
Decreased pancreatic leakage rate in the application of a measurable variable-diameter pancreatic duct catheter in laparoscopic pancreaticoduodenectomy
Introduction
There are 2 steps for laparoscopic pancreaticoduodenectomy (LPD) (1); that is, excision and reconstruction. Pancreaticojejunostomy is the most important concern in reconstruction, as the leakage of pancreaticointestinal anastomosis is one of the most serious complications after LPD and has an incidence rate of 5–25% (2-4). Pancreaticointestinal anastomosis leakage may lead to secondary complications, such as postoperative bleeding and abdominal infection, which is one of the main causes of perioperative death (5). Thus, experts and scholars in China have long been exploring and innovating methods for pancreaticojejunostomy, which include Chen’s technique (6,7), implantation (8), and the Hong’s single-stitch method (9). A previous report on the innovation in pancreaticojejunostomy has further promoted the popularization and development of LPD (10).
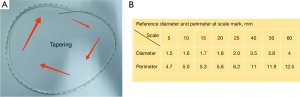
In 2017, our team began to explore the use of the Hong’s single-stitch method in pancreaticojejunostomy. The Hong’s single-stitch method was developed by Professor Defei Hong, and has achieved good results in the clinical practice of LPD (11,12). During the application of the Hong’s single-stitch method, we found that a pancreatic duct catheter was needed to drain pancreatic fluid into the jejunum. However, the common pancreatic duct catheters currently in clinical use have poor versatility. Pancreatic duct catheters are based on a single model with a fixed diameter, and the depth of placement into the pancreatic duct cannot be measured when such catheters are used. Thus, Professor Jianhua Liu developed a measurable variable-diameter pancreatic duct catheter and applied for a patent. This measurable variable-diameter pancreatic duct catheter has diameter changes from thin to thick and is marked with scales. It can be adapted to the different pancreatic duct diameters encountered in clinical practice, and can accurately determine the depth of the catheter stent inserted into the pancreas.
In this study, we conducted a retrospective comparative analysis of the relevant clinical data of 91 patients who underwent LPD with a common pancreatic duct catheter and 111 patients who underwent LPD with measurable variable-diameter pancreatic duct catheter at the Department of Hepatobiliary Surgery, The Second Hospital of Hebei Medical University, from January 2021 to April 2022, to evaluate the preventive effect on pancreatic leakage of the measurable variable-diameter pancreatic duct catheter in LPD. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-478/rc).
Methods
Patients and study design
As illustrated in Figure 1, a retrospective analysis was performed from January 2021 to April 2022 using data from The Second Hospital of Hebei Medical University from the liver surgical team, which used the Hong’s single-stitch method to perform pancreatic duct jejunum anastomosis. All the eligible patients were diagnosed with periampullary tumors or benign masses by preoperative liver function tests, tumor marker tests, ultrasonography, contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT, endoscopic ultrasonography, and duodenoscopy biopsy, and there were no signs of distant metastasis of the tumor. Patients who met all these inclusion criteria were eligible for LPD surgery. Based on the type of catheter used for the pancreaticojejunostomy, the patients who received LPD surgery were divided into the following 2 groups: (I) the normal group; and (II) the variable-diameter group. The patients’ clinical characteristics and perioperative data were collected and compared between these two groups. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Second Hospital of Hebei Medical University (No. 2020-R325), and the requirement of individual consent for this retrospective analysis was waived.
Surgical procedure
Each patient was placed in a supine position and intubated under general anesthesia. First, a laparoscopy was performed on the abdominal cavity and pelvis to exclude distant metastasis. After excision using a routine surgical procedure, the specimen was placed in a bag, and an incision of approximately 5 cm was made under the xiphoid process of the upper abdomen. The specimen was removed, and pneumoperitoneum was re-established. After the abdominal cavity was rinsed, the digestive tract was reconstructed using Child’s method (13). For all of the patients who underwent pancreaticojejunostomy, the Hong’s single-stitch method was adopted. The specific procedures were as follows:
- The neck of the pancreas was separated vertically with an ultrasonic knife, the pancreatic duct was cut with sharp scissors, and for the bleeding points, hemostasis was performed by electrocoagulation or suture. The broken end of the pancreas was dissociated by 1 cm. After identifying the pancreatic duct, a common pancreatic duct catheter or measurable variable-diameter pancreatic duct catheter (see Figure 2A,2B) was inserted. A 4-0 absorbable suture was introduced from the ventral side of the pancreas through the anterior and posterior walls of the pancreatic duct catheter (see Figure 3A). The suture was threaded out from the dorsal side of the pancreas, knotted, and fixed beside the pancreatic duct catheter with a margin >5 mm.
