Cryosurgery of breast cancer
Key words: Breast cancer; cryoablation; cryosurgery; ductal carcinoma-in-situ
Introduction
Breast cancer is the most common type of cancer among women in the world (other than skin cancer). Over 211,000 new cases of invasive breast cancer were diagnosed in the Unites States in 2003. The widespread screening for breast cancer has resulted in more patients with earlier stages and tumors less than 20 mm in size being detected (1). One third of all new cancers, which are less than 10 mm, have an excellent prognosis, with approximately a 90% survival rate at 20 years (2), the diagnosis and treatment of breast cancer have changed rapidly in the past 2 decades, with less invasive procedures replacing more extensive procedures.
For decades, ablative techniques have been applied to malignancies in the liver, lung, bone, kidney, prostate gland, and pancreas (3). The breast is an ideal model for ablative therapies because of its superficial location on the thorax and absence of intervening organs between it and the skin. Several methods are presently being investigated for the nonsurgical ablation of breast cancer, and these methods include radiofrequency ablation, cryosurgery, laser interstitial therapy, high-intensity focused ultrasound surgery, and focused microwave thermotherapy (3,4). Of these modalities, cryoablation of breast cancer has been paid great attention (4,5). Apart from treating small breast cancer, cryoablation has been used for palliation of advanced breast cancer.
Indication
The selection of patients with breast cancer who undergo cryosurgery is based on the following considerations (6,7):
Technology
The following kinds of cryosurgery techniques are used for breast cancer (7,8):
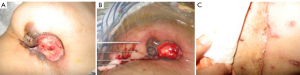
The tumor is first identified by using ultrasound (US) and the most convenient access to the mass was determined. For local anesthesia, 2-5 mL of 1% lidocaine is injected into the deeper tissues proximal to the mass along the expected course of the cryoprobe. Afterward, a variable number (one to two) of cryoprobes (1.4 or 1.7 mm in diameter) are percutaneously placed directly into the breast mass through a small incision and the tip was advanced 1.0-1.5 cm beyond the distal edge of the tumor (Figure 1). Generally, lesions smaller than 15 mm could be reliably frozen with a single, centrally placed, 3-mm probe, and large lesions require multiple probes. Placement of probes within the tumor is confirmed by using US to ensure symmetrical placement of the probe prior to activation of the cryoablation system. Each cryoprobe is cooled to –160 ˚C for 10-15 minutes. The cryoablation procedure consists of two freeze-thaw cycles. With real-time ultrasound, the freeze ball can be seen encompassing the tumor because there exists a highly echogenic interface between frozen and unfrozen tissue. Because the ice ball forms more like an oval than a ball; that is, it is longer in the longitudinal plane along the length of the probe, the diameter of the ice-ball in the longitudinal and transverse planes is measured during each freeze-thaw cycle to ensure appropriate width and length so that the ice-ball encompasses the cancer with an additional “safe border” of at least 5 mm.
If the ice-ball is too close to skin, saline may be injected into the breast tissue between tumor and skin to maintain a suitable distance. Alternatively, room temperature saline or water can be dripped directly onto the skin’s surface to protect it (6).
Percutaneous cryoablation for breast cancer is also performed under guidance of near-real-time open-configuration MR system.
It is specially noted that once the tumor tissue has been destroyed, tumor markers cannot be reliably assessed. Therefore, core biopsy of the breast cancer to determine the presence of estrogen and progesterone receptors, HER-2/neu, and markers of proliferation, apoptosis, differentiation, and cell regulation is a prerequisite for the ablative techniques (6).
Clinical results
Small breast cancer
In 1985, Rand et al. (11) made the first reported a 77-year-old woman with a 1 by 2 cm palpable mass, who underwent cryosurgery under US guidance, and the tumor was then resected. Histopathologic analysis revealed no viable tumor cells. The patient had disease-free during 2-year follow-up. In 1997, Staren (5) reported a 76-year-old lady with two foci of infiltrating lobular carcinoma who received percutaneous cryosurgery. Core needle biopsy at 4 and 12 weeks postablation revealed tissue necrosis, inflammatory cells, and cellular debris but were negative for persistent tumor. This is the only human example of the natural history of cryoablated breast carcinoma because nearly all ablation studies are coupled with postprocedure resection.
