
Preoperative pancreatic CT value is related to pancreatic fistula after pancreaticoduodenectomy: a retrospective study
Highlight box
Key findings
• The preoperative pancreatic computed tomography (CT) value may relate to pancreatic fistula after pancreaticoduodenectomy.
What is known and what is new?
• Pancreatic fistula is a main complication in patients undergoing pancreaticoduodenectomy.
• Pancreatic CT value can indirectly reflect the histological features of pancreas and thus may relate to postoperative pancreatic fistula.
What is the implication, and what should change now?
• Further studies are needed to establish a pancreatic fistula evaluation system based on preoperative CT scans for helping surgeons to decide upon the pancreaticojejunostomy method.
Introduction
Pancreaticoduodenectomy (PD) remains the main surgical treatment for many malignant and benign diseases in the pancreatic and periampullary area. With the development of surgical techniques and the improvement of perioperative management, the perioperative mortality for patients undergoing PD has decreased to 3–5%. However, the incidence of postoperative complications continues to be as high as 40–50%. Among the different complications after PD, pancreatic fistula (PF) remains the most common. According to recent reports (1-3), the incidence of PF ranges from 5% to 30%. PF can not only prolong the time of hospitalization, increase physical pain, and enlarge the medical burden, but it can also cause abdominal infection and massive bleeding in some severe cases.
In recent years, many comprehensive studies have been performed on the pathogenesis, risk factors, prevention, and control measures of PF. Altogether, these results suggest that the risk factors of pancreatic leak can be divided into four aspects: systematic factors (age, sex, preoperative jaundice index, nutritional status, etc.), local factors (pathological type, the texture of the pancreas, the diameter of pancreatic duct, the blood supply of pancreatic stump, the level of external secretion, etc.), intraoperative factors (operation time, blood loss, anastomosis methods, drainage of pancreatic duct, etc.), and postoperative factors (antibiotics, use of somatostatin, nutritional supports, etc.). Among these factors, local factors and intraoperative factors play a critical role in the healing of pancreaticojejunostomy directly and thus are more closely associated with the incidence of PF. Given that the operative methods can be adjusted to pancreatic local conditions, it is crucial to accurately evaluate the pancreas’ condition before operation, as this may have a significant impact on decreasing the incidence of PF.
Computerized tomography (CT) is the main method for diagnosis of pancreatic diseases. It is advisable for patients with pancreatic diseases to accept a dynamic 3-phase scan of spiral CT before operation so that surgeons can make a locational and qualitative diagnosis for the pancreatic lesion; ascertain whether tumors have invaded the important blood vessels (portal vein, superior mesenteric vein and artery), lymph nodes, and the surrounding tissue; stage tumors according to TNM classification; and finally complete preliminary evaluation concerning the resectability of the tumors. CT value represents the absorption of an X-ray when it passes through tissue. Expressed in Hounsfield units, it can reflect the density of certain tissues. In general, the CT value of high-density tissue is higher than that of low-density tissue. In our present study, we attempted to use the unenhanced CT value of pancreas to evaluate its histological characteristics for the first time. We performed an association analysis between the pancreatic CT value and the incidence of postoperative PF. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-23-19/rc).
Methods
Patients
The clinical data and CT images of 127 cases who had undergone PD with end-to-side duct-to-mucosa pancreaticojejunostomy from 2017 to 2021 in Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine were analyzed retrospectively. Among them, 68 cases were male and 59 were female. The average age of these cases was 57 years old (range 12–79 years). There were 59 patients (46.5%) with pancreatic head carcinoma, 8 patients (6.3%) with benign pancreatic head tumor, 6 patients (4.7%) with periampullary carcinoma, 18 patients (14.2%) with duodenal carcinoma, 28 patients (22.0%) with inferior common bile duct carcinoma, and 8 patients (6.3%) with chronic pancreatitis. The physical condition, clinical data, pathological diagnosis, history, and postoperative outcome of all these patients were collected and analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. XHEC-D-2022-265), and individual consent for this retrospective analysis was waived.