- At a jejunal loop, an ultrasonic knife was applied to the mesentery edge for a full thickness bowel wall incision and perforation, with the diameter of the pore approximately equal to that of the pancreatic duct catheter. A purse-string suture was placed around the pore with a 4-0 absorbable suture without knotting (see Figure 3B).
- One end of the 3-0 double-needle vascular suture was placed into the abdominal cavity, and the other end was left outside the body by a trocar. A continuous suture of the jejunal seromuscular layer and ventral pancreas was performed from the foot side to the cephalic side using 1 end of the 3-0 double-needle vascular suture. The needle was introduced from the jejunal seromuscular layer and was placed so that it exited from the pancreas side after 4–5 stitches, with an interval between stitches of 0.5–1 cm. The pancreatic duct catheter was inserted into the intestinal canal, and the purse-string suture from Step 2 was knotted. An assistant inserted a double-needle vascular suture into the abdominal cavity by the same trocar (with the vascular suture partially left outside the body to avoid having an intraperitoneal vascular suture that was too long to operate on) and placed a continuous suture of the jejunal seromuscular layer and ventral pancreas from the foot side to the cephalic side. Generally, the needle was introduced from the pancreas side and exited from the jejunal seromuscular layer after 4–5 stitches, with an interval of 0.5–1 cm between stitches depending on the width of the pancreas (see Figure 3C). The needles were then removed at both ends of the vascular suture, and the 2 ends at the upper margin of pancreaticointestinal anastomosis were knotted. The remaining vascular suture was cut at the lower margin of pancreaticointestinal anastomosis to a suitable length and was slowly tightened, and was then knotted to complete the anastomosis (see Figure 3D).
- End-to-end continuous suturing was used for bilioenteric anastomosis, and side-to-side anastomosis was used for gastrointestinal anastomosis.

Postoperative management
The patients abstained from water in the early postoperative period. The gastric tube was removed after 24 hours of drainage, flatulence, and defecation. After the gastric tube was removed, the patients then tried liquid foods. A routine proton pump inhibitor was administered to protect the gastric mucosa, and the patients also received symptomatic treatments to prevent infections, liver protection, phlegm reduction, hypoproteinemia correction, and nutritional support. After surgery, the patency and drainage of each drainage tube and the amylase content of relevant drainage fluid were checked every day. The highest amylase content of the drainage fluid in the drainage tubes was taken as the amylase value of the patient’s postoperative drainage fluid. The approaches adopted to diagnose and grade pancreatic fistulas (14), diagnose biliary fistulas (15), diagnose gastric emptying disorders (16), and diagnose postoperative hemorrhages (17) have been described previously.
The discharge indicators included the following: stable vital signs, free movement, no complications that required continued hospitalization, a normal body temperature, no signs of infection, normal food intake and defecation, and imaging findings indicating no obvious abnormality in the abdominal cavity.
The preoperative, intraoperative, and postoperative data, and the pathological results of the patients were organized and summarized, and the postoperative complications were recorded in real time.
Statistical analysis
SPSS (version 21.0) was used for the statistical analysis. The Chi-square test or Fisher’s exact test was used to compare the differences between the groups in relation to the categorical variables. Student’s t-test or the Wilcoxon rank-sum test was used to compare differences between two groups in relation to the continuous variables, depending on whether the data analyzed were normally distributed. A two-sided P value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 202 eligible patients (123 male and 79 female) were enrolled in this study, and the patient characteristics are summarized in Table 1. The patients had an average age of 58.79±7.89 years (range, 15–79 years) and an average body mass index (BMI) was 23.55±4.25 kg/m2. In terms of the preoperative complications, 24 patients had coronary heart disease, 39 had hypertension, 37 had diabetes, and 13 had a history of abdominal surgery. Among the jaundice patients, 6 underwent endoscopic nasobiliary drainage and 15 underwent percutaneous transhepatic bile duct drainage before surgery to reduce jaundice. Based on the type of catheter used for pancreaticojejunostomy, the 202 patients were divided into the following 2 groups: (I) the normal group (n=91), who underwent intestinal anastomosis with a normal pancreatic duct catheter; and (II) the variable-diameter group (n=111) who underwent intestinal anastomosis with a measurable variable-diameter pancreatic duct catheter.