Stocks et al. (12) reported 11 patients with invasive ductal carcinoma who underwent cryoablation and then the tumors were excised within 1 to 3 weeks. The tumors ranged from 7 to 22 mm and averaged 13 mm. Ten of the 11 tumors were completely ablated. In one case residual malignant cells were seen at the border of the ablation zone. This study highlights the challenge of eradicating in situ carcinoma with ablative therapy.
Pfleiderer et al. (13) further investigated potential of cryosurgery in the treatment of invasive and in situ breast carcinoma. The 5 tumors <16 mm showed no evidence of invasive cancer. However, two of these five had ductal carcinoma-in-situ (DCIS) in the surrounding tissue. In the 11 tumors >23 mm, histologic examination revealed incomplete necrosis. The results showed that the invasive components of small tumors can be treated using cryotherapy, but that DCIS components may not be detected before ablation and represent a challenging problem.
Roubidoux et al. (14) reported that 9 patients were treated with US-guided tabletop argon gas-based cryoablation system. Mean cancer size was 12 mm. Tumor sites were excised at lumpectomy 2-3 weeks after cryoablation. Seven (78%) of 9 patients had no residual cancer. One patient had a small focus of invasive cancer; one had extensive multifocal ductal carcinoma in situ. No residual invasive cancer occurred in tumors 17 mm or smaller or in cancers without spiculated margins at US. After cryoablation, there was increased echogenicity at US and increased density at mammography. The study shows that tumor size <16 mm, increased mammographic density, and US characteristics without spiculated margins may suggest complete necrosis of the tumor.
Sabel et al. (15) reported 29 patients with primary invasive breast cancer less than 20 mm who underwent ultrasound-guided cryosurgery with a tabletop argon-gas-based cryosurgical system. All cancers <1.0 cm were successfully destroyed. For tumors between 1.0 and 1.5 cm, this success rate was achieved only in patients with invasive ductal carcinoma without a significant DCIS component.
Morin et al. (16) reported that under the guidance of open-configuration MR system, percutaneous cryosurgery was performed in 25 patients with operable invasive breast carcinoma, 4 weeks prior to their scheduled mastectomy. All tumoral tissues included in the cryogenic “ice-ball” were destroyed, with no viable histologic residues. Ablation was total in 13 of the 25 tumors treated.
In Fuda Cancer Hospital Guangzhou, Niu et al. (17) had treated 27 patients with small solitary invasive breast cancers using US-guided cryoablation. Tumor proven by core biopsy had median of 13 mm with range of 8-25 mm in size. All 27 patients underwent lumpectomy an average of 14 days after the cryoablation (8-35 days). Twenty-two of 27 patients had axillary staging by intraoperative lymph node mapping and sentinel lymph node biopsy performed at the same time. Four (14.8%) patients had a positive sentinel lymph node. No viable invasive cancer was discovered in 23 (85.2%) of the 27 patients according to histological findings of specimen from lumpectomy. A DCIS which was present within the normal tissue surrounding the cryozone was discovered in an additional four patients (14.8%), in whom two patients had a small focus of lesion and two cases had multifocal lesions. Among 11 patients whose tumors <15 mm in size successfully underwent cryoablation, with no residual invasive or intraductal carcinoma, whereas the tumors in 4 patients with residual DCIS had 15, 21, 21, and 25 mm in size, respectively.
Advanced breast cancer
For advanced breast cancer, cryosurgery can improve patient’s condition and prolong survival. Korpan (7) showed 52 patients with breast cancer, including 10 primary advanced and 42 recurrent, who underwent cryosurgery. Three- and four-year survival were 40%. This figure is not disappointing given the patients’ severe condition.
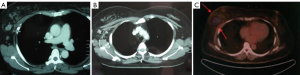
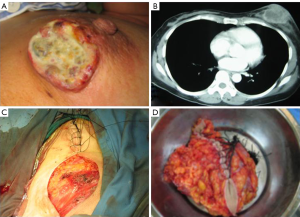
From July to Dec 2005, 42 patients with advanced breast cancer underwent percutaneous cryosurgery in Fuda Cancer Hospital at Guangzhou, China. Of 42 patients, 15 received chemotherapy and 27 chemo/endocrine therapy. Overall 1-, 2-, 3-, and 4-year survival are 72%, 64%, 53% and 45%, as shown in Table 1, and typical cases were illustrated in Figures 1,2,3 (18).