Surgical procedure
All the operations were performed by 3 experienced chief surgeons in our hospital. The reconstruction of digestive tract was performed according to the rule of the Child procedure. End-to-side duct-to-mucosa pancreaticojejunostomies were conducted during PD. The main steps were as follows: after the closing of the jejunum stump, suturing was used to control bleeding in the pancreatic cutting area. A silastic internal drainage tube about 10 cm in length was inserted into the pancreatic duct during the operation and was inserted into the jejunum to support the anastomotic stoma and to drain the pancreatic juice. Two-layered continuous suturing was performed in the pancreatic duct and the jejunal mucosa. Following this, a continuous suture was made in the anterior pancreatic capsule and jejunal seromuscular layer. We placed drainage tubes both below and above the anastomosis after Pancreaticojejunostomy. A stomach tube was also routinely placed.
Perioperative management
We measured the level of amylase in the drainage fluid for all the patients on the third day after operation and recorded the character and volume of the drainage. Standard intravenous fluid therapy and parenteral nutrition support were conducted. We used second-generation cephalosporin to prevent infection for 3 days and proton pump inhibitor to inhibit gastric acid secretion. The drainage tube was removed 7 days after operation if there was no occurrence of PF, seroperitoneum, abdominal abscess. or infection. When PF was suspected, remeasuring the amylase of drainage fluid and an abdominal CT scan were needed to make a definite diagnosis. The treatment depended on the different severity of PF. Postponing removal of the drainage tube, administration of antibiotics and somatostatin, ultrasound-guided percutaneous drainage, and even a second operation were used as alternative methods for treating PF.
The grading of PF
The International Study Group on Pancreatic Fistula (ISGPF) defines PF as a drainage volume from pancreaticojejunostomy of more than 50 mL/d and amylase in the drainage 3 times greater than the normal value of amylase in serum 3 days after PD. We further classified the severity of postoperative PF into 3 grades according to the ISGPF definition (1). Grade A requires little change in management or deviation from the normal clinical pathway, is not associated with a delay in hospital discharge, and is frequently managed by the slow removal of the operatively placed drainage tubes; moreover, CT scan shows no peripancreatic fluid collection. Grade B was defined as a leak resulting in changes in management and delay in discharge from the hospital. Grade C was defined as a leak requiring percutaneous drainage of fluid collection or reexploration in a critically ill patient, and delay in discharge from hospital.
The analysis of pancreatic CT value
We used a dual source 64-row spiral CT system (SOMATON Definition, Siemens) to perform upper abdominal plain plus multiphasic contrast-enhanced scanning for all the patients before surgery. The slice thickness of scan was 6 mm. The results of the scan were reserved in DICOM format and analyzed by related software. The CT value was measured based on the image of pancreatic plain scan. For patients without a dilated pancreatic duct (Figure 1A), we drew the measurement range along pancreatic contour in the broadest layer of the pancreas, excluding the areas of tumors and the splenic artery. The computer would present the CT value of these areas. We also measured the CT value of the next and the last layers and calculated the average value as our final results. The CT value ratios of the pancreas and abdominal aorta (PCT/ACT) was calculated according to our observation in order to calibrate between different imaging scans. For patients with a dilated pancreatic duct, we excluded the area of pancreatic duct when measuring (Figure 1B). The other steps were conducted in similar fashion. We excluded cases with extremely dilated pancreatic ducts or with an atrophied pancreas in which the contour of the pancreas could not be identified clearly.

Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (IBM Corp.). Continuous data are presented as the mean ± SD. The receiver operating characteristic (ROC) curve was obtained using the PCT/ACT ratio, and the most appropriate cut-off was chosen. The independent t test was used to compare the PCT/ACT between the PF and non-PF groups. The value in the different PF severity groups was compared using variance analysis. We evaluated the preoperative risk factors of PF by chi-squared test. Multiple-factor analysis was performed using the logistic regression model. A P value <0.05 was considered as statistically significant.
Results
According to our statistics, 40 patients (31.5%) had postoperative PF. Among them, there were 11 patients with a grade A leak, 23 patients with a grade B leak, and 6 patients with a grade C leak. All the patients were discharged after postoperative therapy, except 1 patient who died from intra-abdominal hemorrhage during the perioperative period.
