
Analysis of clinicopathological features of papillary thyroid carcinoma in solid organ transplant recipients: a retrospective study
Introduction
The past few decades have witnessed a rise in the incidence of thyroid cancer worldwide, mainly on account of the increased incidence of papillary thyroid cancer (PTC), the most common histological type of thyroid cancer. The wide application of ultrasound and other imaging techniques that enables the detection of small (<1 cm), indolent PTC may also have contributed to the increased incidence of PTC (1). However, the etiology of PTC is still poorly understood. Except for female gender, history of radiation exposure, and obesity (2), few other risk factors of PTC have been identified. Since it is almost impossible to prevent PTC without further knowledge of its etiologies, research in thyroid surgery has previously focused more on the treatment strategies for patients with thyroid tumors that have already been diagnosed. Conservative treatment strategies have increasingly been advocated in recent National Comprehensive Cancer Network (NCCN) or Cancer Therapy Advisor (CTA) guidelines.
Compared with the general population, solid organ transplant recipients (SOTR) require closer medical monitoring, with examinations including thyroid gland palpation and computed tomography (CT)/magnetic resonance imaging (MRI) of the neck and chest. In addition, abnormal endocrine-related findings in transplant recipients may lead to further testing and subsequent identification of thyroid tumors (3). Solid organ transplantation (SOT), especially liver and kidney transplantation, is currently the most effective treatment for end-stage liver and kidney diseases, and leads to dramatically optimized survival rates and quality of life (QoL) of those patients. However, with the significantly prolonged graft survival, the aging of transplant recipients, and the use of more potent immunosuppressants, the incidence of malignant tumors after SOT has shown a significant upward trend (4). The incidence of certain types of cancers, especially virus-related cancers (e.g., skin cancer), is particularly higher in SOTR compared to non-recipients, which is attributed to the immunosuppressive drugs intended to prevent immune rejection after transplantation (5). The incidence of thyroid cancer has been found to be increased among SOTR (6), whereas no difference has been observed among patients infected with human immunodeficiency virus (HIV; another immunosuppressed population) (7).
A previous meta-analysis showed that thyroid cancer after renal transplantation all presented PTC in pathological features (8). However, there is a lack of detailed studies reporting the differences in clinicopathological features between post-transplant PTC in SORT and PTC in general population currently. It is still uncertain whether the characteristics of PTC differ between the general population and SOTR. Are PTCs in SOTR larger and more aggressive? Are they more prone to lymph node metastasis? In this study, we aimed to compare the clinicopathological features of PTC between SOTR and the general population, and routine monitoring of thyroid lesions after SOT is of great significance for timely detection and treatment of related diseases and improving the overall QoL in the SORT population. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-431/rc).
Methods
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional committee of the First Affiliated Hospital of Zhejiang University School of Medicine (No. IIT20200464A) and informed consent was taken from all the patients.
Patients
We retrospectively analyzed the data of 798 patients undergoing PTC surgery in the Thyroid Surgery Department of the First Affiliated Hospital of Zhejiang University School of Medicine from July 2013 to April 2019. All patients underwent total thyroidectomy or hemithyroidectomy and central compartment cervical lymph node dissection. Lateral cervical lymph node dissection was dependent on the specific lateral cervical lymph node involvement. The exclusion criteria were as follows: (I) thyroid cancer diagnosed before transplantation; (II) other types of thyroid cancer; (III) a history of surgery for a benign or malignant thyroid tumor; (IV) a history of long-term use of thyroxine or steroids; (V) accompanying immune diseases or a history of immunomodulator use for any reason; and (VI) recipients of other non-solid-organ transplants (e.g., hematopoietic stem cell transplantation).