| Table 1 The results of cryosurgery for 42 patients with advanced breast cancer | |||||
| Therapy | Cases | Survival (%) | |||
| 1-year | 2-year | 3-year | 4-year | ||
| Cryosurgery + Chemotherapy | 15 | 68 | 63 | 56 | 47 |
| Cryosurgery + Chemotherapy + Endocrine therapy | 27 | 74 | 65 | 51 | 44 |
| Total | 42 | 72 | 64 | 53.5 | 45.5 |
Discussion
From surgical resection to ablation
During the last 20 years, the conventional treatment for breast cancer has shifted from radical mastectomy to breast conservation through the combined use of lumpectomy and radiation therapy. It is conclusively demonstrated that mastectomy and lumpectomy have long-term efficacy for the appropriate candidate (19,20). The reduced psychological and cosmetic impact of lumpectomy in comparison to radical mastectomy makes it a much more desirable option (21).
Although lumpectomy is not a major operation, it still is a surgical procedure. Compared with surgical resection, ablative therapies have a number of benefits, including (I) breast tumor ablation without surgical excision may be a less morbid procedure without sacrificing cancer control; (II) the therapy can be performed in the office or ambulatory surgery setting with local anesthesia; (III) because the technique does not resect native tissue, it is less disruptive to the contour of the breast, ablated tissues remain in the breast for resorption over time, hence, this may improve the cosmetic outcome; (IV) these percutaneous techniques do not require incisions, which may speed recovery time (4,5). However, ablative techniques have the drawback of not providing tissue for pathological examination. As a consequence, one cannot be certain that the entire lesion is ablated. Moreover, some tumor characteristics like the microscopic size, grade, hormonal receptor status and margin status are not available.
Comparison between heat-based ablation and cryoablation
Compared with ablation technologies which raise tissue temperature, cryosurgery appears more rational (22). There are following theoretical advantages of cryoablation:
Firstly, in heat-based ablation, the degree of thermal injury depends on temperature and duration of exposure. The time necessary for cell death decreases as temperature increases, At 42 °C, tissue injury occurs, but complete cell necrosis may take several hours to achieve, and at 51 °C, cell death can occur as soon as 2 minutes (23). The large and rapid increases in temperature result in almost instantaneous melting of the lipids in the cellular membrane and protein denaturation, causing instant necrosis. However, the creation of a confluent volume of necrosis with a heat-based ablation technology is technically challenging. Breast tissue is composed of both fatty and stromal elements. These different tissues have different thermal properties (i.e., heat capacity and thermal impedance) which may lead to heating that is not predictable or symmetrical. Also, blood flow will remove heat from the tissue being treated, which can also cause irregular ablation zones (24,25), and the uneven size of ablation volume can lead to over or under treatment (26). Several studies have shown residual viable cancer following radiofrequency ablation (27,28).
During cryoablation, the ablation zone is literally frozen in place. No blood flow occurs into or out of an ice-ball. In addition, the ice-ball is always in a quasi- equilibrated state, which ensures both a symmetric ice-ball and symmetric temperature distribution within the ice-ball, resulting in a symmetric volume of confluent ablation (29).
Secondly, heat ablation often causes pain during and after the procedure (30). Consequently, sedation or general anesthesia is often required with heat- based ablation. A significant volume of anesthetic may alter the heating characteristics of the device and the biologic effect it induces (26).
Pain, as observed with heat-based ablation, is usually not a problem during or after cryoablation, because the natural analgesic effect of cold is a natural analgesic (31).
Thirdly, another drawback of heat-based ablation is the difficulty of real-time guidance for operative procedure with ultrasound, which may impair the targeting precision (26). In contrast, during cryoablation, ultrasound shows the proximal edge of the ice-ball appearing as a clearly visible hyperechoic rim, provides an excellent guidance at the boundary between frozen and unfrozen tissue.
Lastly, for prevention of skin injury, at least a 10-mm separation of the tumor edge and the skin surface is required. With cryoablation, it is possible to inject saline (while monitoring with real-time ultrasound) between the tumor and the skin, increasing their separation. This allows for the treatment of tumors close to the skin (5). However, with heat-based ablation, injection of saline may alter the thermic effect.
Patient selection of cryoablation
Patient selection is key to successful cryoablation. DCIS represents a special problem, the presence of DCIS at the margin of the cryoablation zone results in incomplete tumor necrosis, in other words, DCIS may be a relative contraindication to cryotherapy. However, even using MR imaging, DCIS lesions are only detected in 60-70% of patients with breast cancer (15). This fact makes it impossible to include DCIS components of invasive carcinomas in the therapy planning for such cases.