The association between PCT/ACT and clinical variables
We divided all the cases into 2 groups in terms of sex, age, BMI, the location of tumor, the texture of pancreas, and other variables. The difference of PCT/ACT between these 2 groups were evaluated and shown in Table 1. The PCT/ACT in patients with softer pancreas tissue structure was significantly lower than in patients with stiffer tissue structure. Meanwhile, the PCT/ACT in patients who underwent preoperative endoscopic retrograde cholangiopancreatography (ERCP) was also decreased. In addition, an association was observed between PCT/ACT and the level of obesity,and patients with high BMI tended to have a low PCT/ACT.
Table 1
| Variables | Category | PCT/ACT value (SD) (SD) | t value | P value |
|---|---|---|---|---|
| Gender | Male | 0.99 (0.17) | 0.47 | >0.05 |
| Female | 0.98 (0.12) | |||
| Age (years) | ≤60 | 0.98 (0.12) | 1.13 | >0.05 |
| >60 | 1.01 (0.19) | |||
| BMI (kg/m2) | ≤23 | 0.95 (0.11) | 3.25 | <0.05 |
| >23 | 1.04 (0.18) | |||
| Gland texture | Soft | 0.96 (0.11) (0.11) (0.11) | 2.68 | <0.01 |
| Firm | 1.02 (0.18) (0.18) | |||
| Postoperative ERCP | Yes | 0.93 (0.11) | 2.56 | <0.05 |
| No | 1.01 (0.15) | |||
| Dilated pancreatic duct dilation | Yes | 0.97 (0.13) | 1.34 | >0.05 |
| No | 1.00 (0.16) | |||
| Tumor location | Pancreas | 0.99 (0.12) | 0.18 | >0.05 |
| Other | 0.98 (0.18) | |||
| Benign | 0.98 (0.16) (0.16) (0.11) | |||
| Malignant | 0.99 (0.15) (0.15) |
PCT/ACT, the CT value ratios of the pancreas and abdominal aorta; SD, standard deviation; BMI, body mass index; ERCP, endoscopic retrograde cholangiopancreatography.
Comparison of PCT/ACT between the PF and non-PF group and among the different PF severity groups
In the PF group, the average PCT/ACT was 0.886±0.113, which was significantly lower (t=5.89; P<0.001; Figure 2) than the average of 1.034±0.138 in the non-PF group. The results of variance analysis indicated a rank correlation between PCT/ACT and the severity of PF (P=0.008). The PCT/ACT in patients with severe PF was remarkably lower than that in patients with mild and moderate PF (Figure 3 and Table 2).


Table 2
| Grading | Cases | PCT/ACT value |
|---|---|---|
| A | 11 | 0.95±0.11 |
| B | 23 | 0.89±0.09 |
| C | 6 | 0.78±0.13 |
| F value | – | 5.512 |
| P value | – | 0.008 |
Description PCT/ACT value by using the mean ± standard deviation. PCT/ACT, the CT value ratios of the pancreas and abdominal aorta.
Diagnostic performance of PCT/ACT for postoperative PF
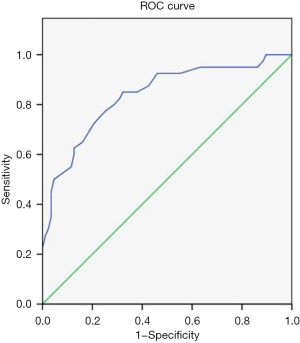
With the PCT/ACT correlating with postoperative PF occurrence, its diagnostic performance for postoperative PF was further investigated. We found that the area under the ROC curve (AUC) was 0.837 (95% CI: 0.759–0.915; P<0.0001; Figure 4). The optimal cutoff value for the PCT/ACT (0.99) was selected based on the best sensitivity and specificity combination.
Univariate and multivariate analysis of preoperative predictive factor of postoperative PF
All the 127 cases were divided into 2 groups according to whether postoperative PF occurred or not. We evaluated the preoperative clinical indices that were likely to be associated with the incidence of PF (Table 3). The results of single-factor analysis indicated that BMI >25, an undilated pancreatic duct in CT imaging, hypoproteinemia before operation, and PCT/ACT <0.99 were correlated with the incidence of PF, while age, sex, preoperative anemia, preoperative jaundice, preoperative diabetes, and the location of tumor were not correlated. A further multifactor analysis identified undilated pancreatic duct in preoperative CT imaging and PCT/ACT <0.99 as independent preoperative predictors.
