
Exploration of prognostic factors and the value of adjuvant chemotherapy in T1a,bN0M0 triple-negative breast cancer: a prospective cohort study based on the SEER database
Introduction
Triple-negative breast cancer (TNBC) is a highly aggressive breast cancer (BC) subtype and has a poor prognosis, which is characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2)/neu (1-5). In TNBC, chemotherapy (CT) remains the cornerstone of treatment, despite its toxic side-effects. Mammographic screening and adjunctive ultrasonography have led to an increase in the number of newly diagnosed BCs with tumor size of ≤1 cm (T1a,b), no lymph node involvement (N0), and no distant metastases (M0) (6-9). Most studies suggest that T1a,bN0M0 TNBC has an excellent long-term prognosis (10-13). According to the National Comprehensive Cancer Network (NCCN) guidelines (14), adjuvant CT is not recommended for patients with T1aN0M0 TNBC, but could be considered for subgroups with high-risk factors, such as young age and high histologic grade. In contrast, for patients with T1bN0M0 TNBC, the NCCN recommends adjuvant CT. Due to the low prevalence of, and limited evidence for, T1a,bN0M0 TNBC, information regarding clinical prognostic factors and the value of adjuvant CT in these patients is limited. Therefore, recommending optimal adjuvant systemic therapy in this patient population remains challenging (10,13).
To better analyze the prognosis of patients with T1a,bN0M0 TNBC and assist physicians with adjuvant systemic therapy decisions, we reviewed all T1a,bN0M0 TNBC cases registered in 2 large database and analyzed their overall survival (OS) and BC-specific mortality (BCSM). We further analyzed the effects of age, tumor size, histologic grade, and adjuvant CT on OS and BCSM in patients with T1a,bN0M0 TNBC. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-409/rc) (15).
Methods
Study population
In this cohort study, we retrospectively reviewed patient data from the Surveillance, Epidemiology, and End Results (SEER) database between January 1, 2010, and December 31, 2015. All included patients fulfilled the following inclusion criteria: female, unilateral BC, BC as the first and only cancer diagnosis, pathologically confirmed invasive BC, ER negative, PR negative, HER2 negative, American Joint Committee on Cancer (AJCC) stage T1a,bN0M0, and reception of definitive surgery. Patients were excluded if they had distant metastasis, received neoadjuvant CT, or had missing data regarding treatment or follow up. Positive ER and PR statuses were defined as >1% of tumor cells with nuclear staining, while positive HER2 status was defined as a score of 3+ with immunohistochemistry staining or a score of 2+ and a positive fluorescence in situ hybridization result. Patients with BC diagnosed before 2010 were not included, because the SEER database did not record data on HER2 status until 2010. Additionally, patients with BC diagnosed after 2015 were not included to ensure adequate follow-up time. Patient demographics, treatment modalities, tumor pathology, and survival characteristics were obtained. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Variables
The following variables were extracted from the SEER databases: age at diagnosis, year of diagnosis, race, marital status, laterality, tumor size, TNM stage (AJCC stage, 7th edition), histologic subtype, histologic grade, ER status, PR status, HER2 status, type of surgery performed, regional nodes examined, radiation treatment status, adjuvant CT treatment status, BCSM, and OS.
To clarify the effect of age at diagnosis on BCSM and OS, we treated age as a categorical variable and divided it into the following age groups: ≤39, 40–49, 50–59, 60–69, and ≥70 years. Regarding histologic grade, patients were divided into grade I (well differentiated), grade II (moderately differentiated), and grade III (poorly differentiated) + IV (undifferentiated; anaplastic) groups. Regarding tumor size, patients were divided into T1a and T1b groups. Patients were divided into the CT and no CT cohorts based on whether adjuvant CT was performed. The extent of axillary staging was classified as 0, 1–5, or ≥6 examined lymph nodes (16).
Statistical analyses
We used SEER Research Plus data submitted in November 2019 with a final follow-up date of December 31, 2018. Data were analyzed in May 2021. The median follow-up time was calculated using the reverse Kaplan-Meier method (17).
A logistic regression model was used to investigate which variables (including demographic, clinical, and pathological) were associated with adjuvant CT treatment in patients with T1a,bN0M0 TNBC in actual clinical practice. OS was measured as the time from the date of BC diagnosis to the date of death from any cause or the date of the last follow up. Cox proportional hazards models were used to evaluate the association between variables (including demographic, clinicopathological, and treatment) and OS. BCSM was measured as the time from the date of BC diagnosis to the date of death due to BC (SEER cause-specific death classification). In the analysis of BCSM, deaths from other causes were considered competing risks. The cumulative incidence function for competing risks method was used to calculate the crude cumulative probabilities of BCSM in the presence of competing risks of non-BC mortality (18,19). We used the Fine-Gray model to evaluate the association between variables (especially age, tumor size, and histologic grade) and BCSM in the total and no CT cohorts (20).
Propensity score matching (PSM) of the CT and no CT cohorts was conducted for baseline characteristics. The CT and no CT cohorts were matched at a ratio of 1:1 using the nearest neighbor method, with a caliper of 0.05 (21). Before and after PSM, we analyzed whether the use of adjuvant CT affected OS and BCSM in patients with T1a,bN0M0 TNBC, and performed an exploratory subgroup analysis on BCSM after PSM. The Kaplan-Meier estimator was used to calculate the 5-year OS of the CT and no CT cohorts, which was then compared using the log-rank test. The Nelson-Aalen estimator was used to calculate the 5-year BCSM of the CT and no CT cohorts, which was then compared using Gray’s test (22,23).
