
Localization of perforators in autologous breast reconstruction with deep inferior epigastric perforator (DIEP)-flap—the indocyanine green angiography perspective
Autologous breast reconstruction with free flap abdominal based tissue has gained increasing popularity over the years and is considered as a preferable choice in the patient with an adequate abdominal donor-site.
Balancing a low donor-site morbidity and preserving adequate tissue perfusion while providing the patient with an acceptable breast in terms of shape, size and symmetry, is fundamental for a successful reconstruction.
Autologous-based breast reconstruction was first described by Fujino in 1976 using a gluteus maximus myocutaneous flap (1), and has since evolved from Holmstrom’s rectus abdominis musculocutaneous free flap to the pedicled transverse rectus abdominis (pTRAM) flap, while today primarily including the free transverse rectus abdominis myocutaneus (TRAM) flap, the muscle-sparing (MS) TRAM flap and the deep inferior epigastric perforator (DIEP) flap (2).
The DIEP-flap was first introduced for autologous breast reconstruction by Allen et al. in 1994 (3) and has since become a workhorse within autologous breast reconstruction (2,4,5).
The DIEP-flap is based on one or several musculocutaneous perforators and generally characterized by a low donor site morbidity and an acceptable aesthetic result (6,7). Perfusion-related complications in any breast reconstruction remains an important issue, making preservation of sufficient perfusion crucial to the surgical and aesthetic outcome (2,8).
The vascular anatomy of the abdominal wall has been studied intensively and various classification systems for perfusion have been described (8,9). Hartrampf, Sheflan and Dinner and later Holm et al., described perfusion by the classical perfusion zones concept (10-13).
The perforasome theory based on the angiosome concept (14), was introduced in 2010 by Saint-Cyr et al. (9), investigating the perforator perfusion by localization in lateral vs. medial rows (15). The DIEP-flap perfusion has since then been described according to localization by rows (8,16,17).
Despite numerous studies on vascular anatomy, quality, size, and location (zones vs. rows) of the abdominal wall perforators, there is no consensus on the ideal DIEP-flap perfusion classification system (18).
Preoperative computed tomography angiography (CTA) has become gold standard for surgical planning including perforator mapping and selection (19). The preoperative CTA provides important information on perforator anatomy supporting the intraoperative clinical judgement of perfusion (20). Yet, this snapshot of vascular anatomy may not be comparable to the intraoperative findings, emphasizing the need for a reliable method to perform a perioperative perfusion assessment (19,20).
Indocyanine green angiography (ICG-A) is a well described imaging method for real-time assessment of tissue perfusion (21). ICG-A has been reported to be associated with lower risk of complications in autologous breast reconstruction (22), making this modality a valuable intraoperative assessment tool for the breast reconstructive surgeon. Though ICG-A is widely used to guide intraoperative procedure adjustments i.e., DIEP-flap resection area and resection margins in tumor surgery, there exists no international consensus on interpretation of perfusion, absolute vs relative values and cut-off score (6,23,24).
Optimal preoperative planning, clinical judgement and intraoperative decision-making is a prerequisite in achieving a successful breast reconstruction. Combining preoperative CTA with intraoperative ICG-A may be crucial to guide and optimize surgical decision-making. Using all available measures to minimize per- and postoperative complications, donor site morbidity and ultimately to provide highest possible patient satisfaction, aesthetic outcome, and quality of life.
We have read the original article by Park, Lee and Woo with great interest (5). Park, Lee and Woo analyzed DIEP-flap perfusion by vertical location of the dominant perforators, in 67 women undergoing unilateral autologous breast reconstruction.
The article assumes that the horizontal line crossing the lower margin of the umbilical stalk act as a threshold dividing perforators into two groups with different characteristics in terms of flap perfusion. In dominant perforators above this line, contralateral perfusion is blocked by umbilical incision for flap elevation. Conversely dominant perforators below this line will have maintained contralateral perfusion.
Most dominant DIEP-flap perforators are in the periumbilical region within 3 cm of the umbilicus, placing the dominant perforator eccentrically in the upper portion of the flap. Furthermore, if the dominant perforator is located beside the umbilical stalk, umbilical incision can interfere with linking vessels resulting in reduced perfusion.
The authors hypothesize that the relative perfusion is reduced when the dominant perforator is located aligned with or above the umbilical stalk. Also, that inclusion of an additional perforator located below the umbilical stalk may increase perfusion.
The analysis of perfusion was based on the dominant perforators’ vertical location above or below the umbilical stalk. In addition, the effect on flap-perfusion when adding an additional perforator was investigated. Quantitative analysis of perioperative perfusion was performed using ICG-A.