Finally, 411 PTC patients were included in this study cohort. These patients were divided into two groups (Table 1): non-transplant recipients (group A, n=380) and transplant recipients (group B, n=31). The transplant recipients underwent SOT including liver transplantation, kidney transplantation, and combined liver-kidney transplantation. We retrospectively analyzed the clinicopathological characteristics of both groups including age, gender, time interval after transplantation, time of the detection of nodules, maximum tumor diameter, extent of surgery, multifocality, distribution of nodules, number of involved lymph nodes in the central and lateral compartments, blood levels of parathyroid hormone (PTH), thyroid stimulating hormone (TSH), calcium, and phosphorus, and recurrence rate. All patients underwent unilateral or bilateral central lymph node dissection. In cases where one side was identified as malignant and the contralateral side was with or without nodules [thyroid imaging reporting and data system (TI-RADS) category 3 or below], whether surgery should be performed was decided by the surgeon based on the pre- and intra-operative findings. Ultrasound was performed in all patients. When the preoperative lymph node biopsy confirmed the presence of metastases in the cervical lymph nodes, therapeutic neck dissection at nodal levels IIa, III, IV, and Vb was performed. Routine postoperative ultrasound was performed to assess the possible recurrence. Disease recurrence requiring reoperation was defined as a structural recurrence of the malignancy identified by ultrasound and confirmed by needle biopsy. Multifocality was defined as more than one focus of invasive carcinoma, regardless of whether these lesions were located on one side or both sides.
Table 1
| Parameters | Group A (n=380) | Group B (n=31) | t/χ2 | P value |
|---|---|---|---|---|
| Gender | 4.589 | 0.032 | ||
| Male | 103 (27.1) | 14 (45.2) | ||
| Female | 277 (72.9) | 17 (54.8) | ||
| Multifocality | 9.292 | 0.002 | ||
| No | 281 (73.9) | 15 (48.4) | ||
| Yes | 99 (26.1) | 16 (51.6) | ||
| Extraglandular invasion | 1.488 | 0.222 | ||
| No | 335 (88.2) | 25 (80.6) | ||
| Yes | 45 (11.8) | 6 (19.4) | ||
| Central compartment lymph node metastasis | 5.022 | 0.025 | ||
| No | 237 (62.4) | 13 (41.94) | ||
| Yes | 143 (37.6) | 18 (58.06) | ||
| Number of involved lymph nodes in central compartment | 1.64±3.78 | 2.03±5.24 | −0.535 | 0.593 |
| Age (years) | 45.36±12.07 | 45.77±9.58 | −0.184 | 0.854 |
| TSH (mU/L) | 2.74±6.97 | 2.01±1.37 | 0.582 | 0.561 |
| Maximum tumor diameter (cm) | 0.89±0.58 | 0.68±0.43 | 1.971 | 0.049 |
Data are expressed as n (%) or mean ± standard deviation. The statistical analysis methods used are independent sample t-test and χ2 test. A value of P<0.05 was considered statistically significant. SPSS software package (version 21.0) was used. TSH, thyroid stimulating hormone.
Statistical analysis
Statistical analyses were performed using the SPSS 25.0 (IBM Corp., Armonk, NY, USA). Measurement data were expressed as mean ± standard deviation (SD), independent sample Student’s t-test was used for comparison between groups with normal distribution, Chi-square test was used for comparison of measurement data with non-normal distribution, and count data were expressed as percentage. χ2 test or Fisher’s exact test was used for comparison between groups. Stepwise binary logistic regression was used for logical analysis. P value <0.05 was considered statistically significant.
Results
Table 1 summarizes the clinicopathological characteristics of the enrolled patients. The two groups were matched for age and TSH level at disease onset. There were more females in group A (72.9%), whereas the ratio of men to women was similar in group B. These two groups had significant differences in tumor multifocality (P=0.002), maximum tumor diameter (P=0.049), and lymph node metastasis (P=0.025), but no significant difference in the number of involved lymph nodes and the extraglandular invasion.
Binary logistic regression multivariate analysis showed that these two groups were significantly different in central compartment lymph nodes metastasis (P=0.005), multifocality (P=0.003), and maximum tumor diameter (P=0.007). In other words, patients in group B were more likely to have multifocality and lymph node metastasis, although their tumors were smaller in size. Gender, age, extraglandular invasion, preoperative TSH, number of involved lymph nodes in the central compartment, and lymph node metastasis in the lateral compartments were not significantly different between groups A and B (Table 2).