Table 1
| Events | All (n=202) | Variable-diameter group (n=111) |
Normal catheter group (n=91) |
P value |
|---|---|---|---|---|
| Sex | 0.293 | |||
| Male | 123 (60.89%) | 62 (55.86%) | 61 (67.03%) | |
| Female | 79 (39.11%) | 49 (44.14%) | 30 (32.97%) | |
| Age (years, mean ± SEM) | 58.79±7.89 | 59.34±8.90 | 58.12±6.44 | 0.275 |
| BMI (kg/m2, mean ± SEM) | 23.55±4.25 | 23.75±3.99 | 23.23±4.68 | 0.394 |
| Combined underlying diseases | 113 (55.94%) | 59 (53.15%) | 54 (59.34%) | 0.378 |
| Hypertension | 39 (19.31%) | 23 (20.72%) | 16 (17.58%) | 0.574 |
| Coronary heart disease | 24 (11.88%) | 10 (9.01%) | 14 (15.38%) | 0.164 |
| Type 2 diabetes | 37 (18.32%) | 16 (14.41%) | 21 (23.08%) | 0.113 |
| Hepatitis | 13 (6.44%) | 10 (9.01%) | 3 (3.30%) | 0.100 |
BMI, body mass index; SEM, the standard error of the mean.
The clinical characteristics between the 2 groups were compared, and no statistically significant differences were found in terms of age, sex, BMI, or combined underlying diseases (P>0.05; see Table 1).
The pancreatic fistula rate in the variable-diameter catheter group was significantly lower than that in the normal catheter group
The patients’ perioperative data are shown in Table 2. The differences in the perioperative data between these 2 groups were compared, and no statistically significant differences were found in terms of preoperative bilirubin, the proportion of preoperative jaundice reduction, the operation time, intraoperative blood loss, pancreatic texture, biliary fistulas, postoperative bleeding, or disorders of gastric emptying (P>0.05; see Table 2). For both groups, the biliary fistulas healed spontaneously after conservative treatment. In the normal catheter group, 5 patients had delayed gastric emptying, including 3 Grade B patients and 2 Grade C patients. In the variable-diameter catheter group, 7 patients had delayed gastric emptying in, including 6 Grade B patients and 1 Grade C patient. For both groups, gastrointestinal decompression, the prolongation of the time of gastric intubation, and the provision of parenteral nutrition support, gastric motility therapy, and psychotherapy improved all the relevant symptoms, and the patients were all discharged successfully. In the normal catheter group, 2 patients suffered from gastrointestinal bleeding and 2 patients suffered from abdominal bleeding after surgery; the bleeding sites were the mesenteric root vessels and the broken end arterioles of the pancreas. In the variable-diameter catheter group, 3 patients suffered from intraperitoneal hemorrhages after surgery, but postoperative pancreatic fistulas were not considered. The exploratory laparotomies indicated that the pancreaticointestinal anastomoses had healed well. Bleeding points were found in all 3 cases (1 in the gastric stump and 1 in the mesentery of the small intestine) and hemostasis was successful. These 3 patients were discharged successfully after the re-operations.