To investigate this hypothesis, preoperative CTA was performed in all cases to identify and select the dominant perforator. Perforators were then divided into 2 zones based on which level the perforators penetrated the anterior rectus sheath. Vertical zone 1: perforators penetrating the anterior rectus sheath at or above the lower margin of the umbilical stalk. Vertical zone 2: perforators penetrating the anterior rectus sheath below the lower margin of the umbilical stalk (Figure 1).
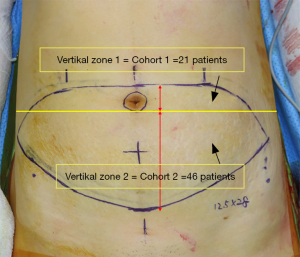
Perioperative ICG-A was performed in two stages to assess and analyze the quantitative perfusion. Stage 1: after completion of intramuscular dissection of the dominant perforator and clamping of other perforators. Stage 2: assessment of perfusion right before pedicle division, to evaluate the effect of additional perforators.
Park, Lee and Woo found that in 68.7% of the included 67 cases, the dominant perforator was located below the umbilical stalk (zone 2, cohort 2), with the remaining 31.3% located above the umbilicus (zone 1, cohort 1).
Cohort 2 showed significantly higher flap inset rate, with flap inset rate defined as proportion of inset flap to harvested flap weight (5). Furthermore, perfusion outcomes (perfused area, perfused proportion and maximal distance of midline cross) in DIEP-flaps based on a single dominant perforator located in zone 2, was found significantly higher compared to zone 1.
In order to analyze the effect on perfusion when adding an additional perforator, preoperative CTA identified additional perforators in 49%, resulting in 33 cases included for analysis. All additional perforators were harvested from zone 2.
The vertical-spacing group was defined as cases with the dominant perforator in zone 1 and the additional perforator in zone 2. Cases with both the dominant perforator and the additional perforator in zone 2, were defined as no-vertical-spacing group. The vertical-spacing group constituted 48.5%.
Of all cases with an identified additional perforator, the flap inset rate was higher in the no-vertical-spacing group compared to the vertical-spacing group (61.8% vs. 54.7%). Inset rates of both groups were comparable to the overall inset rate of cohort 1 (55%) and cohort 2 (62%).
Though inset rates were not increased by adding an additional perforator, the perfused proportion increased significantly in both groups. The addition increased perfusion from 56% to 73% in the vertical-spacing group and from 70% to 75% in the no-vertical-spacing group.
In conclusion, in cases requiring a large flap with the dominant perforator located in zone 1, adding an additional perforator from zone 2 should be considered, increasing the perfused proportion from 56% to 73%. Alternatively, if no suitable additional perforators from zone 2 is located on the preoperative CTA, a bipedicled flap should be considered. Cases requiring a smaller flap/lower inset rate (<56%), can be based on a single perforator from zone 1. Single dominant perforators from zone 2 can perfuse larger flaps up to 70%, with potential to increase the perfused proportion to 75% when adding an additional perforator.
As the authors acknowledge the study has some limitations. The study is a retrospective study of a prospectively collected cohort, corresponding to evidence level II (25). In addition, the study cohort consists of a population with a relatively low body mass index (BMI; kg/m2) of 24.2.
According to the World Health Organization, >14% of the world’s population is classified as obese with a BMI >25. With an increasing obese population, it is expected that a higher flap inset rate (proportion of inset flap to harvested flap weight) will be needed within breast reconstructive surgery due to larger mastectomy specimen weight.
Given the premise that approximately 1/3 of dominant DIEP-flap perforators are located in zone 1, and that zone 1 dominant perforators have an inset rate of 55%, consequently, there will be an increased need for flaps based on > one perforator, alternatively a bipedicled- or supercharged-flap. An additional perforator from zone 2 (vertical-spacing-group) may increase perfused proportion with 17% (from 56% to 73%).
Furthermore, as the authors state, the use and assessment of ICG-A is subjective and international consensus on the intraoperative application and interpretation has yet to be established. Several stages of applying ICG-A for autologous breast reconstruction, including the intraoperative dose of ICG, interpretation of perfusion (i.e., fluorescence intensity) and cut-off scores (absolute- or relative values), remain unstandardized.