Table 3
| Variable | Category | Group | χ2 | Univariate P value | OR (95% CI) | Multivariate P value | |
|---|---|---|---|---|---|---|---|
| PF | Non-PF | ||||||
| Age (years) | <60 | 11 | 28 | 0.050 | 0.850 | ||
| ≥60 | 29 | 59 | |||||
| Sex | Male | 22 | 46 | 1.685 | 0.281 | ||
| Female | 18 | 41 | |||||
| BMI (kg/m2) | ≥25 | 19 | 23 | 5.492 | 0.019 | 1.8 (0.658–4.7) | 0.260 |
| <25 | 21 | 64 | |||||
| Dilated pancreatic duct | No | 25 | 35 | 5.453 | 0.02 | 2.8 (1.1–6.8) | 0.028 |
| Yes | 15 | 52 | |||||
| Alb | <30 | 8 | 16 | 9.284 | 0.002 | 1.4 (0.449–4.4) | 0.558 |
| ≥30 | 32 | 71 | |||||
| Tb | ≥117 | 18 | 31 | 1.015 | 0.314 | ||
| <117 | 22 | 56 | |||||
| PCT/ACT | <0.985 | 34 | 28 | 30.592 | <0.001 | 11.3 (4.0–31.6) | <0.001 |
| ≥0.985 | 6 | 59 | |||||
| Tumor location | Pancreas | 19 | 48 | 1.206 | 0.752 | ||
| Bile duct | 13 | 21 | |||||
| Duodenum | 6 | 12 | |||||
| Other | 2 | 6 | |||||
| Hb | <110 | 12 | 21 | 0.490 | 0.518 | ||
| ≥110 | 28 | 66 | |||||
| Fasting plasma glucose | ≥7.4 | 15 | 29 | 0.210 | 0.691 | ||
| <7.4 | 25 | 58 | |||||
PF, pancreatic fistula; BMI, body mass index; Alb, albumin; Tb, total bilirubin; PCT/ACT, the CT value ratios of the pancreas and abdominal aorta; Hb, hemoglobin; OR, odds ratio; CI, confidence interval.
Discussion
Postoperative PF is a particularly common complication in patients undergoing PD. A large number of studies have shown the texture of pancreas relates closely to the incidence of PF. Marchegiani et al. reported in their study that the incidence of PF in patients with softer pancreas tissue texture was 20.4 times higher than that in patients with stiffer tissue texture (2). Hatano et al. reported that a softer pancreas often had a stronger secretive function. They further indicated that some small pancreatic ducts in the cutting surface were not ligated during PD, which probably caused the postoperative leak of pancreatic juice (3). Therefore, a softer pancreas may be an independent risk factor for pancreatic leak. However, no unified standard evaluation for the stiffness of the pancreas has been developed thus far. Different surgeons have different subjective criteria, and we can only obtain quantitative results of pancreatic stiffness through intraoperative touch. Therefore, establishing an objective and accurate evaluation mechanism is urgently needed.
CT value represents the absorption of an X-ray when it passes through tissue. Expressed in Hounsfield units, it can reflect the density of certain tissues. In general, the CT value of high-density tissue is higher than that of low-density tissue. In our study, we compared the preoperative CT value of the pancreas with the intraoperative stiffness of the pancreas. Our results indicated the patients with high PCT/ACT often had a stiffer pancreas. We thus surmised that the PCT/ACT could accurately reflect the stiffness of the pancreas. Meanwhile, there are also other factors that could affect this value: the pancreatic edema caused by preoperative ERCP could decrease the value of PCT/ACT, and patients with obesity tend to have a lower PCT/ACT probably because of the fatty infiltration in the pancreas (4). We still need more further studies to investigate the relationship between PCT/ACT and the stiffness of the pancreas so as to further elucidate its mechanism.