All statistical analyses were performed using R software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), and differences with a P value of <0.05 were considered statistically significant.
Results
SEER patient characteristics and predictors of CT
Between 2010 and 2015, 3,065 women with T1a,bN0M0 TNBC were enrolled in the SEER database (Figure 1). Patient demographics, and their clinical and pathological characteristics, are shown in Table 1. Of the 3,065 patients, 1,534 (50.0%) received adjuvant CT, 2,215 (72.3%) had T1b tumors, and 1,926 (62.8%) had grade III + IV tumors.
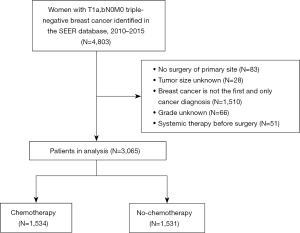
Table 1
| Characteristics | Total cohort (n=3,065), n (%) | CT cohort (n=1,534), n (%) | No CT cohort (n=1,531), n (%) |
|---|---|---|---|
| Age at diagnosis (years) | |||
| ≤39 | 105 (3.4) | 87 (5.7) | 18 (1.2) |
| 40–49 | 395 (12.9) | 279 (18.2) | 116 (7.6) |
| 50–59 | 808 (26.4) | 500 (32.6) | 308 (20.1) |
| 60–69 | 963 (31.4) | 505 (32.9) | 458 (29.9) |
| ≥70 | 794 (25.9) | 163 (10.6) | 631 (41.2) |
| Year of diagnosis | |||
| 2010–2012 | 1,522 (49.7) | 726 (47.3) | 796 (52.0) |
| 2013–2015 | 1,543 (50.3) | 808 (52.7) | 735 (48.0) |
| Race | |||
| White | 2,326 (75.9) | 1,150 (75.0) | 1,176 (76.8) |
| Black | 513 (16.7) | 275 (17.9) | 238 (15.6) |
| Others | 226 (7.4) | 109 (7.1) | 117 (7.6) |
| Marital status | |||
| Married | 1,817 (59.3) | 971 (63.3) | 846 (55.3) |
| Not married | 1,248 (40.7) | 563 (36.7) | 685 (44.7) |
| Laterality | |||
| Right | 1,494 (48.7) | 735 (47.9) | 759 (49.6) |
| Left | 1,571 (51.3) | 799 (52.1) | 772 (50.4) |
| Histologic type | |||
| Infiltrating duct carcinoma | 2,721 (88.8) | 1,411 (92.0) | 1,310 (85.6) |
| Other | 344 (11.2) | 123 (8.0) | 221 (14.4) |
| Histologic grade | |||
| I | 182 (5.9) | 32 (2.1) | 150 (9.8) |
| II | 957 (31.2) | 373 (24.3) | 584 (38.2) |
| III + IV | 1,926 (62.8) | 1,129 (73.6) | 797 (52.1) |
| Tumor size | |||
| T1a | 850 (27.7) | 212 (13.8) | 638 (41.7) |
| T1b | 2,215 (72.3) | 1,322 (86.2) | 893 (58.3) |
| Breast surgery strategies | |||
| Mastectomy | 548 (17.9) | 249 (16.2) | 299 (19.5) |
| Breast-conserving surgery | 2,188 (71.4) | 1,099 (71.6) | 1,089 (71.1) |
| Reconstruction | 329 (10.7) | 186 (12.1) | 143 (9.3) |
| Regional nodes examined (n) | |||
| 0 | 130 (4.2) | 36 (2.4) | 94 (6.1) |
| 1–5 | 2,553 (83.3) | 1,304 (85.0) | 1,249 (81.6) |
| ≥6 | 382 (12.5) | 194 (12.7) | 188 (12.3) |
| Radiation therapy | |||
| Yes | 1,899 (62.0) | 989 (64.5) | 910 (59.4) |
| No | 1,166 (38.0) | 545 (35.5) | 621 (40.6) |
| Vital status | |||
| Alive | 2,866 (93.5) | 1,466 (95.6) | 1,400 (91.4) |
| Breast cancer-specific mortality | 96 (3.1) | 53 (3.5) | 43 (2.8) |
| Other cause-specific mortality | 103 (3.4) | 15 (1.0) | 88 (5.8) |
CT, chemotherapy.
Younger age at diagnosis, higher histologic grade, larger tumor size, more recent treatment periods, infiltrating ductal carcinoma, breast-conserving surgery, and adjuvant radiotherapy were all associated with an increased probability of receiving adjuvant CT (P<0.05 for each predictor) (Table S1).
OS of SEER patients
Of the 3,065 T1a,bN0M0 TNBC cases in the SEER database, 96 and 103 deaths resulted from BC and other causes, respectively. The median follow up was 57 months (interquartile range: 39–75 months). The 5-year OS estimates for the patient subgroups are presented in Table 2. For the total cohort, the 5-year OS of patients with T1a or T1b tumors not treated with adjuvant CT exceeded 90%. For patients aged ≤39 years, the 5-year OS exceeded 93%, regardless of whether they received adjuvant CT. Among the grade III + IV patients, the 5-year OS was 94.9% and 91.3% for patients receiving and not receiving adjuvant CT, respectively.