Table 2
| Parameters | β | Standard error | Wald | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Gender | −0.606 | 0.407 | 2.209 | 0.137 | 0.246–1.213 |
| Extraglandular invasion | 0.843 | 0.545 | 2.393 | 0.122 | 0.798–6.766 |
| Multifocality | 1.213 | 0.406 | 8.904 | 0.003 | 1.516–7.461 |
| Age | 0.011 | 0.019 | 0.350 | 0.554 | 0.975–1.049 |
| TSH | −0.079 | 0.126 | 0.392 | 0.531 | 0.723–1.182 |
| Maximum tumor diameter | −1.581 | 0.589 | 7.194 | 0.007 | 0.065–0.653 |
| Number of involved lymph nodes in central compartment | −0.103 | 0.114 | 0.810 | 0.368 | 0.721–1.129 |
| Central compartment lymph node metastases | 1.454 | 0.513 | 8.029 | 0.005 | 1.566–11.701 |
| Lateral compartment lymph node metastasis | −0.989 | 1.158 | 0.730 | 0.393 | 0.038–3.599 |
Logistic regression analysis was used for statistical analysis. A value of P<0.05 was considered statistically significant. SPSS software package (version 21.0) was used. TSH, thyroid stimulating hormone; OR, odds ratio; CI, confidence interval.
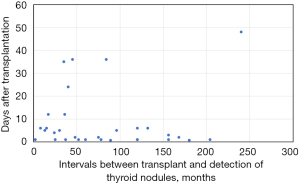
In group B, the time interval from the detection of nodules to the diagnosis of thyroid cancer was within 10 months (Figure 1). Although the tumors occurred at various time points after the transplantation, most patients had the PTC diagnosed 4–8 years after the surgery.
Univariate analysis of the patients with metastasis to central compartment lymph nodes in group B (Table 3) showed that immunosuppressant use was the only risk factor for central cervical lymph node metastasis (P=0.014) and total thyroidectomy showed no significant difference (P=0.073). Although lymph node metastasis was more common in group B, there was no significant difference between the groups with or without lymph node metastasis in terms of multifocality, extraglandular invasion, maximum tumor diameter, and proportions of unilateral and bilateral PTC. In addition, the baseline data, including age, gender, age at transplantation, and preoperative blood calcium, phosphorus, PTH, and TSH levels, showed no significant differences.
Table 3
| Parameters | No metastasis (n=13) | Metastasis (n=18) | P value |
|---|---|---|---|
| Gender | 0.717 | ||
| Male | 5 (38.5) | 9 (50.0) | |
| Female | 8 (61.5) | 9 (50.0) | |
| Extraglandular invasion | 1.000 | ||
| No | 11 (84.6) | 14 (77.8) | |
| Yes | 2 (15.4) | 4 (22.2) | |
| Multifocality | 0.285 | ||
| No | 8 (61.5) | 7 (38.9) | |
| Yes | 5 (38.5) | 11 (61.1) | |
| Total thyroidectomy | 0.073 | ||
| No | 9 (69.2) | 6 (33.3) | |
| Yes | 4 (30.8) | 12 (66.7) | |
| Unilateral cancer | 0.462 | ||
| No | 4 (30.8) | 9 (50.0) | |
| Yes | 9 (69.2) | 9 (50.0) | |
| Immunosuppressant regimens | 0.014 | ||
| Mycophenolate mofetil + tacrolimus | 6 (46.2) | 16 (88. 9) | |
| Rapamycin + tacrolimus | 4 (30.8) | 2 (11.1) | |
| Others | 3 (23.1) | 0 (0.0) | |
| Age (years) | 48.08±9.70 | 44.11±9.42 | 0.262 |
| Interval after transplant (months) | 87.77±73.56 | 75.78±57.17 | 0.613 |
| Time to the detection of nodules (months) | 8.31±15.26 | 8.80±11.29 | 0.919 |
| PTH (ng/L) | 250.23±428.08 | 132.95±186.01 | 0.307 |
| TSH (mU/L) | 2.22±1.51 | 1.87±1.30 | 0.495 |
| Blood calcium level (mmol/L) | 2.42±0.33 | 2.37±0.22 | 0.616 |
| Blood phosphorus level (mmol/L) | 1.14±0.42 | 1.02±0.26 | 0.334 |
| Maximum tumor diameter (cm) | 0.58±0.31 | 0.74±0.50 | 0.317 |
Data are expressed as n (%) or mean ± standard deviation. Independent sample t-test and χ2 test were used for statistical analysis. A value of P<0.05 was considered statistically significant. SPSS software package (version 21.0) was used. PTH, papillary thyroid hormone; TSH, thyroid stimulating hormone.
Since there was only one patient with cervical lymph node metastasis in group B, the sample size was too small, so we did not perform statistical analysis on the risk factors for cervical lymph node metastasis of PTC in group B.