In addition to the density of the pancreas, CT value can also provide information regarding the tissue characteristics of the pancreas. Histologically, the pancreas consists of many lobes separated by thin fibrous barriers. It is a dual-function organ with both exocrine and endocrine cells. The endocrine tissue only accounts for 5% of the total organ, and the vast bulk of the pancreas is composed of exocrine tissue. Secretions from the exocrine cells are reserved in each acinus and ultimately flow into a series of pancreatic ducts. Because the pancreatic juice shows low density in CT images, a pancreas with abundant acini corresponds to a low CT value. Since we requested all the patients to keep an empty stomach before the CT scan, the influence of food on the pancreatic secretion was eliminated. Consequently, we believe the pancreases of the patients with a lower CT value are often much richer in acini and have a stronger secretive function. For these patients, it is easier to damage acini and cause the leak of pancreas juice when we suture the tissue. This leaked fluid can also accumulate around the anastomotic stoma which can elevate the tension of the anastomotic stoma and result in anastomotic fistula.
Pathologically, the CT value increases when the tissue develops fibrosis. It was reported the severity of liver fibrosis has a positive correlation with the CT value of the liver (4). Xia et al. also found that severe lung fibrosis could lead to an increased CT value in the corresponding area (5). Therefore, the same correlation might exist between pancreatic fibrosis and increased CT value. Kakita et al. investigated the association among the fibrotic level of pancreatic stump, postoperative exocrine function, and the defect of pancreaticojejunostomy (6). The results showed the level of pancreatic fibrosis related closely to pancreatic exocrine function and could be a critical factor of pancreaticojejunostomy outcome. Bassi et al. performed a randomized controlled study, with the results indicating that patients with a fibrotic pancreas had a lower incidence of anastomotic fistula (7). Thus, the increased CT value might indicate the pancreas had developed fibrosis, which would decrease the risk of PF significantly. On the other hand, the CT value will decrease when pancreas develops edema, necrosis, and fatty infiltration as was demonstrated in our results. These pathologic changes are also deleterious to the healing of the anastomotic stoma. Liu et al. reported the key to the healing of pancreaticojejunostomy was the appearance and rapid growth of granulation tissue around the wound surface 3 days after operation (8), which produced collagenous fiber and substrate, strengthening the anastomotic stoma mechanically. When the pancreas develops edema, necrosis, or fatty infiltration, this can make suturing during the operation difficult and cause the PF in the early stage after the operation. Moreover, insufficient blood supply in the anastomotic stoma caused by these changes can also inhibit the increase of cells and granulation tissue, leading to delayed healing and PF.
The method of pancreaticojejunostomy is another critical factor affecting the incidence of PF. The main methods used currently include end-to-end or end-to-side invaginated pancreaticojejunostomy, duct-to-mucosa pancreaticojejunostomy, and binding pancreaticojejunostomy(9). It is still not clear which method works best for preventing postoperative PF. In our hospital, we prefer performing duct-to-mucosa pancreaticojejunostomy, which can keep good patency of the anastomosis and maintain good pancreas function. Direct anastomosis of the pancreatic duct and jejunal mucosa contributes to a rapid healing of the mucosa and prevents the contamination of the pancreatic juice from the cutting surface, which consequently decreases the incidence of PF. Although it is a technically demanding procedure, it can be performed successfully even in patients with pancreatic ducts less than 0.3 cm in diameter due to the development of microsurgical techniques and the improvement of surgical instruments. Furthermore, although duct-to-mucosa pancreaticojejunostomy is a mature anastomotic technique, our anastomotic methods can be adjusted according to the variable stiffness of the pancreas and the diameter of the pancreatic ducts. Suzuki et al. indicated duct-to-mucosa anastomosis was suitable in low-risk patients with dilated pancreatic ducts or with pancreatic fibrosis, while side-to-side invaginated anastomosis would be safer in patients with undilated pancreatic ducts or softer pancreases (10). Bai et al. also suggested that end-to-end invaginated pancreaticojejunostomy can reduce PF rate in patients with softer pancreases or smaller diameters of the pancreatic ducts. The authors reported a 0% PF rate after the completion of 150 cases using a binding pancreaticojejunostomy they had developed (11). Therefore, preoperative CT value can help to inform the choice of anastomotic methods during operation. However, further studies are needed for us to determine whether invaginated or binding pancreaticojejunostomy can decrease the incidence of PF for patients with a low pancreatic CT value before operation.