Table 2
| Characteristics | 5-year OS (%) and 95% CI | 5-year cumulative probabilities of BCSM (%) and 95% CI | |||||
|---|---|---|---|---|---|---|---|
| Total cohort (n=3,065) | CT cohort (n=1,534) | No CT cohort (n=1,531) | Total cohort (n=3,065) | CT cohort (n=1,534) | No CT cohort (n=1,531) | ||
| All patients | 93.6 (92.6–94.6) | 95.3 (94.1–96.6) | 91.9 (90.4–93.5) | 3.3 (2.6–4.1) | 3.9 (2.8–5.0) | 2.8 (1.9–3.7) | |
| Age at diagnosis (years) | |||||||
| ≤39 | 94.0 (88.8–99.4) | 93.1 (87.2–99.3) | 100.0 (100.0–100.0) | 6.0 (0.7–11.4) | 6.9 (0.8–13.0) | 0.0 (0.0–0.0) | |
| 40–49 | 95.7 (93.3–98.2) | 96.9 (94.3–99.5) | 93.2 (88.0–98.7) | 3.9 (1.6–6.3) | 3.1 (0.5–5.7) | 5.7 (0.7–10.6) | |
| 50–59 | 96.1 (94.5–97.6) | 95.9 (93.9–98.0) | 96.3 (94.0–98.8) | 3.5 (2.0–4.9) | 3.9 (1.9–5.9) | 2.8 (0.7–4.9) | |
| 60–69 | 94.6 (93.0–96.3) | 95.1 (93.0–97.3) | 94.1 (91.6–96.6) | 2.9 (1.8–4.1) | 3.8 (1.9–5.7) | 2.0 (0.6–3.4) | |
| ≥70 | 88.8 (86.3–91.4) | 92.6 (88.1–97.2) | 87.9 (85.0–90.9) | 3.1 (1.8–4.4) | 3.8 (0.4–7.2) | 2.9 (1.5–4.4) | |
| Histologic grade | |||||||
| I | 97.1 (94.2–100.0) | 100.0 (100.0–100.0) | 96.4 (93.0–100.0) | 1.5 (0.0–3.8) | 0.0 (0.0–0.0) | 1.9 (0.0–4.6) | |
| II | 93.5 (91.7–95.3) | 96.2 (94.1–98.4) | 91.7 (89.1–94.4) | 2.7 (1.5–3.8) | 3.2 (1.2–5.2) | 2.3 (0.9–3.7) | |
| III + IV | 93.3 (92.1–94.6) | 94.9 (93.4–96.4) | 91.3 (89.1–93.5) | 3.8 (2.8–4.8) | 4.2 (2.8–5.6) | 3.3 (2.0–4.7) | |
| Tumor size (stage) | |||||||
| T1a | 95.3 (93.6–96.9) | 98.5 (96.9–100.0) | 94.2 (92.1–96.3) | 1.8 (0.8–2.8) | 1.5 (0.0–3.1) | 1.9 (0.7–3.2) | |
| T1b | 93.0 (91.8–94.3) | 94.8 (93.5–96.2) | 90.5 (88.3–92.7) | 3.9 (3.0–4.8) | 4.2 (3.0–5.5) | 3.4 (2.1–4.7) | |
| Tumor size (mm) | |||||||
| 2 | 90.9 (85.9–6.1) | 100.0 (100.0–100.0) | 89.4 (83.8–95.4) | 3.8 (0.4–7.3) | 0.0 (0.0–0.0) | 4.5 (0.5–8.4) | |
| 3 | 98.1 (96.0–100.0) | 100.0 (100.0–100.0) | 97.7 (95.1–100.0) | 0.6 (0.0–1.8) | 0.0 (0.0–0.0) | 0.7 (0.0–2.2) | |
| 4 | 95.3 (92.2–98.6) | 97.9 (93.8–100.0) | 94.4 (90.4–98.6) | 1.1 (0.0–2.5) | 2.1 (0.0–6.3) | 0.7 (0.0–2.1) | |
| 5 | 96.1 (93.6–98.7) | 98.0 (95.2–100.0) | 95.2 (91.7–98.8) | 1.9 (0.2–3.6) | 2.0 (0.0–4.9) | 1.9 (0.0–4.0) | |
| 6 | 93.5 (90.5–96.7) | 96.1 (93.0–99.3) | 90.9 (85.8–96.3) | 2.8 (0.9–4.8) | 3.9 (0.7–7.1) | 1.7 (0.0–3.9) | |
| 7 | 94.7 (92.0–97.4) | 95.1 (91.5–98.9) | 94.1 (90.1–98.2) | 3.2 (1.1–5.3) | 4.3 (0.8–7.8) | 2.1 (0.0–4.4) | |
| 8 | 93.6 (91.0–96.2) | 93.8 (90.5–97.2) | 93.2 (89.4–97.3) | 4.1 (2.0–6.1) | 4.9 (1.9–8.0) | 2.9 (0.3–5.6) | |
| 9 | 94.2 (91.7–96.8) | 96.8 (94.7–99.0) | 90.2 (85.0–95.8) | 2.7 (1.0–4.3) | 2.8 (0.7–4.9) | 2.3 (0.0–5.1) | |
| 10 | 90.4 (87.7–93.1) | 93.4 (90.6–96.3) | 85.1 (79.8–90.7) | 5.7 (3.6–7.8) | 4.9 (2.4–7.4) | 7.0 (3.3–10.8) | |
CI, confidence interval; CT, chemotherapy.
For the total cohort (n=3,065), Cox multivariable analysis revealed several predictors of worse OS, including age ≥60 years at diagnosis, breast mastectomy surgery, unmarried status, and 0 axillary nodes examined (P<0.05 for each predictor) (Table 3). In the no CT cohort (n=1,531), another Cox multivariable analysis revealed several predictors of worse OS, including age ≥60 years at diagnosis, unmarried status, omission of radiotherapy, and higher histologic grade (P<0.05 for each predictor) (Table 3).