Univariate analysis was performed on the risk factors of bilateral cancer in group B (Table 4). It was found that extraglandular invasion (P=0.059), multifocality (P<0.001), central compartment lymph node metastasis (P=0.093), and blood phosphorus level (P=0.019) significantly differed between the two groups. However, there were no statistical differences in gender, age, the detection time of nodules, time interval after transplantation, immunosuppressant regimen, blood PTH, TSH, and calcium levels, and maximum tumor diameter.
Table 4
| Parameters | Unilateral cancer (n=18) | Bilateral cancer (n=13) | P value |
|---|---|---|---|
| Gender | 0.157 | ||
| Male | 6 (33.3) | 8 (61.5) | |
| Female | 12 (66.7) | 5 (38.5) | |
| Extraglandular invasion | 0.059 | ||
| No | 17 (94.4) | 8 (61.5) | |
| Yes | 1 (5.6) | 5 (38.5) | |
| Multifocality | <0.001 | ||
| No | 15 (83.3) | 0 (0.0) | |
| Yes | 3 (16.7) | 13 (100.0) | |
| Total thyroidectomy | <0.001 | ||
| No | 15 (83.3) | 0 (0.0) | |
| Yes | 3 (16.7) | 13 (100.0) | |
| Re-operations | 0.497 | ||
| No | 16 (88.9) | 13 (100.0) | |
| Yes | 2 (11.1) | 0 (0.0) | |
| Immunosuppressant regimens | 0.183 | ||
| Mycophenolate mofetil + tacrolimus | 13 (72.2) | 11 (84.6) | |
| Rapamycin + tacrolimus | 5 (27.8) | 1 (7.7) | |
| Mycophenolate mofetil | 0 (0.00) | 1 (7.7) | |
| Age (years) | 45.67±10.39 | 45.92±8.75 | 0.944 |
| Interval after transplant (months) | 86.83±70.85 | 72.46±53.78 | 0.602 |
| Time to the detection of nodules (months) | 9.69±14.59 | 7.08±10.38 | 0.586 |
| PTH (ng/L) | 217.26±380.76 | 130.78±163.45 | 0.449 |
| TSH (mU/L) | 2.23±1.17 | 1.72±1.62 | 0.316 |
| Blood calcium level (mmol/L) | 2.39±0.24 | 2.39±0.32 | 1.000 |
| Blood phosphorus level (mmol/L) | 1.19±0.38 | 0.91±0.16 | 0.019 |
| Maximum tumor diameter (cm) | 0.62±0.46 | 0.76±0.40 | 0.385 |
| Number of involved lymph nodes in central compartment | 0.83±1.38 | 2.62±4.09 | 0.093 |
Data are expressed as n (%) or mean ± standard deviation. Independent sample χ2 test was used for statistical analysis. A value of P<0.05 was considered statistically significant. SPSS software package (version 21.0) was used. PTC, papillary thyroid carcinoma; PTH, papillary thyroid hormone; TSH, thyroid stimulating hormone.
Binary logistic stepwise regression analysis of risk factors for bilateral cancer in group B revealed that both extraglandular invasion (P=0.04) and serum phosphorus level (P=0.04) were independent risk factors for bilateral cancer (Table 5). More specifically, serum phosphorus level was positively correlated with bilateral cancer. Patients with low blood phosphorus levels were more likely to develop bilateral cancer; extraglandular invasion was positively correlated with the risk of bilateral cancer, and patients with extraglandular invasion were prone to bilateral cancer.
Table 5
| Parameters | β | Standard error | Wald | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Extraglandular invasion | −2.67 | 1.28 | 4.32 | 0.04 | 0.006–0.859 |
| Blood phosphorus level | 4.89 | 2.38 | 4.21 | 0.04 | 1.247–14,286.754 |
A value of P<0.05 was considered statistically significant. SPSS software package (version 21.0) was used. OR, odds ratio; CI, confidence interval.
