
Neoadjuvant pyrotinib plus trastuzumab and vinorelbine for HER2-positive locally advanced breast cancer patient who was initially resistant to HP therapy: a case report and literature review
Highlight box
Key findings
• As second-line neoadjuvant therapy, the patient received pyrotinib plus trastuzumab and vinorelbine, which was evaluated as a partial response (PR), and finally underwent modified radical mastectomy.
What is known and what is new?
• For patients who have developed resistance to HP neoadjuvant therapy, there is currently no approved second-line neoadjuvant therapy.
• This is the first report to present a case of neoadjuvant pyrotinib plus trastuzumab and vinorelbine in a HER2-positive locally advanced BC patient who have developed resistance to HP neoadjuvant therapy.
What is the implication, and what should change now?
• Neoadjuvant pyrotinib plus trastuzumab and vinorelbine provide a novel treatment option for individuals who have developed resistance to HP neoadjuvant therapy.
• Well-designed clinical trials are needed to further confirm the effectiveness of this treatment regimen.
Introduction
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer (BC) accounts for 20–30% of all BCs (1). This subtype of breast cancer has an aggressive clinical behavior with high rates of recurrence and metastasis, a poor prognosis, and a 5-year survival rate of less than 30% (2). Anti-HER2 targeted therapy has significantly improved the prognosis of patients with HER2-positive BC. Trastuzumab (H) and pertuzumab (P) plus chemotherapy is the standard guideline-recommended neoadjuvant treatment for inoperable HER2-positive, locally advanced BC, which can be used for tumor downgrading, improving patients’ chances of surgical treatment, and obtaining sensitivity information of treatment drugs. However, in the NeoSphere and PEONY trials, around 12% of patients developed primary drug resistance and did not respond to treatment (3,4). For these patients, stronger regimens are required to increase efficacy. However, no standard second-line neoadjuvant treatment approach is currently available for these individuals.
Pyrotinib is a novel oral, irreversible tyrosine kinase inhibitor (TKI) drug that potently targets HER1, HER2, and HER4, blocking the activation of downstream signaling pathways, inhibiting tumor cell growth, and exerting strong anti-tumor activity (5). A phase II study (6) showed that in HER2-positive metastatic breast cancer (MBC), pyrotinib plus capecitabine significantly increased the objective response rate (ORR) of patients compared with lapatinib plus capecitabine (78.5% vs. 57.1%, respectively); The progression-free survival (PFS) was also significantly better than that of the lapatinib group (18.1 vs. 7.0 months, respectively). In the phase III PHOEBE study (7), pyrotinib plus capecitabine in the treatment of HER2-positive MBC had a median PFS of 12.5 months and an ORR of 67.2%, which was significantly better than the lapatinib plus capecitabine group. Vinorelbine, a semi-synthetic antimitotic, microtubule destabilizing drug, has been shown to be effective and well-tolerated in the treatment of MBC (8). Furthermore, in clinical trials for patients with HER2-positive MBC, vinorelbine demonstrated effectiveness and tolerability when combined with trastuzumab, lapatinib, or neratinib (9-11). This supports the evaluation of vinorelbine in combination with other anti-HER2 medicines, such as pyrotinib. In a multicenter retrospective study, HER2-positive MBC was treated with pyrotinib with vinorelbine. The data demonstrate that the therapy was well tolerated and had a median PFS of 7.8 months (12). Vinorelbine and pyrotinib have both shown efficacy in MBC. To the best of our knowledge, no study has yet assessed the efficacy of pyrotinib in combination with trastuzumab and vinorelbine in neoadjuvant therapy. Here, we present the case of a patient with locally advanced HER2-positive BC who was given pyrotinib plus trastuzumab and vinorelbine as second-line neoadjuvant treatment after developing resistance to HP therapy. The patient’s tumor shrank significantly after treatment, and the goal of the downstaging surgery was eventually met. We present the following article in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-751/rc).