Table 3
| Variables | Total cohort | No CT cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age at diagnosis (years) | |||||||||||
| ≤39 | 1.768 (0.730–4.281) | 0.207 | 1.477 (0.599–3.641) | 0.397 | – | – | – | – | |||
| 40–49 | 1.094 (0.574–2.087) | 0.784 | 1.032 (0.538–1.978) | 0.924 | 1.490 (0.542–4.101) | 0.44 | 1.452 (0.525–4.012) | 0.472 | |||
| 50–59 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||||
| 60–69 | 1.655 (1.043–2.627) | 0.033 | 1.688 (1.059–2.690) | 0.028 | 2.019 (0.987–4.129) | 0.054 | 2.181 (1.065–4.467) | 0.033 | |||
| ≥70 | 3.790 (2.475–5.804) | <0.001 | 3.178 (2.014–5.016) | <0.001 | 4.356 (2.262–8.389) | <0.001 | 4.081 (2.112–7.885) | <0.001 | |||
| Year of diagnosis | |||||||||||
| 2010–2012 | 1 (Reference) | – | 1 (Reference) | – | |||||||
| 2013–2015 | 0.900 (0.639–1.267) | 0.545 | – | – | 0.848 (0.545–1.320) | 0.466 | – | – | |||
| Race | |||||||||||
| White | 1 (Reference) | – | 1 (Reference) | – | |||||||
| Black | 1.307 (0.925–1.846) | 0.129 | – | – | 1.298 (0.837–2.013) | 0.244 | – | – | |||
| Others | 0.634 (0.323–1.242) | 0.184 | – | – | 0.743 (0.345–1.599) | 0.447 | – | – | |||
| Marital status | |||||||||||
| Married | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||||
| Not married | 1.862 (1.407–2.463) | <0.001 | 1.582 (1.189–2.106) | 0.002 | 2.034 (1.428–2.898) | <0.001 | 1.766 (1.232–2.532) | 0.002 | |||
| Laterality | |||||||||||
| Right | 1 (Reference) | – | 1 (Reference) | – | |||||||
| Left | 1.216 (0.919–1.608) | 0.171 | – | – | 1.177 (0.835–1.659) | 0.353 | – | – | |||
| Histologic type | |||||||||||
| Infiltrating duct carcinoma | 1 (Reference) | – | 1 (Reference) | – | |||||||
| Others | 0.877 (0.558–1.379) | 0.57 | – | – | 0.708 (0.413–1.212) | 0.208 | – | – | |||
| Histologic grade | |||||||||||
| I | 1 (Reference) | – | 1 (Reference) | 1 (Reference) | |||||||
| II | 2.686 (1.083–6.662) | 0.033 | – | – | 2.991 (1.197–7.473) | 0.019 | 2.810 (1.123–7.035) | 0.027 | |||
| III + IV | 2.459 (1.006–6.012) | 0.048 | – | – | 2.720 (1.098–6.737) | 0.031 | 2.698 (1.088–6.692) | 0.032 | |||
| Tumor size (mm) | |||||||||||
| T1a (2–5) | 1 (Reference) | – | 1 (Reference) | – | |||||||
| T1b (6–10) | 1.274 (0.911–1.781) | 0.157 | – | – | 1.424 (0.983–2.061) | 0.061 | – | – | |||
| Breast surgery strategies | |||||||||||
| Mastectomy | 1 (Reference) | 1 (Reference) | 1 (Reference) | – | |||||||
| Breast-conserving surgery | 0.498 (0.364–0.683) | <0.001 | 0.528 (0.382–0.728) | <0.001 | 0.619 (0.423–0.904) | 0.013 | – | – | |||
| Reconstruction | 0.679 (0.419–1.101) | 0.117 | 1.107 (0.662–1.852) | 0.699 | 0.398 (0.178–0.890) | 0.025 | – | – | |||
| Regional nodes examined (n) | |||||||||||
| 0 | 1 (Reference) | 1 (Reference) | 1 (Reference) | – | |||||||
| 1–5 | 0.335 (0.208–0.541) | <0.001 | 0.468 (0.287–0.764) | 0.002 | 0.423 (0.241–0.740) | 0.003 | – | – | |||
| ≥6 | 0.432 (0.242–0.770) | 0.004 | 0.513 (0.283–0.931) | 0.028 | 0.550 (0.275–1.097) | 0.09 | – | – | |||
| CT | – | – | |||||||||
| Yes | 1 (Reference) | 1 (Reference) | – | – | |||||||
| No | 1.895 (1.414–2.540) | <0.001 | 1.201 (0.867–1.664) | 0.271 | – | – | – | – | |||
| Radiation therapy | |||||||||||
| Yes | 1 (Reference) | – | – | 1 (Reference) | 1 (Reference) | ||||||
| No | 1.698 (1.286–2.242) | <0.001 | – | – | 1.463 (1.039–2.061) | 0.029 | 1.589(1.125–2.246) | 0.009 | |||
In the ≤39-year subgroup of the no CT cohort, there were no death. Therefore, when we performed the univariable and multivariable analyses in the no CT cohort, the ≤39- and 40–49-year subgroups were combined into 1 group. CT, chemotherapy; HR, hazard ratio; CI, confidence interval.