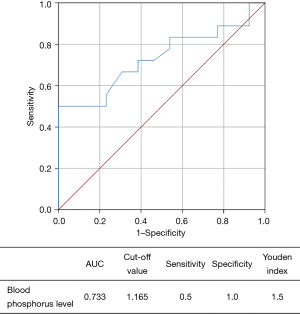
Similarly, the receiver operating characteristic (ROC) curve demonstrated the diagnostic value of blood phosphorus level for bilateral thyroid cancer in group B (Figure 2). The analysis showed that the optimal cut-off point of blood phosphorus was 1.165 and the area under the ROC curve (AUC) was 0.733; the sensitivity and specificity of the optimal cut-off value were 0.5 and 1.0, respectively, with a Youden index of 1.5.
Discussion
The incidences and types of malignant tumors after SOT vary widely across countries and centers in different periods. A meta-analysis of 15 articles on malignant tumors after renal transplantation showed the incidence of malignant tumors following renal transplantation ranged from 0.56% to 4.2% (9); the overall incidence was 1.5%, and the incidences of post-transplant malignant tumors were 3.1–16.5% (10,11), which were markedly lower than those reported in other countries. In addition to factors such as race, geographic area, and lifestyle, other factors including organ transplantations which were carried out late in most Chinese centers, short follow-up duration, large number of patients lost to follow-up, inaccurate statistics, and less use of potent immunosuppressants (e.g., anti-lymphocyte globulin and anti-thymocyte globulin), may have also contributed to such low incidences in China.
The main post-transplant malignancies include non-melanoma skin cancer and post-transplant lymphoproliferative disorders (PTLD), and their incidences are approximately 10 times those of the general population (12). Other types include colorectal cancer, lung cancer, head and neck cancer, urinary system tumors, and cervical cancer. In China, the most prevalent newly-onset malignancies following transplantation are gastrointestinal cancers and PTLD, followed by liver cancer, head and neck cancer, and lung cancer. Among the head and neck tumors, thyroid cancer is the most common type. The significant diversity in the spectrum of post-transplant tumors may be due to the differences in race/ethnicity and lifestyle among European, American, and Asian populations. Kitahara et al. used a retrospective study to assess potential risk factors for SOTR involving 229,300 patients with a median follow-up of 3.9 years (range, 1.4 to 7.9 years) (6). Of these, 356 transplant recipients were eventually diagnosed with thyroid cancer (with an incidence rate of 29.2/100,000 person-years). In terms of pathological types, 91% were PTCs, 5% were follicular type, 2% were medulla type, 1% were anaplastic type, and the rest were other types. The incidence of thyroid cancer in transplant recipients was 2.50 times higher than in the general population [95% confidence interval (CI): 2.25–2.77]. According to the analysis of different organ transplant recipients, patients who underwent organ transplantation for hypertension-related renal sclerosis had a higher risk of thyroid cancer than the average [incidence rate ratio (IRR) =1.41, 95% CI: 1.03–1.94]. Diabetes mellitus was associated with a lower risk of thyroid cancer (IRR =0.78, 95% CI: 0.56–1.09), whereas cholestatic liver disease or cirrhosis was associated with an average risk (IRR =1.69, 95% CI: 1.09–2.63) (6). Pond et al. retrospectively analyzed the data of 10,689 kidney recipients in Australia and New Zealand between 1963 and 31 March 2002, and 23 cases (0.22%) were diagnosed with thyroid cancer after transplantation. The median time to thyroid cancer diagnosis after transplantation was 68 months (range, 3 to 253 months) compared to 102 months (range, 3 to 363 months; P=0.004) in non-thyroid cancers (3). The higher incidence of newly-onset thyroid cancer and the shorter time to thyroid cancer development in transplant recipients may be due to the following reasons: (I) transplant recipients are more likely to receive close medical monitoring via thyroid ultrasound and thyroid function testing (13,14), which increases the detection rate of thyroid cancer; and (II) long-term use of immunosuppressants suppresses the immune system and facilitates tumor escape from immune surveillance (15). Studies have shown that cyclosporine can induce adenocarcinoma cell invasion through a transforming growth factor-β (TGF-β)-dependent mechanism. Compared with low-dose cyclosporine, normal doses of cyclosporine have a higher carcinogenic risk (16), suggesting that cyclosporine has a direct carcinogenic effect (17). It was also found that tacrolimus could enhance the activity of TGF-β, increase the expression of TGF-β in vivo and in vitro in immunodeficient rats, and promote the invasion of kidney cancer cells to the lungs (18). In the present study (Table 3), all patients with lymph node metastasis had been treated with tacrolimus, which again suggests that tacrolimus may increase PTC aggressiveness. The carcinogenic effect of mycophenolate mofetil remains controversial. Research has shown that mycophenolate mofetil could prevent the progression of adhesion receptor-dependent tumors (19), but there has also been evidence that it could increase the invasiveness of tumor cells (20). We will verify the effects of these immunosuppressants on tumors in subsequent animal experiments.