Case presentation
Diagnosis and treatment in local hospital
A 58-year-old Chinese female attended a local hospital on December 2019 because of a right breast mass that had been found for more than 3 months. She had no family history of BC, and no history of other diseases. A needle biopsy of the right breast mass revealed the following: (right breast) invasive ductal carcinoma, immunohistochemistry (IHC): estrogen receptor (ER; −), progesterone receptor (PR; −), HER-2 (2+), and Ki-67 (about 15%+). Fluorescence in situ hybridization (FISH) detection revealed HER2 gene amplification. Right axillary lymph node biopsy revealed metastatic breast cancer. After a complete examination, the clinical stage was T4N2M0 IIIB stage. From December 2019 to March 2020, 4 cycles of docetaxel, carboplatin, and trastuzumab (TCbH) regimen (docetaxel 75 mg/m2 day 1 every 21 days + carboplatin AUC 6 day 1 every 21 days + trastuzumab 8 mg/kg day 1 followed by 6 mg/kg day 1 every 21 days) were performed. The patient reported that the BC shrank after treatment, but she stopped the treatment on March 2020 due to personal reasons.
Diagnosis and treatment in our hospital
In June 2020, the patient came to our hospital. We conducted a physical examination and found that there was a hard mass of about 8 cm × 7 cm under the right nipple, with poor borderline and poor mobility; the right nipple was inverted, and there was edema on the skin around the right nipple, the range was about 15 cm × 13 cm; the right axillary lymph nodes were enlarged, about 7 cm × 6 cm, hard, fused, and had poor mobility. The clinical stage was cT4N2M0 IIIB stage. The docetaxel, carboplatin, trastuzumab, and pertuzumab (TCbHP) regimen (docetaxel 75 mg/m2 day 1 every 21 days + carboplatin AUC 6 day 1 every 21 days + trastuzumab 8 mg/kg day 1 followed by 6 mg/kg day 1 every 21 days + pertuzumab 840 mg day 1 followed by 420 mg day 1 every 21 days) was initiated in June 2020, and stable disease (SD) was evaluated after 2 and 4 cycles (Figure 1).
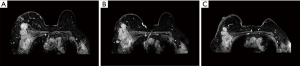
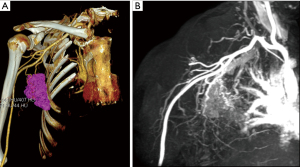
After 4 cycles of TCbHP treatment, the patient had locally advanced BC that was inoperable and refused T-DM1 treatment. Therefore, in September 2020, the regimen was changed to 6 cycles of pyrotinib (400 mg days 1–21 every 21 days) and trastuzumab (trastuzumab 8 mg/kg day 1 followed by 6 mg/kg day 1 every 21 days) plus vinorelbine (25 mg/m2 days 1, 8 every 21 days) regimen. The patient tolerated this treatment well, with mild symptoms of diarrhea. After 2, 4, and 6 cycles of treatment, the right breast tumor and the right axillary lymph node were significantly smaller than before, and the edema was significantly reduced. The curative effect was evaluated as partial response (PR) (Figure 2). Considering that the patient’s tumor had shrunk significantly, surgical treatment was planned. In addition, computed tomography angiography/magnetic resonance angiography (CTA/MRA) was performed before surgery to understand the relationship between axillary lymph nodes and blood vessels, and to evaluate the feasibility of surgery (Figure 3).

The patient underwent a modified radical mastectomy for BC on 22 February 2021. Postoperative pathological biopsy (after neoadjuvant chemotherapy for right BC) revealed right breast invasive carcinoma, no special type, histological grade: III, chemotherapy response grade (Miller-payne system) G2, tumor thrombus in the vessel, nerve invasion (−), the tumor infiltrated into the muscle tissue, about 0.5 mm from the basal resection margin; no exact cancer involvement was seen at the resection margin, the nipple was not affected. Cancer metastasis was seen in regional lymph nodes (6/9), 1/1 in the upper right axillary group, 4/7 in the middle right axillary group, and 1/1 in the right intermuscular area. The results of IHC revealed ER(−), PR(−), HER-2(2+), Ki-67(++70%). The FISH test revealed no amplification of HER2 gene.