This study emphasizes the importance of applying both preoperative CTA and perioperative ICG-A in order to optimize all aspects of surgical planning- and decision-making in order to provide the breast reconstructive patient with best possible reconstructive outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gland Surgery. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-745/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated or resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fujino T, Harashina T, Enomoto K. Primary breast reconstruction after a standard radical mastectomy by a free flap transfer. Case report. Plast Reconstr Surg 1976;58:371-4. [Crossref] [PubMed]
- Chang DW. Breast Reconstruction with Microvascular MS-TRAM and DIEP Flaps. Arch Plast Surg 2012;39:3-10. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Lee KT, Eom Y, Jeon BJ, et al. Vertical Spacing of Perforators in Deep Inferior Epigastric Perforator Flap Breast Reconstruction Can Affect the Outcomes. Plast Reconstr Surg 2018;142:319-29. [Crossref] [PubMed]
- Park JW, Lee MK, Woo KJ. Influence of vertical location and spacing of perforators on perfusion in deep inferior epigastric artery perforator flap breast reconstruction: quantitative analysis using indocyanine green angiography. Gland Surg 2022;11:1851-63. [Crossref] [PubMed]
- Momeni A, Sheckter C. Intraoperative Laser-Assisted Indocyanine Green Imaging Can Reduce the Rate of Fat Necrosis in Microsurgical Breast Reconstruction. Plast Reconstr Surg 2020;145:507e-13e. [Crossref] [PubMed]
- Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg 2014;30:121-5. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Le Roux CM, et al. The perforator angiosome: a new concept in the design of deep inferior epigastric artery perforator flaps for breast reconstruction. Microsurgery 2010;30:1-7. [Crossref] [PubMed]
- Saint-Cyr M, Wong C, Schaverien M, et al. The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg 2009;124:1529-44. [Crossref] [PubMed]
- Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982;69:216-25. [Crossref] [PubMed]
- Scheflan M, Dinner MI. The transverse abdominal island flap: part II. Surgical technique. Ann Plast Surg 1983;10:12-9. [Crossref] [PubMed]
- Scheflan M, Dinner MI. The transverse abdominal island flap: part I. Indications, contraindications, results and complications. Ann Plast Surg 1983;10:24-35. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg 2006;117:37-43. [Crossref] [PubMed]
- Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987;40:113-41. [Crossref] [PubMed]
- Wong C, Saint-Cyr M, Mojallal A, et al. Perforasomes of the DIEP flap: vascular anatomy of the lateral versus medial row perforators and clinical implications. Plast Reconstr Surg 2010;125:772-82. [Crossref] [PubMed]
- Hallock GG. Perforasomes, Venosomes, and Perfusion Zones of the DIEAP Flap. Plast Reconstr Surg 2010;126:2282-3. [Crossref] [PubMed]
- Mohan AT, Saint-Cyr M. Anatomic and physiological fundamentals for autologous breast reconstruction. Gland Surg 2015;4:116-33. [Crossref] [PubMed]
- Ludolph I, Bettray D, Beier JP, et al. Leaving the perfusion zones? Individualized flap design in 100 free DIEP and ms-TRAM flaps for autologous breast reconstruction using indocyanine green angiography. J Plast Reconstr Aesthet Surg 2022;75:52-60. [Crossref] [PubMed]
- Boer VB, van Wingerden JJ, Wever CF, et al. Concordance between preoperative computed tomography angiographic mapping and intraoperative perforator selection for deep inferior epigastric artery perforator flap breast reconstructions. Gland Surg 2017;6:620-9. [Crossref] [PubMed]
- Pestana IA, Zenn MR. Correlation between abdominal perforator vessels identified with preoperative CT angiography and intraoperative fluorescent angiography in the microsurgical breast reconstruction patient. Ann Plast Surg 2014;72:S144-9. [Crossref] [PubMed]
- Lauritzen E, Bredgaard R, Bonde C, et al. Indocyanine green angiography in breast reconstruction: a narrative review. Ann Breast Surg 2022;6:6.
- Lauritzen E, Damsgaard TE. Use of Indocyanine Green Angiography decreases the risk of complications in autologous- and implant-based breast reconstruction: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2021;74:1703-17. [Crossref] [PubMed]
- Hembd AS, Yan J, Zhu H, et al. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast Reconstr Surg 2020;146:1e-10e. [Crossref] [PubMed]
- Lauritzen E, Bredgaard R, Bonde C, et al. An observational study comparing the SPY-Elite® vs. the SPY-PHI QP System in breast reconstructive surgery. Ann Breast Surg 2022; [Crossref]
- Sullivan D, Chung KC, Eaves FF 3rd, et al. The level of evidence pyramid: indicating levels of evidence in Plastic and Reconstructive Surgery articles. Plast Reconstr Surg 2011;128:311-4. [Crossref] [PubMed]


