donor sites have also been utilized for microvascular oncoplastic reconstruction. Zaha et al. described a laparoscopically-harvested free omental flap for immediate partial breast reconstruction that maintained size and contour through adjuvant radiation (28). However, as the omentum does not provide skin for resurfacing, this flap is not ideal for delayed reconstructions that often have some component of a cutaneous defect from scar contracture. The medial thigh has also been utilized with smaller transverse and diagonal gracilis flaps as well as profunda artery perforator (PAP) flaps (18,19). These donor sites can provide adequate flap volume and pedicle length for most partial defects. However, the medial thigh is also a common donor site for total autologous reconstruction in thinner patients that lack abdominal volume. Finally, the back has also been described for free tissue transfer in the form of thoracodorsal artery perforator (TDAP) flaps (19), which similarly can provide excellent volume and pedicle length for partial defects. Issues of desired scar placement as well as sacrifice of pedicled bail out options, however, should also be considered at this site.
The abdominal donor site can also be utilized while still preserving the majority of abdominal tissue for future reconstruction, if needed. Planning a low transverse incision and a “mini-flap” of limited height (Figure 3) allows for preservation of the DIEP flap donor site while still providing adequate tissue based either on the SIEA or superficial circumflex iliac artery (SCIA) systems for partial breast reconstruction. However, this technique does have volume limitations in order to minimize the height of the flap and preserve the abdominal donor site. While some volume can be increased by flap folding, defects significantly larger than 100 g require alternative donor sites or procedures. Additionally, certain studies have elucidated concerns over superficial-dominant drainage of DIEP flaps. As the mini-flap does require sacrifice of the SIEV, this may require adjustments in perforator selection in the case of a “superficial dominant” flap.
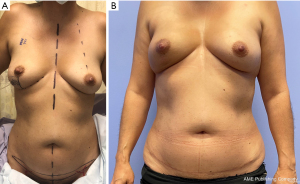
Intraoperative considerations
Recipient vessels
All procedures performed in this work were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal. Recipient vessel access is likely the most important consideration in microvascular oncoplastic reconstruction (Table 2). Limited incisions, partial defects and limited pedicle length depending on flap choice can provide significant challenges for free tissue transfer and require appropriate planning of incisions and flap design. In the medial chest, the internal mammary vessel perforators serve excellent recipient vessels when preserved during the partial mastectomy. While the proper internal mammary vessels are ideally preserved for potential future total autologous reconstruction (29), use of the internal mammary vessels for partial reconstruction still allows for their proximal/distal utilization beyond intact branches, anastomosis to the prior pedicle, or lateral chest wall vessels, if needed (19). The lateral chest wall has an abundance of vessels that can be used as recipients including the intercostal perforators, serratus branch and lateral thoracic vessels. The thoracodorsal vessels are typically avoided, as possible, to preserve the latissimus flap as a future potential backup option in the future.
Table 2
| Recipient vessel location |
| Medial |
| Internal mammary perforators |
| Internal mammary vessels |
| Lateral |
| Intercostal perforators |
| Lateral thoracic vessels |
| Serratus branch |
| Thoracodorsal vessels |
Flap choice and defect location also have significant implications for recipient vessel usability. The DIEP, medial thigh-based and TDAP flaps should provide pedicle length to reach either the medial or lateral chest from most breast defect locations. Smaller SIEA and SCIA-based flaps, however, have shorter pedicles that may have difficulty reaching medial or lateral recipients from central defects in wide-chested patients, necessitating the use of vascular grafts.
Flap design
Flap design should take into consideration the necessary volume to eliminate deadspace and restore shape, the need for skin, and the optimization of pedicle length for the desired recipient vessels. In immediate reconstruction, final flap size should be based on the final size and/or weight of the lumpectomy specimen. In delayed cases, this can be more challenging due to fibrosis of subcutaneous tissue and gland as well as contracture of the skin envelope that may require design of a larger flap, including a skin component, after thorough scar release.
Typically, volume goals for the therapeutic breast in oncoplastic reconstruction are made slightly larger than the contralateral to account for radiation fibrosis and shrinkage (30). Spiegel et al. designed flaps around 20% larger than the respective defect to account for radiation changes in immediate cases (20) whereas Smith et al. advocate for achieving breast symmetry without oversizing regardless of timing (19). The latter authors recommend addressing radiation volume loss by reducing the contralateral breast; however, patient desiring to preserve as much breast size as possible may benefit from oversizing to avoid contralateral reductions. Excess volume present after completion of radiation therapy easily be addressed with suction lipectomy.