Table 2
| Events | All (n=202) | Variable-diameter group (n=111) |
Normal catheter group (n=91) |
P value |
|---|---|---|---|---|
| Total bilirubin 1 d before surgery [μmol/L, median (Q1, Q3)] | 78.50 (43.16, 112.71) | 73.05 (38.53, 117.81) | 81.88 (46.63, 109.82) | 0.549 |
| Preoperative jaundice reduction | 21 (10.40%) | 13 (11.71%) | 8 (8.79%) | 0.499 |
| Operation time (min, mean ± SEM) | 313.75±69.56 | 318.85±64.00 | 307.54±75.69 | 0.251 |
| Intraoperative blood loss [mL, median (Q1, Q3)] | 400 (100, 600) | 300 (100, 500) | 400 (100, 600) | 0.09 |
| Pancreatic texture | 0.422 | |||
| Soft | 143 (70.79%) | 76 (68.47%) | 67 (73.63%) | |
| Hard | 59 (29.21%) | 35 (31.53%) | 24 (26.37%) | |
| Pancreatic fistula | 12 (5.94%) | 3 (2.70%) | 9 (9.89%) | 0.032 |
| Biliary fistula | 10 (4.95%) | 6 (5.41%) | 4 (4.40%) | 0.742 |
| Postoperative bleeding | 7 (3.47%) | 3 (2.70%) | 4 (4.40%) | 0.513 |
| Disorders of gastric emptying (gastroparesis) | 12 (5.94%) | 7 (6.31%) | 5 (5.50%) | 0.808 |
| Postoperative length of stay (d), median (Q1, Q3) | 15 (13, 18) | 15 (12, 17) | 16 (13, 19) | 0.005 |
SEM, the standard error of the mean.
Notably, the pancreatic fistula rate of the variable-diameter catheter group was significantly lower than that of the normal catheter group (2.70% vs. 9.89%, P=0.032). In the normal catheter group, 6 patients had Grade B pancreatic fistulas and 3 patients had Grade C pancreatic fistulas. In the variable-diameter catheter group, 3 patients had Grade B pancreatic fistulas, but no patients had Grade C pancreatic fistulas. The postoperative length of stay in the variable-diameter catheter group was less than that in the normal group (15 vs. 16 days), and the difference was statistically significant (P=0.005). There were no deaths in either group.
Discussion
The Hong’s single-stitch method was invented by Professor Defei Hong, and has been applied in several centers in China, and all of the surgeries have achieved good results (11,12). Based on Hong’s single-stitch method, our team formed our own views on the location of jejunal perforation, insertion and fixation of pancreatic duct catheter and purse string suture (9). There are two main purposes for inserting pancreatic duct catheter: one is to drain pancreatic juice, and another is to promote the formation of artificial fistula between pancreatic duct and jejunum along the pancreatic duct catheter. However, at present, most medical centers use local materials for the common pancreatic duct catheters required for pancreaticoenterostomy, such as scalp needles with appropriate diameters, sputum suction tubes, and anesthesia pump extension tubes (18). Thus, there is a lack of dedicated pancreatic duct catheter.
Thus, 2 problems arise in clinical work: (I) the local pancreatic duct catheter does not accurately match the diameter of the pancreatic duct; thus, the catheter with the closest size to the pancreas duct has to be selected; and (II) most of the stent catheter surfaces have no scales, and thus it is impossible to accurately measure the implantation depth during the operation. To address these issues, Professor Jianhua Liu developed a measurable variable-diameter pancreatic duct catheter to replace the original ordinary pancreatic duct catheter (see Figure 2A,2B). This measurable variable-diameter pancreatic duct catheter is marked with scales, its diameter changes from thin to thick, and it can be adapted to the different pancreatic duct diameters encountered in clinical practice. Further, the specific position of the tube body scale corresponds to the fixed value of the tube diameter so that the thickness of the pancreatic duct can be accurately measured during the operation, and the depth of the catheter stent inserted into the pancreas can be accurately determined.
The measurable variable-diameter pancreatic duct catheter has 2 main advantages. First, because the diameter of the catheter can be measured from thin to thick, the process of inserting the catheter into the pancreatic duct is much smoother. It is more suitable for the anatomical morphology of the pancreatic duct and can be inserted deeper. In the past, if the diameter of the non-variable pancreatic duct catheter matches the diameter of the pancreatic duct at the end of the pancreas fracture, it is difficult for the duct to be inserted deep enough because the closer it is to the tail of the pancreas, the thinner the pancreatic duct. Consequently, the following 2 issues often arise: (I) the diameter of the non-variable pancreatic duct catheter is difficult to intubate to an effective depth, so it can easily fall off; and (II) after forced insertion, it can be too tight, which may affect the secretion function of some pancreatic duct branches, resulting in pancreatitis. Conversely, if a pancreatic duct catheter with a diameter smaller than the diameter of the broken pancreatic duct is selected, there is a gap, which increases the risk of leakage. The use of the measurable variable-diameter pancreatic duct catheter can cut out the corresponding stent section on the measurable variable-diameter pancreatic duct according to the specific pancreatic duct diameter during the operation, which effectively solves these problems.