The incidence of thyroid cancer has been rising in various countries. The number of organ transplantation surgeries has been surging with the increase in the number of organ donors. In our studies, however, the number of graft recipients undergoing thyroid surgery has not increased remarkably, which may be explained by the fear of re-operation among the transplant recipients, the concern about the possible damage to the liver and kidney function after the use of anesthesia and thyroid drugs before and after the re-operation, and the increasingly conservative recommendations on thyroid cancer treatment in current guidelines. Therefore, the number of transplant recipients with thyroid cancer was relatively small in our center for the duration of the study period (from 2013 to 2019).
The extent of surgical resection for thyroid cancer has long been controversial due to its relatively “gentle” biological behaviors and good prognosis. During long-term follow-up, the 10-year survival rate reached up to 96% in the low-risk groups (2,3). Among them, a large proportion of patients had unilateral PTC, for whom the disabling total thyroidectomy is often considered an overtreatment. The feasibility of total thyroidectomy should be carefully evaluated for transplant recipients, especially for cases where one side is diagnosed with malignancy, but the contralateral side has no nodules or has only TI-RADS category 3 or below nodules. On the one hand, there is concern that taking immunosuppressive agents may cause recurrence of thyroid cancer after surgery, and mental stress can also occur during nodule follow-up; on the other hand, there is concern that parathyroid function and recurrent laryngeal nerve damage after total thyroidectomy will affect the QoL and that the loss of thyroid function may lead to the lifelong use of thyroxine tablets, resulting in damage to normal organ function.
Through univariate analysis, we found that thyroid cancer is more common in women in the general population, which is consistent with the incidence of thyroid cancer; however, the proportions of males and females were not significantly different in the transplant recipients (Table 1), which may be explained by the fact that the indications of organ transplantation are not markedly different between males and females. In addition, multivariate analysis of the clinicopathological parameters between our two groups (Table 2) showed that, compared with the general population, the transplant recipients had higher risks of multifocality and lymph node metastasis but smaller maximum tumor diameter (Table 1). These tumors had more aggressive biological behaviors, as observed in patients with Hashimoto’s thyroiditis with thyroid cancer (21,22): there are multiple small tumor foci, which more readily spread and metastasize inside the gland, no matter whether the multifocal tumors arise from the same clone or from different clones. Pasieka et al. reported that the incidence of bilateral differentiated thyroid cancer after total thyroidectomy was 43% (23). Giles et al. also demonstrated that multifocal thyroid cancer is more likely to undergo malignant transformation, recurrence, and metastasis than unifocal lesions (24). In our study, 9 patients with bilateral TI-RADS category 4 nodules detected by preoperative ultrasound were confirmed to have bilateral PTC and 7 patients with unilateral TI-RADS category 4 nodules (no nodules on the contralateral side) were diagnosed with bilateral cancer after the operation; of the 6 patients undergoing unilateral resection, contralateral suspicious malignant nodules were found in 2 cases during the follow-up period (4 and 18 months after operation, respectively) and were confirmed as bilateral PTC after re-operation. Thus, the probability of bilateral thyroid cancer is high in the transplant recipients. Total thyroidectomy may benefit most patients if the function of the recurrent laryngeal nerve and parathyroid glands can be well preserved.
In addition, we found that group B was more likely to experience central compartment lymph node metastasis than group A (Tables 1,2). Lymph node metastasis, mainly in the central compartment, is the most important form of PTC metastasis (25). According to the literature, the incidence of central compartment lymph node metastasis accounts for an average of 35–45% among all PTC metastases (26), whereas the incidence of central lymph node metastasis of papillary thyroid microcarcinoma is 24.1–64.1% (27). In our study, the overall incidence of central compartment lymph node metastasis of PTC was 58.06% in group B, which was significantly higher than that in group A; however, the number of involved lymph nodes in group B was not significantly higher than that in group A. In addition, univariate analysis of central compartment lymph node metastasis in group B showed no other risk factors except for immunosuppressant use (Table 3), which may be due to the different extent of lymph node dissection in the central compartment by different operators. Therefore, for transplant recipients using tacrolimus, a thorough bilateral central lymph node dissection following total thyroidectomy is recommended. Since transplant recipients are more likely to have lymph node metastasis, bilateral central lymph node dissection is also recommended for some high-risk recipients using other immunosuppressant regimens under the premise that the recurrent laryngeal nerve and parathyroid glands are adequately protected.