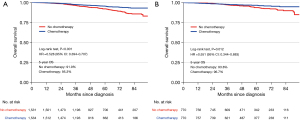
After PSM, the baseline characteristics of the CT and no CT cohorts were balanced (Table S2). Before PSM, the estimated 5-year OS was 95.3% in the CT cohort and 91.9% in the no CT cohort [hazard ratio (HR): 0.53; 95% confidence interval (CI): 0.39–0.71; P<0.001] (Figure 2A). After PSM, the estimated 5-year OS was 96.7% in the CT cohort and 93.8% in the no CT cohort (HR: 0.55; 95% CI: 0.34–0.88; P=0.012) (Figure 2B).
BCSM in SEER patients
Table 2 lists the 5-year cumulative BCSM estimates for the SEER patients treated/not treated with adjuvant CT. In all patients, the 5-year cumulative BCSM did not exceed 3.9%. In the no CT cohort, the 5-year cumulative BCSM was the highest in the 40–49-year group (5.7%), and similar within the 50–59-, 60–69-, and ≥70-year groups (2.8%, 2.0%, and 2.9%, respectively). There were no BCSM events in the ≤39-year group. In the no CT cohort, the 5-year cumulative BCSM was 1.9%, 2.3%, and 3.3% for histologic grades I, II, and III + IV, respectively. In the no CT cohort, among the different subgroups with tumor diameters of 2–10 mm, the 10-mm subgroup had the highest 5-year cumulative BCSM (7.0%).
For the total cohort (n=3,065), the Fine-Gray model revealed only 2 predictors of lower cumulative BCSM, which were smaller tumor size (P=0.039) and breast-conserving surgery (P=0.001) (Table 4). For the no CT cohort (n=1,531), the Fine-Gray model also revealed 2 predictors of lower cumulative BCSM, which were married status (P=0.012) and breast-conserving surgery (P=0.008) (Table 4).
Table 4
| Variables | Total cohort (n=3,065) | No CT cohort (n=1,531) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age at diagnosis (years) | |||||||||||
| ≤39 | 1.893 (0.744–4.815) | 0.180 | – | – | – | – | – | – | |||
| 40–49 | 1.090 (0.479–2.481) | 0.836 | – | – | 1.562 (0.533–4.580) | 0.416 | |||||
| 50–59 | 1 (Reference) | – | 1 (Reference) | – | |||||||
| 60–69 | 1.001 (0.575–1.742) | 0.998 | – | – | 1.006 (0.375–2.695) | 0.991 | – | – | |||
| ≥70 | 1.059 (0.571–1.967) | 0.855 | – | – | 1.350 (0.578–3.153) | 0.488 | – | – | |||
| Year of diagnosis | |||||||||||
| 2010–2012 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||||||
| 2013–2015 | 0.496 (0.290–0.850) | 0.011 | 0.548 (0.293–1.026) | 0.060 | 0.341 (0.162–0.716) | 0.004 | 0.398 (0.158–1.002) | 0.051 | |||
| Race | |||||||||||
| White | 1 (Reference) | – | 1 (Reference) | – | |||||||
| Black | 1.624 (0.933–2.829) | 0.087 | – | – | 1.539 (0.687–3.447) | 0.295 | – | – | |||
| Others | 0.576 (0.212–1.571) | 0.282 | – | – | 0.544 (0.129–2.287) | 0.406 | – | – | |||
| Marital status | |||||||||||
| Married | 1 (Reference) | – | 1 (Reference) | 1 (Reference) | |||||||
| Not married | 1.431 (0.927–2.209) | 0.106 | – | – | 1.940 (1.060–3.550) | 0.032 | 2.644 (1.239–5.642) | 0.012 | |||
| Laterality | |||||||||||
| Right | 1 (Reference) | 1 (Reference) | 1 (Reference) | – | |||||||
| Left | 1.498 (1.071–2.096) | 0.018 | 1.503 (0.964–2.343) | 0.072 | 1.604 (0.867–2.967) | 0.132 | – | – | |||
| Histologic type | |||||||||||
| Infiltrating duct carcinoma | 1 (Reference) | – | 1 (Reference) | 1 (Reference) | |||||||
| Others | 0.494 (0.227–1.075) | 0.075 | – | – | 0.246 (0.062–0.972) | 0.045 | 0.361 (0.063–2.080) | 0.254 | |||
| Histologic grade | |||||||||||
| I | 1 (Reference) | – | 1 (Reference) | – | |||||||
| II | 2.407 (0.571–10.141) | 0.231 | – | – | 1.870 (0.422–8.283) | 0.410 | – | – | |||
| III + IV | 3.349 (0.838–13.391) | 0.087 | – | – | 2.468 (0.596–10.229) | 0.213 | – | – | |||
| Tumor size (mm) | |||||||||||
| T1a (2–5) | 1 (Reference) | 1 (Reference) | 1 (Reference) | – | |||||||
| T1b (6–10) | 2.099 (1.255–3.509) | 0.005 | 1.806 (1.030–3.168) | 0.039 | 1.898 (0.981–3.673) | 0.057 | – | – | |||
| Breast surgery strategies | |||||||||||
| Mastectomy | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||||||
| Breast–conserving surgery | 0.376 (0.216–0.654) | <0.001 | 0.390 (0.221–0.688) | 0.001 | 0.383 (0.179–0.818) | 0.013 | 0.328 (0.144–0.747) | 0.008 | |||
| Reconstruction | 1.146 (0.599–2.195) | 0.681 | 1.243 (0.660–2.341) | 0.501 | 0.654 (0.253–1.689) | 0.380 | 1.079 (0.368–3.161) | 0.890 | |||
| Regional nodes examined (n) | |||||||||||
| 0 | 1 (Reference) | – | 1 (Reference) | – | |||||||
| 1–5 | 0.458 (0.151–1.389) | 0.168 | – | – | 0.427 (0.139–1.312) | 0.137 | – | – | |||
| ≥6 | 0.551 (0.142–2.131) | 0.388 | – | – | 0.510 (0.128–2.025) | 0.339 | – | – | |||
| CT | – | – | |||||||||
| Yes | 1 (Reference) | – | – | – | |||||||
| No | 0.788 (0.532–1.168) | 0.236 | – | – | – | – | – | – | |||
| Radiation therapy | |||||||||||
| Yes | 1 (Reference) | – | 1 (Reference) | – | |||||||
| No | 1.912 (0.956–3.824) | 0.067 | – | – | 2.000 (0.940–4.257) | 0.072 | – | – | |||
In the ≤39-year subgroup of the no CT cohort, there were no deaths. Therefore, when we performed the univariable and multivariable analyses in the no CT cohort, the ≤39- and 40–49-year subgroups were combined into 1 group. CT, chemotherapy; HR, hazard ratio; CI, confidence interval.