Flap design in abdominally-based reconstructions is particularly critical if attempting to preserve the abdomen for potential future total breast reconstruction. Rizzuto et al. initially described the lower SIEA design in partial mastectomy reconstruction to avoid transposing the umbilicus (17). This principle can be taken a step further to design the flap as a narrow strip of lower abdominal tissue in the underwear line based on either the dominant SIEA or SCIA system, while preserving the majority of abdominal tissue for future DIEP flaps, as needed. In these instances, it is critical to design flaps eccentrically around their respective pedicle to maximize pedicle length.
Postoperative considerations
Small series in the literature have demonstrated low rates of complications with microvascular oncoplastic reconstruction in the immediate, delayed-immediate and delayed settings (17,19,20). Expeditious completion of radiation therapy in immediate cases is critical to maintaining the efficacy of breast conserving therapy (27), and any wound healing complications regardless of how minor should be addressed aggressively. Patients should continue routine postoperative cancer monitoring according to oncologic guidelines similar to traditional oncoplastic procedures. Spiegel et al. reported no cases of recurrence in 12 patients with a mean follow-up length of 5 years (20). Secondary revisions can include fat grafting, liposuction, skin paddle excision and contralateral procedures as needed. Smith et al. reported overall revision rates of 27% in free flap oncoplastic reconstructions, comparable to 20% in traditional pedicled volume replacement procedures (19).
Conclusions
Volume replacement techniques in oncoplastic breast reconstruction have traditionally been limited to regional pedicled flaps; however, ongoing data has suggested a role for free tissue transfer in these cases as well. Consideration free tissue transfer options after breast conserving surgery only expands the potential tools in oncoplastic breast reconstruction through a collaborative approach that may optimize aesthetic results in patients that may not otherwise be good candidates for volume replacement techniques. Microsurgical oncoplastic breast reconstruction is particularly well-suited for small-breasted patients desiring to preserve breast size, those with a paucity of regional tissue and for medial breast defects in the immediate, delayed-immediate and delayed settings. Important considerations include aesthetically designed incisions, recipient vessel access, maximizing pedicle length and ensuring flap design preserves autologous options for potential future total breast reconstruction (Table 3). Larger comparative studies will help refine indications for this procedure within the overall algorithm of oncoplastic reconstruction.
Table 3
| Key points |
| Design aesthetic incisions for oncologic and recipient vessel access |
| Confirm final resection size prior to flap isolation |
| Preserve options for total autologous breast reconstruction |
| Maximize pedicle length (eccentric flap design) |
| Define recipient vessels prior to final flap design |
| Design flap slightly larger than lumpectomy size if maintaining breast size is desired |
| Consider delayed-immediate approach in high-risk cases for recurrence (i.e., DCIS) |
| Team-based approach with oncologic surgeon in all cases |
| Shared decision-making and preoperative counseling on expected outcomes is critical |
DCIS, ductal carcinoma in situ.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gland Surgery for the series “Advances in Microsurgical Breast Reconstruction”. The article was sent for external peer review organized by the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-561/coif). The series “Advances in Microsurgical Breast Reconstruction” was commissioned by the editorial office without any funding or sponsorship. KMP serves as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Gland Surgery from February 2015 to August 2024. AAS serves as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this work were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Christiansen P, Mele M, Bodilsen A, et al. Breast-Conserving Surgery or Mastectomy? Impact on Survival. Ann Surg Open 2022;3:e205. [Crossref]
- Losken A, Kapadia S, Egro FM, et al. Current Opinion on the Oncoplastic Approach in the USA. Breast J 2016;22:437-41. [Crossref] [PubMed]
- Spiegel AJ, Khan FN. An Intraoperative algorithm for use of the SIEA flap for breast reconstruction. Plast Reconstr Surg 2007;120:1450-9. [Crossref] [PubMed]
- Benjamin MA, Sinnott C, Bawa S, et al. Re-excision Rate after Partial Mastectomy in Oncoplastic Breast-Conserving Surgery: A Single-Institutional Experience and Review of the Literature. Ann Plast Surg 2019;82:S170-2. [Crossref] [PubMed]