Second, in relation to measurability, the diameter of the pancreatic duct and the depth of the pancreatic duct insertion can be measured. Indeed, the diameter of the pancreatic duct can be measured directly using the measurable variable-diameter pancreatic duct catheter in the operation. When the measurable variable-diameter pancreatic duct catheter is perfectly matched with the pancreatic duct, the accurate value of the pancreatic duct diameter can be obtained by reading out the marked value on the pancreatic duct catheter. Currently, the diameter of the pancreatic duct is measured by preoperative CT and MRI, but the broken end of the pancreas selected during pancreaticoenterostomy surgery is not necessarily the thickest part of the pancreatic duct (19). Thus, an accurate measurement is still needed.
When selecting a suitable pancreatic duct catheter, the thinner end can be inserted into the pancreatic duct first. After determining the insertable depth and diameter of the pancreatic duct, a pancreatic duct catheter with a suitable diameter and length can be selected for insertion (20). The depth of insertion into the pancreatic duct can also be measured. Due to the magnification effect and visual deception effect of laparoscopy, it is often difficult to judge the length of the catheter inserted into the pancreas during surgery, and sometimes, it must even be pulled out and reinserted after visual inspection. A catheter with a variable-diameter can be used to measure the pancreatic duct, which completely solves this problem, as it has a marked scale on its surface and can be used to accurately measure the length of the inserted pancreatic duct, which makes the operation more accurate and improves the operation efficiency.
The length of the pancreatic duct of patients at our center is generally approximately 10 cm, and the depth of the pancreatic duct is approximately 4–5 cm. If the depth is <3 cm, the probability of prolapse shortly after surgery is high, and the intestinal segment is approximately 5 cm. If it is too long, it will not be conducive to endoscopic operation. Additionally, there is the possibility of missing the intestinal wall or encountering resistance in the intestinal wall in the intestinal cavity, which leads to an increase in the pancreatic duct pressure and pancreatic juice leakage. To address this issue, our team injects water into the intestine before inserting the pancreatic duct catheter into the intestine for lubrication. If pancreaticoenterostomy is performed by laparotomy, the length of the pancreatic duct in the intestinal segment can be 6–7 cm.
Among the indices that may affect the occurrence of postoperative pancreatic leakage in the 2 groups, there were no significant differences in 3 of the items; that is, the softness and hardness of the pancreas texture, the preoperative total bilirubin, and whether it is necessary to reduce jaundice before surgery (21). However, the number of Grade B and Grade C pancreatic fistulas in the variable-diameter group was lower than that in the normal group, and there was no significant difference in the incidence of pancreatic leakage between the 2 groups, which shows that the measurable variable-diameter pancreatic duct catheter reduces the occurrence of pancreatic leakage compared to the normal pancreatic duct catheter.
This study had some limitations. First, the sample size of the cohort was limited, and thus it is necessary to further expand the sample size. Second, this was a single-center retrospective study, and clinical randomized controlled studies need to be conducted to improve the relevant experimental conclusions. Third, strict follow-up should be carried out to observe the incidence of long-term complications after surgery.
Conclusions
Compared to the pancreatic duct catheter commonly used in clinical practice, the measurable variable-diameter pancreatic duct catheter has 2 advantages. First, it has a variable diameter. Second, it is measurable. Thus, it can better meet the needs of precise surgery. It could also decrease the pancreatic fistula rate and postoperative median length of stay in laparoscopic duodenectomy, and it is worthy of further clinical application and research.
Acknowledgments
We would like to thank Dr. Aimin Zhang from The Second Hospital of Hebei Medical University for his hand-painting of the schematic diagram of the surgical procedures for this manuscript, and Dr. Lihong Wu from Burning Rock Biotech for her assistance and suggestions for the drafting of the manuscript.