After surgery, the patient was advised to receive adjuvant therapy with trastuzumab emtansine (T-DM1) for 1 year, but she declined the option because she could not afford the cost. It was considered that the IHC showed ER(−), PR(−), HER-2(2+), Ki-67(++70%) in the postoperative pathological examination, and there was no HER2 gene amplification. Therefore, patients received 6 cycles of capecitabine therapy and a total of 1 year of pyrotinib plus trastuzumab therapy. In addition, the patient also received postoperative adjuvant radiotherapy during treatment. Fortunately, after 23 months of follow-up, there was no recurrence or metastasis of breast cancer. A timeline is shown in Figure 4.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Patient perspective
During the treatment, I experienced mild side effects, had good tolerance and was satisfied with the therapeutic effect.
Discussion
Pyrotinib plus capecitabine exhibited clinically meaningful results and acceptable tolerability in patients with HER2-positive MBC in phase I, phase II, and phase III studies (6,7,13), which also promote its development in neoadjuvant therapy. For early and locally advanced HER2-positive BC, Xuhong et al. conducted a phase II trial (14) using neoadjuvant pyrotinib plus H and epirubicin/cyclophosphamide/docetaxel (AC-T). The findings revealed that the total pathological complete response (tpCR) rate was as high as 73.7%. Zhong et al. also reported a clinical study of neoadjuvant pyrotinib plus H and chemotherapy (albumin paclitaxel) for HER2-positive BC, the results of which showed that tpCR can reach 57.1% (15). In a multicenter phase II trial of pyrotinib and H plus docetaxel/carboplatin (TCb) regimen as neoadjuvant treatment, Liu et al. reported that the tpCR was 55.1% (16). In addition, Wu et al. described the randomized, double-blind, multicenter, phase III trial (PHEDRA) (17), which found that pyrotinib and H plus docetaxel dramatically raised the tpCR (41.0% vs. 22.0%, respectively) when compared to placebo and H plus docetaxel. Meanwhile, vinorelbine combined with pyrotinib also showed good efficacy in HER2-positive MBC (12). Based on the current medical evidence, pyrotinib plus trastuzumab and vinorelbine is a good option for second-line neoadjuvant treatment.
In the case report, When the patient was first diagnosed, the clinical stage was T4N2M0 IIIB, which was inoperable HER2-positive locally advanced BC, suitable for neoadjuvant therapy. She was initially given neoadjuvant treatment with the TCbH regimen and reported that the tumor had shrunk, and the breast lesions continued to grow after the treatment was interrupted for personal reasons. When she came to our hospital for treatment, considering that the previous TCbH regimen had been effective, she was treated with TCbHP regimen, and the effect was not good after 4 cycles. Therefore, this patient innovatively used neoadjuvant pyrotinib and trastuzumab plus vinorelbine after developing resistance to HP neoadjuvant therapy. To the best of our knowledge, the patient presented herein is the first case report of neoadjuvant pyrotinib plus trastuzumab and vinorelbine in a patient with HER2-positive locally advanced BC. After 6 cycles of neoadjuvant therapy, this patient was evaluated as PR and finally underwent modified radical mastectomy. Although the postoperative pathological evaluation did not achieve pCR, it achieved the initial surgical objective.
Pathological examination after surgery revealed residual invasive carcinoma, Miller-Payne grade G2, no pCR. Furthermore, the HER2 gene was not amplified in the remaining tumor specimens, which contradicted the findings at the time of initial diagnosis. This could be related to tumor heterogeneity, and it could also explain why some tumor cells are resistant to anti-HER2 treatment. Several clinical trials and meta-analyses have shown that capecitabine adjuvant therapy significantly improves disease-free survival and overall survival in triple-negative breast cancer (18-21). The patient was given 6 cycles of capecitabine intense adjuvant therapy based on the postoperative findings of ER(−), PR(−), HER-2(2+), Ki-67(++70%), and FISH(−). Fortunately, there was no recurrence or metastasis of breast cancer after 23 months of follow-up.
Conclusions
There is presently no standard second-line neoadjuvant treatment strategy available for these patients who develop resistance to HP neoadjuvant therapy. This case of neoadjuvant pyrotinib plus trastuzumab and vinorelbine offers these patients a novel therapy alternative. However, well-designed clinical trials will be required to demonstrate the efficacy of this therapy regimen.