Generally, DCIS is often seen in tumors >15 mm. Cryoablation is adaptable for tumors <10 mm, for primary tumors 10 to 15 mm, only those without an extensive intraductal component are destroyed, while tumors over 15 mm are not reliably eradicated with cryoablation.
Core biopsy is helpful to detect the presence of DCIS. Specifically, the noncalcified type of DCIS causes the most treatment failures in the patients with larger tumors. It is suggested that cryosurgery should be limited to invasive ductal cancers <1.5 cm and with <25% DCIS on the core biopsy. Some breast cancers, such as invasive lobular carcinoma and significant intraductal carcinoma, tend to be multifocal and may include foci too small to be detected through imaging, making them unsuitable for in situ ablation. Tumors that present with more than the most minimal degree of microcalcification should also be excluded, since the extent of these lesions on mammography often can not be detected (5).
It is believed that as diagnostic tools improve, specifically those such as MRI and PET, may allow for accurate mapping of all cancer and DCIS within the breast. In addition, the improvement of cryoablation procedure is important. Use of multiple cryoprobes can partially overcome the pitfall of single cryoprobe ablation (29).
Follow-up after cryoablation
Since the tissue is not excised, complete pathology of the margins cannot be achieved, requiring vigilant follow-up to show if there is residual or recurrent cancer in untreated tissue of the breast. However, it is not yet known what the optimal guiding option for patient is who underwent cryoablation. Serial radiological follow-up should be able to detect residual growth or recurrent cancer. The combined use of imaging technologies such as mammography, ultrasound, gadolinium-enhanced MRI, and PET may be helpful to decrease misdiagnosis of cancer recurrence (5).
Use of cryoablation for advanced breast cancer
There are few data of cryoablation for advanced breast cancer. For such cases whose tumors can’t be resected, cryosurgery seems to be a wise choice. Our primary experience is encouraging. As a cytoreductive technique, cryoablation can provide a basis for subsequent resection and other modalities such as chemotherapy, radiation and molecular targeted therapy.
It is suggested that the immune system of the host becomes sensitized to the tumor being destroyed by the cryosurgery. As the body resorbs the necrotic tissue, an active immunity is developed for the tumor tissue. Any primary tumor tissue undamaged by the cryosurgery and the metastases can be destroyed by the immune system after cryosurgery (32,33). The “cryoimmunological response”, obviously, results in therapeutic benefits for advanced breast cancer.
Sugiyama et al. (34) treated a total of 6 breast cancer patients, 5 with local recurrent tumors on their anterior chest wall and 1 with far advanced primary breast tumor, using multimodal therapy in which included cryosurgery, locoregional immunotherapy and systemic chemotherapy. The results showed that the tumor burden decreased markedly in 2 patients and rapid tumor growth was suppressed in 1 patient, even though the diameter of tumor was over 5 cm in all cases.
Conclusions
For early stage breast cancer, cryoablation is a safe, well-tolerated office-based procedure. Ability to find appropriate candidates for this type of procedure will determine its usefulness. Candidates that have unifocal breast cancer with margins that are accurately defined with imaging studies will benefit from this new modality. For advanced breast cancer, cryosurgery is one of the combined therapies that has a good palliative effect.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ghafoor A, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity. CA Cancer J Clin 2003;53:342-55.
- Korourian S, Klimberg S, Henry-Tillman R, et al. Assessment of proliferating cell nuclear antigen activity using digital image analysis in breast carcinoma following magnetic resonance-guided interstitial laser photocoagulation. Breast J 2003;9:409-13.
- Mirza AN, Fornage BD, Sneige N, et al. Radiofrequency ablation of solid tumors. Cancer J 2001;7:95-102.
- Simmons RM, Dowlatshahi K, Singletary SE, et al. Ablative therapies for breast cancer. Contemp Surg 2002;58:61-72.
- Huston TL, Simmons RM. Ablative therapies for the treatment of malignant diseases of the breast. Am J Surg 2005;189:694-701.
- Staren ED, Sabel MS, Gianakakis LM, et al. Cryosurgery of breast cancer. Arch Surg 1997;132:28-33.
- Tanaka S. Cryosurgery for Advanced Breast Cancer. In: Korpan NN. eds. Basics of Cryosurgery. New York: Wein NewYork, 2001:117-120.