Preoperative CT can provide more valuable information concerning the risk evaluation of PF compared to CT value alone. The CT image can inform us whether the pancreatic ducts are dilated. Our study also found that a narrow pancreatic duct is one of the risk factors for the incidence of PF. Moreover, we were able to indirectly evaluate the blood supply around the pancreatic cutting surface through dynamic enhanced CT scanning. This is because the tissue rich in capillaries shows strong CT enhancement, and sufficient blood supply is critical to improving the healing of anastomotic stoma. Thus, with more detailed studies, we might establish an evaluation system for the risk of postoperative PF based on the CT value of the pancreas, diameter of the pancreatic ducts, and enhanced CT value. According to those systems, we will be able to adjust the intraoperative anastomosis and perioperative management so as to reduce the incidence of PF and improve the prognosis of patients undergoing PD (12).
Conclusions
The preoperative plain scan pancreatic CT value can indirectly reflect the histological features of pancreas and thus may related to PF. Patients who have a lower PCT/ACT are more likely to develop postoperative PF. Further studies are needed to establish a PF evaluation system based on preoperative CT scans that can aid surgeons in selecting a pancreaticojejunostomy method.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 82272691); Key Specialty Construction Project of Pudong Health Commission of Shanghai (No. PWZzk2022-09); Clinical project of Shanghai Health Commission (No. 201940224).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-23-19/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-19/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-23-19/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-23-19/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. XHEC-D-2022-265), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Marchegiani G, Bassi C. Prevention, prediction, and mitigation of postoperative pancreatic fistula. Br J Surg 2021;108:602-4. [Crossref] [PubMed]
- Hatano M, Watanabe J, Kushihata F, et al. Quantification of pancreatic stiffness on intraoperative ultrasound elastography and evaluation of its relationship with postoperative pancreatic fistula. Int Surg 2015;100:497-502. [Crossref] [PubMed]
- Bandula S, Punwani S, Rosenberg WM, et al. Equilibrium contrast-enhanced CT imaging to evaluate hepatic fibrosis: initial validation by comparison with histopathologic sampling. Radiology 2015;275:136-43. [Crossref] [PubMed]
- Xia L, Lu F, Wang Y, et al. Compute tomography-based quantitative evaluation of pneumoconiosis. Nan Fang Yi Ke Da Xue Xue Bao 2012;32:1768-72.
- Kakita A, Yoshida M, Takahashi T. History of pancreaticojejunostomy in pancreaticoduodenectomy: development of a more reliable anastomosis technique. J Hepatobiliary Pancreat Surg 2001;8:230-7. [Crossref] [PubMed]
- Bassi C, Falconi M, Molinari E, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery 2003;134:766-71. [Crossref] [PubMed]
- Liu YB, Zhu JH, Wang JW, et al. Comparison of wound healing after pancreaticojejunostomy with three anastomotic methods in piglets. Zhonghua Wai Ke Za Zhi 2006;44:339-43.
- Li Q, Zhou X, Duan J, et al. Decreased pancreatic leakage rate in the application of a measurable variable-diameter pancreatic duct catheter in laparoscopic pancreaticoduodenectomy. Gland Surg 2022;11:1546-54. [Crossref] [PubMed]
- Suzuki Y, Fujino Y, Tanioka Y, et al. Selection of pancreaticojejunostomy techniques according to pancreatic texture and duct size. Arch Surg 2002;137:1044-7; discussion 1048. [Crossref] [PubMed]
- Bai MD, Rong LQ, Wang LC, et al. Experimental study on operative methods of pancreaticojejunostomy with reference to anastomotic patency and postoperative pancreatic exocrine function. World J Gastroenterol 2008;14:441-7. [Crossref] [PubMed]
- Yin J, Zhu Q, Zhang K, et al. Development and validation of risk prediction nomogram for pancreatic fistula and risk-stratified strategy for drainage management after pancreaticoduodenectomy. Gland Surg 2022;11:42-55. [Crossref] [PubMed]
(English Language Editor: J. Gray)