Acknowledgments
Funding: This study was supported by the Special Project of Scientific and Technological Innovation for Social Undertakings and People’s Livelihood Security of Chongqing (No. cstc2017shmsA130093) and the Chongqing Natural Science Foundation (No. CSTB2022NSCQ-MSX0842).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-751/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-751/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-751/coif). Both authors report that this study was supported by the Special Project of Scientific and Technological Innovation for Social Undertakings and People’s Livelihood Security of Chongqing (No. cstc2017shmsA130093) and the Chongqing Natural Science Foundation (No. CSTB2022NSCQ-MSX0842). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Testa U, Castelli G, Pelosi E. Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-Specific Treatments. Med Sci (Basel) 2020;8:18. [Crossref] [PubMed]
- Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA 2019;321:288-300. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Shao Z, Pang D, Yang H, et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:e193692. [Crossref] [PubMed]
- Gourd E. Pyrotinib shows activity in metastatic breast cancer. Lancet Oncol 2017;18:e643. [Crossref] [PubMed]
- Ma F, Ouyang Q, Li W, et al. Pyrotinib or Lapatinib Combined With Capecitabine in HER2-Positive Metastatic Breast Cancer With Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study. J Clin Oncol 2019;37:2610-9.
- Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. [Crossref] [PubMed]
- Romero A, Rabinovich MG, Vallejo CT, et al. Vinorelbine as first-line chemotherapy for metastatic breast carcinoma. J Clin Oncol 1994;12:336-41. [Crossref] [PubMed]
- Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 2011;29:264-71. [Crossref] [PubMed]
- Janni W, Sarosiek T, Karaszewska B, et al. A phase II, randomized, multicenter study evaluating the combination of lapatinib and vinorelbine in women with ErbB2 overexpressing metastatic breast cancer. Breast Cancer Res Treat 2014;143:493-505. [Crossref] [PubMed]
- Awada A, Dirix L, Manso Sanchez L, et al. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol 2013;24:109-16. [Crossref] [PubMed]
- Li Y, Qiu Y, Li H, et al. Pyrotinib Combined With Vinorelbine in HER2-Positive Metastatic Breast Cancer: A Multicenter Retrospective Study. Front Oncol 2021;11:664429. [Crossref] [PubMed]
- Ma F, Li Q, Chen S, et al. Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol 2017;35:3105-12. [Crossref] [PubMed]
- Xuhong J, Qi X, Tang P, et al. Neoadjuvant Pyrotinib plus Trastuzumab and Chemotherapy for Stage I-III HER2-Positive Breast Cancer: A Phase II Clinical Trial. Oncologist 2020;25:e1909-20. [Crossref] [PubMed]
- Zhong X, He P, Chen J, et al. Neoadjuvant pyrotinib plus trastuzumab and nab-paclitaxel for HER2-positive early or locally advanced breast cancer: an exploratory phase II trial. Gland Surg 2022;11:216-25. [Crossref] [PubMed]
- Liu Z, Wang C, Chen X, et al. Pathological response and predictive role of tumour-infiltrating lymphocytes in HER2-positive early breast cancer treated with neoadjuvant pyrotinib plus trastuzumab and chemotherapy (Panphila): a multicentre phase 2 trial. Eur J Cancer 2022;165:157-68. [Crossref] [PubMed]
- Wu J, Liu Z, Yang H, et al. Abstract PD8-08: Pyrotinib in combination with trastuzumab and docetaxel as neoadjuvant treatment for HER2-positive early or locally advanced breast cancer (PHEDRA): A randomized, double-blind, multicenter, phase 3 study. Cancer Res 2022;82:PD8-08.
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147-59. [Crossref] [PubMed]
- Wang X, Wang SS, Huang H, et al. Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA 2021;325:50-8. [Crossref] [PubMed]
- Huo X, Li J, Zhao F, et al. The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer 2021;21:78. [Crossref] [PubMed]
- Li Y, Zhou Y, Mao F, et al. Adjuvant addition of capecitabine to early-stage triple-negative breast cancer patients receiving standard chemotherapy: a meta-analysis. Breast Cancer Res Treat 2020;179:533-42.