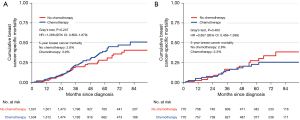
Before PSM, the estimated 5-year cumulative BCSM was 3.9% in the CT cohort and 2.8% in the no CT cohort (HR: 1.27; 95% CI: 0.86–1.88; P=0.247) (Figure 3A). After PSM, the estimated 5-year cumulative BCSM was 2.3% in the CT cohort and 2.8% in the no CT cohort (HR: 0.86; 95% CI: 0.46–1.60; P=0.463) (Figure 3B). For the post-PSM population, an exploratory subgroup analysis also did not find a statistically significant improvement in BCSM with adjuvant CT in any subgroup (Figure S1).
Discussion
In the present study, we explored the prognostic predictors and the value of adjuvant CT in patients with T1a,bN0M0 TNBC in the SEER database. The findings of the study indicated that 50% of patients with T1a,bN0M0 TNBC underwent adjuvant CT, and subtypes with larger tumors, younger age, and higher histologic grade were more likely to receive adjuvant CT. These findings are consistent with the NCCN recommendations. The findings of the present study also indicated that higher histologic grade and larger tumor size are predictors of poor prognosis, although the effect of age was complex. We did not identify a significant BCSM advantage for adjuvant CT in patients with T1a,bN0M0 TNBC. We found that the overall T1a,bN0M0 TNBC population had an excellent prognosis, with a 5-year OS of 93.6% and 5-year cumulative BCSM of only 3.3%. For patients with T1a,bN0M0 TNBC, both undertreatment and overtreatment should be avoided.
In both the total and no CT cohorts, we did not find a statistical association between younger age and worse OS or increased BCSM, which conflicts with the findings of previous studies (10,24-26). Two retrospective studies found that in patients with TNBC, age <40 years at diagnosis was an independent adverse prognostic factor in a multivariable analysis (24,25). Conversely, our study found that the risks of all-cause mortality were higher in the 60–69- and ≥70-year groups when the 50–59-year group was used as the reference group, whereas the risks of all-cause mortality in the ≤39- and 40–49-year groups were similar to those of the reference group. Interestingly, the 5-year cumulative BCSM was only 2.9% and 3.1% for the 60–69- and ≥70-year subgroups, respectively, both being lower than the average for the overall population (3.3%). Therefore, worse OS in patients with T1a,bN0M0 TNBC aged ≥60 years is mainly caused by increased non-BCSM (cardiovascular death and others). In some younger subgroups, the 5-year cumulative BCSM could reach twice the mean, but did not reach a statistical difference, which adds to the complexity of the effect of age on survival. In summary, the findings of our study did not fully elucidate the effect of age on T1a,bN0M0 TNBC, and further prospective, large-scale studies are needed to explore the underlying mechanism.
Regarding the effect of age on the prognosis of T1a,bN0M0 TNBC, current studies tended to reach different conclusions, with the following possible explanations for these discrepancies. First, the study enrollment criteria were inconsistent. We enrolled only patients with T1a,bN0M0 TNBC, whereas most previous studies enrolled patients with non-metastatic TNBC and did not consider T and N staging. TNBC is a very heterogeneous subtype. Younger patients with TNBC have a higher proportion of higher histologic grade, increased nodal involvement, and larger tumor size, which present as confounding factors when assessing the effect of age on survival (24,26-28). Second, the proportion of young patients varied. In our study, the ≤39-year group represented only 3.4% of the total population, which might also have influenced the statistical analysis. Third, the study endpoints were different. When examining the effect of age on survival, BCSM is a more appropriate endpoint than OS due to the influence of non-BC mortality events (20). Fourth, the statistical methods used were different. When BCSM is the study endpoint and the frequency of competing events is high, the Cox proportional hazards model is not an appropriate analytical method, and the Fine–Gray model should be used (20,29). Fifth, the confounding effects of adjuvant CT were excluded. We performed statistical analyses mainly in the no CT cohort, expecting to observe the direct effect of age on BCSM and to identify the true prognostic factors.