- Roubidoux MA, Sabel MS, Bailey JE, et al. Small (<2.0-cm) breast cancers: mammographic and US findings at US-guided cryoablation--initial experience. Radiology 2004;233:857-67.
- Kaufman CS, Rewcastle JC. Cryosurgery for breast cancer. Technol Cancer ResTreat 2004;3:165-75.
- Bland KL, Gass J, Klimberg VS. Radiofrequency, cryoablation, and other modalities for breast cancer ablation. Surg Clin North Am 2007;87:539-50, xii.
- Rand RW, Rand RP, Eggerding F, et al. Cryolumpectomy for carcinoma of the breast. Surg Gynecol Obstet 1987;165:392-6.
- Stocks LH, Chang HR, Kaufman CS, et al. Pilot study of minimally invasive ultrasound-guided cryoablation in breast cancer. American Society of Breast Surgeons Meeting 2002.
- Pfleiderer SO, Freesmeyer MG, Marx C, et al. Cryotherapy of breast cancer under ultrasound guidance: initial results and limitations. Eur Radiol 2002;12:3009-14.
- Roubidoux MA, Bailey JE, Wray LA, et al. Invasive cancers detected after breast cancer screening yielded a negative result: relationship of mammographic density to tumor prognostic factors. Radiology 2004;230:42-8.
- Sabel MS, Kaufman CS, Whitworth P, et al. Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann Surg Oncol 2004;11:542-9.
- Morin J, Traoré A, Dionne G, et al. Magnetic resonance-guided percutaneous cryosurgery of breast carcinoma: technique and early clinical results. Can J Surg 2004;47:347-51.
- Niu LZ, Xu KC, He WB, et al. Efficacy of percutaneous cryoablation for small solitary breast cancer in term pathologic evidence. Technol Cancer Res Treat 2007;6:460-1.
- Xu KC, Niu LZ. Cryosurgery for Cancer. In: Xu KC, Niu LZ, Liang B. et al. Breast Cancer. Shanghai: Shanghai Science-Technology-Education Pub,2007:138-56.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41.
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32.
- Engel J, Kerr J, Schlesinger-Raab A, et al. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. Breast J 2004;10:223-31.
- Whitworth PW, Rewcastle JC. Cryoablation and cryolocalization in the management of breast disease. J Surg Oncol 2005;90:1-9.
- Izzo F, Thomas R, Delrio P, et al. Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer 2001;92:2036-44.
- Böhm T, Hilger I, Müller W, et al. Saline-enhanced radiofrequency ablation of breast tissue: an in vitro feasibility study. Invest Radiol 2000;35:149-57.
- Lee FT Jr, Haemmerich D, Wright AS, et al. Multiple probe radiofrequency ablation: pilot study in an animal model. J Vasc Interv Radiol 2003;14:1437-42.
- Burak WE Jr, Agnese DM, Povoski SP, et al. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer 2003;98:1369-76.
- Michaels MJ, Rhee HK, Mourtzinos AP, et al. Incomplete renal tumor destruction using radio frequency interstitial ablation. J Urol 2002;168:2406-9; discussion 2409-10.
- Rendon RA, Kachura JR, Sweet JM, et al. The uncertainty of radio frequency treatment of renal cell carcinoma: findings at immediate and delayed nephrectomy. J Urol 2002;167:1587-92.
- Rewcastle JC, Sandison GA, Muldrew K, et al. A model for the time dependent three-dimensional thermal distribution within iceballs surrounding multiple cryoprobes. Med Phys 2001;28:1125-37.
- Fornage BD, Sneige N, Ross MI, et al. Small (
- Tovar EA, Roethe RA, Weissig MD, et al. Muscle-sparing minithoracotomy with intercostal nerve cryoanalgesia: an improved method for major lung resections. Am Surg 1998;64:1109-15.
- Johnson JP. Immunologic aspects of cryosurgery: potential modulation of immune recognition and effector cell maturation. Clin Dermatol 1990;8:39-47.
- Sabel MS, Nehs MA, Su G, et al. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat 2005;90:97-104.
- Sugiyama Y, Saji S, Miya K, et al. Therapeutic effect of multimodal therapy, such as cryosurgery, locoregional immunotherapy and systemic chemotherapy against far advanced breast cancer. Gan To Kagaku Ryoho 2001;28:1616-9.