Funding: This work was supported by the S&T Program of Hebei—People’s Livelihood Science and Technology Special Project (No. 20377772D).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-478/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-478/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-478/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Second Hospital of Hebei Medical University (No. 2020-R325), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pan S, Qin T, Yin T, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: study protocol for a multicentre randomised controlled trial. BMJ Open 2022;12:e057128. [Crossref] [PubMed]
- Akizuki E, Kimura Y, Nobuoka T, et al. Prospective nonrandomized comparison between pylorus-preserving and subtotal stomach-preserving pancreaticoduodenectomy from the perspectives of DGE occurrence and postoperative digestive functions. J Gastrointest Surg 2008;12:1185-92. [Crossref] [PubMed]
- Zhang Z, Yin T, Qin T, et al. Comparison of laparoscopic versus open pancreaticoduodenectomy in patients with resectable pancreatic ductal adenocarcinoma: A propensity score-matching analysis of long-term survival. Pancreatology 2022;22:317-24. [Crossref] [PubMed]
- Naffouje SA, Pointer DT Jr, Satyadi MA, et al. Surgical approach to pancreaticoduodenectomy for pancreatic adenocarcinoma: uncomplicated ends justify the means. Surg Endosc 2022;36:4912-22. [Crossref] [PubMed]
- Pugalenthi A, Protic M, Gonen M, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol 2016;113:188-93. [Crossref] [PubMed]
- Yin XM, Li YF, Cheng W, et al. Application of Chen's pancreaticojejunostomy technique in laparoscopic pancreaticoduodenectomy (116 cases report). Zhonghua Wai Ke Za Zhi 2020;58:114-8. [PubMed]
- Sun XQ, Wang LC, Ma JH, et al. Different techniques of pancreaticojejunostomy in laparoscopic pancreaticoduodenectomy for patients with slim pancreatic ducts. Chinese Journal of Hepatobiliary Surgery 2019;12:838-41.
- Ma CY, Zhu F, Xiao GQ, et al. Application of Imbedding Pancreaticojejunostomy in Pure Laparoscopic Pancreaticoduodenectomy. Chinese Journal of Bases and Clinics in General Surgery 2016;4:388-92.
- Hong DF, Liu YH, Zhang YH, et al. The role of Hong's single-stitch duct to mucosa pancreaticojejunostomy in laparoscopic pancreaticoduodenectomy. Zhonghua Wai Ke Za Zhi 2017;55:136-40. [PubMed]
- Wang Z, Wang X, Ke N. A different suturing method of the duct-to-mucosa pancreaticojejunostomy for the normal pancreatic duct in laparoscopic pancreaticoduodenectomy. J Minim Access Surg 2021;17:412-4. [Crossref] [PubMed]
- Liu J, Xu SF, Yang FH, et al. Laparoscopic pancreaticoduodenectomy: A report of 340 cases. Chinese Journal of Practical Surgery 2020;2:203-7.
- Chen QM, Wang YC, Liu SY, et al. Application of Hong's pancreaticojejunostomy in laparoscopic pancreaticoduodenectomy on 184 patients. Chinese Journal of Hepatobiliary Surgery 2019;11:842-5.
- Ielpo B, Sanchez P, Grande L, et al. Laparoscopic pancreatoduodenectomy: how we have standardized the technique (with video). Updates Surg 2022;74:1479-81. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [Crossref] [PubMed]
- Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20-5. [Crossref] [PubMed]
- Ma H, Wang J, Liu L, et al. Clinical application of pancreatic-duct-jejunum end-to-side continuous suture anastomosis in total laparoscopic pancreaticoduodenectomy. Surg Endosc 2022;36:5366-73. [Crossref] [PubMed]
- Cai H, Ji B, Liu S, et al. Outcomes of laparoscopic pancreaticoduodenectomy using a modified technique346 cases from a single center. Asian J Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- He YG, Yang XM, Peng XH, et al. Association of a Modified Blumgart Anastomosis With the Incidence of Pancreatic Fistula and Operation Time After Laparoscopic Pancreatoduodenectomy: A Cohort Study. Front Surg 2022;9:931109. [Crossref] [PubMed]
- Zhang P, Gong S, Wu N, et al. Effect of total laparoscopic versus open pancreaticoduodenectomy on short-term and oncological outcomes: a single-institution comparative study. Langenbecks Arch Surg 2022; Epub ahead of print. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