Consistent with previous studies (10,12,13,30), our study also found that patients with T1a,bN0M0 TNBC with lower histologic grade and smaller tumors tended to have a better prognosis. A review found that, among the several possible negative prognostic predictors of T1a,bN0M0 BC, a high histologic grade was most consistently shown to be associated with poor long-term prognosis (13). Similar to a previous study (31), we found that, among patients with T1a,bN0M0 BC, tumors with a 1-cm diameter were associated with an overwhelmingly poor prognosis. This could be because, as the tumor diameter increases, the tumor volume increases significantly, and larger tumor volumes are theoretically more likely to develop distant metastases (31). Also, due to inadvertent “rounding down” of tumor size by the pathologist when measuring the tumor diameter, a portion of tumors with true diameters >1 cm were incorrectly recorded as 1 cm (31). Our findings of the present study indicate that 1-cm T1bN0M0 TNBC tumors should be treated differently from the remaining subtypes.
Consistent with previous studies (32,33), the findings of the present study showed no association between adjuvant CT and OS or BCSM in the multivariable analysis. However, this outcome could be influenced by a number of confounding factors, such as life expectancy, comorbidities, and socioeconomic factors. Therefore, we should be careful when interpreting and applying this conclusion. In addition, we performed a direct comparison of 5-year OS and BCSM between the CT and no CT cohorts before and after PSM. Our findings indicated that the CT cohort had a significantly better 5-year OS than the no CT cohort, both before and after PSM. However, no statistical difference was observed in the 5-year cumulative BCSM between the CT and no CT cohorts, both before and after PSM. While contradictory, this result was inevitable. In our study, the selection of adjuvant CT was not randomized; patients with a higher risk of recurrence were more likely to receive adjuvant CT. This resulted in a particularly high risk of recurrence at baseline in the CT cohort. Although adjuvant CT theoretically improved BCSM, the final data showed a higher rate of BCSM in the CT cohort than in the no CT cohort (3.9% vs. 2.8% before PSM). After PSM, the baseline risk of recurrence was balanced between the CT and no CT cohorts, allowing us to explore the true role of adjuvant CT (34). The baseline risk of recurrence in the CT cohort was somewhat diluted after PSM, as corroborated by the difference in the 5-year cumulative BCSM in the CT cohort before and after PSM (3.9% vs. 2.3%). After PSM, we found a 0.5% absolute reduction in the 5-year cumulative BCSM with adjuvant CT, although this did not reach statistical significance, most likely due to the sample size.
Overall, our study does not fully elucidate whether adjuvant CT is beneficial in patients with T1a,bN0M0 TNBC. To date, we lack tools, such as genetic testing, to accurately predict the extent of adjuvant CT benefit in T1a,bN0M0 TNBC; therefore, we rely heavily on traditional clinicopathological indicators, such as histologic grade and tumor size. Therefore, when decisions regarding adjuvant CT are made for patients with T1a,bN0M0 TNBC, baseline risk of recurrences, benefits and risks of adjuvant CT, patient preferences, life expectancy, and comorbidities need to be taken into consideration.
The present study has some limitations. First, the CT variable was categorized as either “yes” or “no/unknown” in the SEER database. The increasing number of patients undergoing CT outside the hospital settings has resulted in the CT treatment not being accurately recorded in the SEER database, but only registered as “no/unknown” (35). Therefore, we must acknowledge that conclusions based on CT variables and related analyses could be inaccurate and misleading. Second, this study had a relatively short follow-up time, and the median follow-up was only 5 years. Nevertheless, previous studies have found that recurrences and metastases in TNBC occur mainly in the first 5 years after diagnosis (36-38). Third, the SEER database does not contain information regarding Ki-67. Furthermore, the SEER database does not provide detailed information on the adjuvant CT regimens used, and we were unable to analyze the risk–benefit ratio for the different CT regimens. Fourth, the results could be affected by selection bias from excluded and incomplete data. Finally, the sample size of the CSCO BC database was too small to validate the analytical results of the SEER database.
Conclusions
Women with T1a,bN0M0 TNBC had an excellent prognosis, with or without adjuvant CT. In this population, higher histologic grade and larger tumor size were found to be predictors of poor prognosis, although the effect of age was complex. Further studies are needed to explore the underlying mechanisms. Our data did not support using adjuvant CT in patients with T1a,bN0M0 TNBC. However, due to the limitations of the present study, we must approach this result with caution. When considering adjuvant CT for high-risk T1a,bN0M0 TNBC patients, a risk–benefit discussion should be undertaken on a case-by-case basis.