In group B, univariate analysis showed that bilateral PTC was associated with extraglandular invasion, multifocality, total thyroidectomy, blood phosphorus level, and number of involved lymph nodes in the central compartment (Table 4). Since multifocality and central compartment lymph node metastasis were more common in transplant recipients (Table 1), it was natural that there were more bilateral cancer cases after total thyroidectomy. With regard to the relationship between blood phosphorus level and bilateral cancer, we speculate that most of our patients were kidney transplant recipients who need to receive a long period of abdominal dialysis or hemodialysis before undergoing transplantation surgery, which could easily lead to the imbalance of calcium and phosphorus metabolism. Nevertheless, the specific relationship between blood phosphorus and bilateral PTC deserves further laboratory and clinical investigations. The number of involved lymph nodes in the central compartment directly determines the N stage in the TNM staging system of PTC. The higher number of involved lymph nodes indicates more lymph node metastases, which is also one of the characteristics of PTC.
In the present study, binary logistic stepwise regression multivariate analysis was performed to assess the independent risk factors of bilateral PTC based on the relationships of bilateral PTC with extraglandular invasion, multifocality, total thyroidectomy, blood phosphorus level, and number of involved lymph nodes in the central compartment. It was found that only extraglandular invasion and blood phosphorus level were the independent risk factors for bilateral PTC. The ROC curve analysis identified that the optimal cut-off point of blood phosphorus was 1.165 (Figure 1), which was helpful for the diagnosis of bilateral PTC.
The tumor invasion outside the glands can be judged by both preoperative ultrasound and intraoperative macroscopic observation (28). Based on the preoperative blood phosphorus level, intraoperative frozen-section pathological examination is needed to further determine the pathological characteristics (e.g., the number of lesions on one side of the gland and the number of metastases in the lymph nodes). When the blood phosphorus level is below 1.165 and/or there is extraglandular invasion, total thyroidectomy is recommended for cases where one side is diagnosed with PTC, but the contralateral side has no nodules or has only TI-RADS category 3 or below nodules.
Conclusions
In summary, the incidence of bilateral PTC is high in SOTR patients, and a reduced extent of resection is associated with higher probability of residual tumor in the remaining gland. For patients with confirmed unilateral PTC along with extraglandular invasion and/or hypophosphatemia (the contralateral side has no nodules or has only TI-RADS category 3 or below nodules) or in patients with suspicious nodules on both sides, total thyroidectomy is recommended to rule out the possibility of bilateral PTC. Under the premise that the laryngeal nerve and parathyroid glands are adequately protected, thorough bilateral central compartment lymph node dissection should be performed to avoid a missed diagnosis, improve the diagnosis rate of bilateral PTC, reduce the rate of re-operations, prolong disease-free survival, and ultimately maximize the benefits for the SOTR patients.