- Crown A, Scovel LG, Rocha FG, et al. Oncoplastic breast conserving surgery is associated with a lower rate of surgical site complications compared to standard breast conserving surgery. Am J Surg 2019;217:138-41. [Crossref] [PubMed]
- Veiga DF, Veiga-Filho J, Ribeiro LM, et al. Quality-of-life and self-esteem outcomes after oncoplastic breast-conserving surgery. Plast Reconstr Surg 2010;125:811-7. [Crossref] [PubMed]
- Chatterjee A, Gass J, Patel K, et al. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3436-44. [Crossref] [PubMed]
- Salibian AA, Olson B, Shauly O, et al. Oncoplastic breast reconstruction: Principles, current techniques, and future directions. J Surg Oncol 2022;126:450-9. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Monstrey S, et al. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg 2004;57:531-9. [Crossref] [PubMed]
- Schaverien MV, Kuerer HM, Caudle AS, et al. Outcomes of Volume Replacement Oncoplastic Breast-Conserving Surgery Using Chest Wall Perforator Flaps: Comparison with Volume Displacement Oncoplastic Surgery and Total Breast Reconstruction. Plast Reconstr Surg 2020;146:14-27. [Crossref] [PubMed]
- Youssif S, Hassan Y, Tohamy A, et al. Pedicled local flaps: a reliable reconstructive tool for partial breast defects. Gland Surg 2019;8:527-36. [Crossref] [PubMed]
- Levine JL, Soueid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg 2005;116:762-7. [Crossref] [PubMed]
- Mangialardi ML, Baldelli I, Salgarello M, et al. Thoracodorsal artery perforator flap in partial breast reconstruction: a systematic review. Plast Reconstr Surg Glob Open 2020;8:e3104. [Crossref] [PubMed]
- Gatto A, Parisi P, Brambilla L, et al. Thoracodorsal artery perforator flap, muscle-sparing latissimus dorsi, and descending branch latissimus dorsi: A multicenter retrospective study on early complications and meta-analysis of the literature. J Plast Reconstr Aesthet Surg 2022;75:3979-96. [Crossref] [PubMed]
- Lee JW, Kim MC, Park HY, et al. Oncoplastic volume replacement techniques according to the excised volume and tumor location in small- to moderate-sized breasts. Gland Surg 2014;3:14-21. [PubMed]
- Adler N, Carmon E, Chapchay K, et al. Anterior intercostal artery perforator flap for immediate reconstruction following breast conservation surgery. Microsurgery 2023;43:20-6. [Crossref] [PubMed]
- Rizzuto RP, Allen RJ. Reconstruction of a partial mastectomy defect with the superficial inferior epigastric artery (SIEA) flap. J Reconstr Microsurg 2004;20:441-5; discussion 446. [Crossref] [PubMed]
- McCulley SJ, Macmillan RD, Rasheed T. Transverse Upper Gracilis (TUG) flap for volume replacement in breast conserving surgery for medial breast tumours in small to medium sized breasts. J Plast Reconstr Aesthet Surg 2011;64:1056-60. [Crossref] [PubMed]
- Smith ML, Molina BJ, Dayan E, et al. Defining the Role of Free Flaps in Partial Breast Reconstruction. J Reconstr Microsurg 2018;34:185-92. [Crossref] [PubMed]
- Spiegel AJ, Eldor L. Partial breast reconstruction with mini superficial inferior epigastric artery and mini deep inferior epigastric perforator flaps. Ann Plast Surg 2010;65:147-54. [Crossref] [PubMed]
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005;6:145-57. [Crossref] [PubMed]
- Hamdi M, Spano A, Landuyt KV, et al. The lateral intercostal artery perforators: anatomical study and clinical application in breast surgery. Plast Reconstr Surg 2008;121:389-96. [Crossref] [PubMed]
- McCulley SJ, Schaverien MV, Tan VK, et al. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:686-91. [Crossref] [PubMed]
- Carrasco-López C, Julian Ibañez JF, Vilà J, et al. Anterior intercostal artery perforator flap in immediate breast reconstruction: Anatomical study and clinical application. Microsurgery 2017;37:603-10. [Crossref] [PubMed]
- Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg 2006;117:1-11; discussion 12-4. [Crossref] [PubMed]
- Losken A, Styblo TM, Carlson GW, et al. Management algorithm and outcome evaluation of partial mastectomy defects treated using reduction or mastopexy techniques. Ann Plast Surg 2007;59:235-42. [Crossref] [PubMed]
- Shurell E, Olcese C, Patil S, et al. Delay in radiotherapy is associated with an increased risk of disease recurrence in women with ductal carcinoma in situ. Cancer 2018;124:46-54. [Crossref] [PubMed]
- Zaha H, Inamine S, Naito T, et al. Laparoscopically harvested omental flap for immediate breast reconstruction. Am J Surg 2006;192:556-8. [Crossref] [PubMed]
- Halim AS, Alwi AA. Internal mammary perforators as recipient vessels for deep inferior epigastric perforator and muscle-sparing free transverse rectus abdominis musculocutaneous flap breast reconstruction in an Asian population. Ann Plast Surg 2014;73:170-3. [Crossref] [PubMed]
- Losken A, Brown CA. How to Optimize Aesthetics for the Partial Mastectomy Patient. Aesthet Surg J 2020;40:S55-65. [Crossref] [PubMed]