Acknowledgments
Funding: The present study was supported by grant from Henan Province Medical Science and Technology Tackling Program Joint Co-Construction Project (No. LHGJ20210188).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-409/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-409/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hernandez-Aya LF, Chavez-Macgregor M, Lei X, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol 2011;29:2628-34. [Crossref] [PubMed]
- Denkert C, Liedtke C, Tutt A, et al. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017;389:2430-42. [Crossref] [PubMed]
- Abramson VG, Lehmann BD, Ballinger TJ, et al. Subtyping of triple-negative breast cancer: implications for therapy. Cancer 2015;121:8-16. [Crossref] [PubMed]
- Lv M, Li J, Guo H, et al. Impact of Ipsilateral Supraclavicular Lymph Node Dissection (ISLND) for Breast Cancer Patients and a Nomogram for Predicting Ipsilateral Supraclavicular Pathological Complete Response (ispCR). Ann Surg Oncol 2021;28:5098-109. [Crossref] [PubMed]
- Lyu MH, Ma YZ, Tian PQ, et al. Development and validation of a nomogram for predicting survival of breast cancer patients with ipsilateral supraclavicular lymph node metastasis. Chin Med J (Engl) 2021;134:2692-9. [Crossref] [PubMed]
- Luke C, Nguyen AM, Priest K, et al. Female breast cancers are getting smaller, but socio-demographic differences remain. Aust N Z J Public Health 2004;28:312-6. [Crossref] [PubMed]
- Fracheboud J, Otto SJ, van Dijck JA, et al. Decreased rates of advanced breast cancer due to mammography screening in The Netherlands. Br J Cancer 2004;91:861-7. [Crossref] [PubMed]
- Vacek PM, Geller BM, Weaver DL, et al. Increased mammography use and its impact on earlier breast cancer detection in Vermont, 1975-1999. Cancer 2002;94:2160-8. [Crossref] [PubMed]
- Harada-Shoji N, Suzuki A, Ishida T, et al. Evaluation of Adjunctive Ultrasonography for Breast Cancer Detection Among Women Aged 40-49 Years With Varying Breast Density Undergoing Screening Mammography: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open 2021;4:e2121505. [Crossref] [PubMed]
- Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, et al. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol 2007;25:4952-60. [Crossref] [PubMed]
- Theriault RL, Litton JK, Mittendorf EA, et al. Age and survival estimates in patients who have node-negative T1ab breast cancer by breast cancer subtype. Clin Breast Cancer 2011;11:325-31. [Crossref] [PubMed]
- Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol 2014;32:2142-50. [Crossref] [PubMed]
- Hanrahan EO, Valero V, Gonzalez-Angulo AM, et al. Prognosis and management of patients with node-negative invasive breast carcinoma that is 1 cm or smaller in size (stage 1; T1a,bN0M0): a review of the literature. J Clin Oncol 2006;24:2113-22. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Abraham J, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:452-78. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. [Crossref] [PubMed]
- Greene FL PD, Fleming ID, et al. AJCC Cancer Staging Handbook: TNM Classification of Malignant Tumors (ed 6). New York, NY, John Wiley & Sons, 2002.
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- Andersen PK, Geskus RB, de Witte T, et al. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861-70. [Crossref] [PubMed]
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389-430. [Crossref] [PubMed]
- de Glas NA, Kiderlen M, Vandenbroucke JP, et al. Performing Survival Analyses in the Presence of Competing Risks: A Clinical Example in Older Breast Cancer Patients. J Natl Cancer Inst 2016;108:djv366. [Crossref] [PubMed]
- Geldof T, Popovic D, Van Damme N, et al. Nearest Neighbour Propensity Score Matching and Bootstrapping for Estimating Binary Patient Response in Oncology: A Monte Carlo Simulation. Sci Rep 2020;10:964. [Crossref] [PubMed]
- Li Y, Lu S, Zhang Y, et al. Loco-regional recurrence trend and prognosis in young women with breast cancer according to molecular subtypes: analysis of 1099 cases. World J Surg Oncol 2021;19:113. [Crossref] [PubMed]
- Leonardi MC, Scognamiglio IR, Maisonneuve P, et al. Mastectomy alone for pT1-2 pN0-1 breast cancer patients: when postmastectomy radiotherapy is indicated. Breast Cancer Res Treat 2021;188:511-24. [Crossref] [PubMed]
- Liedtke C, Hess KR, Karn T, et al. The prognostic impact of age in patients with triple-negative breast cancer. Breast Cancer Res Treat 2013;138:591-9. [Crossref] [PubMed]
- Liedtke C, Rody A, Gluz O, et al. The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res Treat 2015;152:667-73. [Crossref] [PubMed]
- Han W, Kim SW, Park IA, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer 2004;4:82. [Crossref] [PubMed]
- Paluch-Shimon S, Cardoso F, Partridge AH, et al. ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann Oncol 2020;31:674-96. [Crossref] [PubMed]
- Liu YR, Jiang YZ, Yu KD, et al. Different patterns in the prognostic value of age for breast cancer-specific mortality depending on hormone receptor status: a SEER population-based analysis. Ann Surg Oncol 2015;22:1102-10. [Crossref] [PubMed]
- van de Water W, Markopoulos C, van de Velde CJ, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA 2012;307:590-7. [Crossref] [PubMed]
- Chia SK, Speers CH, Bryce CJ, et al. Ten-year outcomes in a population-based cohort of node-negative, lymphatic, and vascular invasion-negative early breast cancers without adjuvant systemic therapies. J Clin Oncol 2004;22:1630-7. [Crossref] [PubMed]
- Fehrenbacher L, Capra AM, Quesenberry CP Jr, et al. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol 2014;32:2151-8. [Crossref] [PubMed]
- de Nonneville A, Gonçalves A, Zemmour C, et al. Adjuvant chemotherapy in pT1ab node-negative triple-negative breast carcinomas: Results of a national multi-institutional retrospective study. Eur J Cancer 2017;84:34-43. [Crossref] [PubMed]
- Steenbruggen TG, van Werkhoven E, van Ramshorst MS, et al. Adjuvant chemotherapy in small node-negative triple-negative breast cancer. Eur J Cancer 2020;135:66-74. [Crossref] [PubMed]
- Lv M, Yuan P, Ma Y, et al. Evaluation of whether adjuvant chemotherapy can be safely omitted for older female patients with ER-positive, HER2-negative N1 breast cancer: a study based on the SEER database. Ann Transl Med 2021;9:1082. [Crossref] [PubMed]
- Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care 2016;54:e55-64. [Crossref] [PubMed]
- Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 2008;14:1368-76. [Crossref] [PubMed]
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [Crossref] [PubMed]
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275-81. [Crossref] [PubMed]
(English Language Editor: R. Scott)