Acknowledgments
Funding: The study was supported by the Natural Science Foundation of Zhejiang Province (No. LY19H070004).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-431/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-431/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-431/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional committee of the First Affiliated Hospital of Zhejiang University School of Medicine (No. IIT20200464A) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kangelaris GT, Kim TB, Orloff LA. Role of ultrasound in thyroid disorders. Otolaryngol Clin North Am 2010;43:1209-27. vi. [Crossref] [PubMed]
- Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab 2011;96:3390-7. [Crossref] [PubMed]
- Pond F, Serpell JW, Webster A. Thyroid cancer in the renal transplant population: epidemiological study. ANZ J Surg 2005;75:106-9. [Crossref] [PubMed]
- Janus N, Launay-Vacher V, Ferrero JM, et al. Risk of cancer in patients following chronic dialysis and kidney graft. Bull Cancer 2012;99:285-93. [Crossref] [PubMed]
- Karczewski M, Stronka M, Karczewski J, et al. Skin cancer following kidney transplantation: a single-center experience. Transplant Proc 2011;43:3760-1. [Crossref] [PubMed]
- Kitahara CM, Yanik EL, Ladenson PW, et al. Risk of Thyroid Cancer Among Solid Organ Transplant Recipients. Am J Transplant 2017;17:2911-21. [Crossref] [PubMed]
- Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59-67. [Crossref] [PubMed]
- Karamchandani D, Arias-Amaya R, Donaldson N, et al. Thyroid cancer and renal transplantation: a meta-analysis. Endocr Relat Cancer 2010;17:159-67. [Crossref] [PubMed]
- Kyllönen L, Pukkala E, Eklund B. Cancer incidence in a kidney-transplanted population. Transpl Int 1994;7:S350-2. [Crossref] [PubMed]
- Rademacher S, Seehofer D, Eurich D, et al. The 28-year incidence of de novo malignancies after liver transplantation: A single-center analysis of risk factors and mortality in 1616 patients. Liver Transpl 2017;23:1404-14. [Crossref] [PubMed]
- Burra P, Rodriguez-Castro KI. Neoplastic disease after liver transplantation: Focus on de novo neoplasms. World J Gastroenterol 2015;21:8753-68. [Crossref] [PubMed]
- Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891-901. [Crossref] [PubMed]
- Tessari G, Naldi L, Boschiero L, et al. Incidence of primary and second cancers in renal transplant recipients: a multicenter cohort study. Am J Transplant 2013;13:214-21. [Crossref] [PubMed]
- Kluijfhout WP, Drake FT, Pasternak JD, et al. De novo thyroid cancer following solid organ transplantation-A 25-year experience at a high-volume institution with a review of the literature. J Surg Oncol 2017;115:105-8. [Crossref] [PubMed]
- Marcén R, Galeano C, Fernández-Rodriguez A, et al. Effects of the new immunosuppressive agents on the occurrence of malignancies after renal transplantation. Transplant Proc 2010;42:3055-7. [Crossref] [PubMed]
- Suthanthiran M, Hojo M, Maluccio M, et al. Post-transplantation malignancy: a cell autonomous mechanism with implications for therapy. Trans Am Clin Climatol Assoc 2009;120:369-88. [PubMed]
- Kauffman HM. Malignancies in organ transplant recipients. J Surg Oncol 2006;94:431-3. [Crossref] [PubMed]
- Maluccio M, Sharma V, Lagman M, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation 2003;76:597-602. [Crossref] [PubMed]
- Wang K, Zhang H, Li Y, et al. Safety of mycophenolate mofetil versus azathioprine in renal transplantation: a systematic review. Transplant Proc 2004;36:2068-70. [Crossref] [PubMed]
- Vasudev B, Hariharan S. Cancer after renal transplantation. Curr Opin Nephrol Hypertens 2007;16:523-8. [Crossref] [PubMed]
- Kim SS, Lee BJ, Lee JC, et al. Coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma: the influence of lymph node metastasis. Head Neck 2011;33:1272-7. [Crossref] [PubMed]
- Liu Y, Lv H, Zhang S, et al. The Impact of Coexistent Hashimoto's Thyroiditis on Central Compartment Lymph Node Metastasis in Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne) 2021;12:772071. [Crossref] [PubMed]
- Pasieka JL, Thompson NW, McLeod MK, et al. The incidence of bilateral well-differentiated thyroid cancer found at completion thyroidectomy. World J Surg 1992;16:711-6; discussion 716-7. [Crossref] [PubMed]
- Giles Y, Boztepe H, Terzioglu T, et al. The advantage of total thyroidectomy to avoid reoperation for incidental thyroid cancer in multinodular goiter. Arch Surg 2004;139:179-82. [Crossref] [PubMed]
- Viola D, Materazzi G, Valerio L, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab 2015;100:1316-24. [Crossref] [PubMed]
- Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (Oxf) 2012;76:131-6. [Crossref] [PubMed]
- Zhang L, Wei WJ, Ji QH, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab 2012;97:1250-7. [Crossref] [PubMed]
- He Y, Li Z, Yang Y, et al. Preoperative Visualized Ultrasound Assessment of the Recurrent Laryngeal Nerve in Thyroid Cancer Surgery: Reliability and Risk Features by Imaging. Cancer Manag Res 2021;13:7057-66. [Crossref] [PubMed]
(English Language Editor: J. Jones)


