
The prognosis outcomes of autologous fat transfer for breast reconstruction after breast cancer surgery: a systematic review and meta-analysis of cohort studies
Introduction
Autologous fat transfer (AFT) is a minimally invasive technique that removes suctioned fat tissue from a patient’s body and transplants it into their breasts (1). According to the American Society of Plastic Surgeons, 62% of plastic surgeons applied AFT in breast reconstruction in 2018, predominately for restoring volume defects in the upper quadrant of the breast to improve the aesthetic results (2). Considering the enhancement of breast contour and improvement of aesthetic outcomes, most female breast cancer patients are willing to receive AFT surgery (3,4). Although thousands of patients undergo AFT every year, there is still some concern over the oncological safety of AFT for breast reconstruction following breast cancer surgery.
The use of AFT to correct contour deformities in reconstructed breasts has obtained favorable results in recent years (5). Tayeh et al. found that cancer relapse and complications did not occur in breast cancer patients who underwent AFT (6). Several studies have reported AFT in combination with breast reconstruction after breast cancer treatment is safe oncologically, particularly in terms of not increasing the risk of locoregional recurrence (LRR) (7-9). Meanwhile, a case-control study (10) by Berti et al. showed an increased risk of local recurrence (LR) after AFT in women who were treated for invasive breast cancer. Chung et al. also found a significantly higher risk of cancer recurrence in a population of breast cancer patients who underwent immediate reconstruction (11). However, a monocentric cohort study (12) found a low incidence rate of tumor recurrence and metastasis following the use of AFT, and no evidence of increased risk in any of the survival outcomes was identified from another study evaluating the oncologic safety of AFT after breast cancer surgical treatment (13). The safety of AFT in the context of breast reconstruction is still a matter of controversy. A meta-analysis, as a statistical analysis method of evidence-based medicine, aims to increase the sample size by comprehensively analyzing the research results of multiple small samples on the same subject, thus improving the research efficiency of the original results and making the conclusions more representative (14). It is crucial to offer breast cancer patients information on the benefits and risks of AFT to further improve their quality of life.
Herein, we performed a meta-analysis based on eligible cohort studies to systematically explore the oncological safety of AFT treatment in breast cancer patients, which may help clinicians, policymakers, and steering committees in decision-making and application. We present the following article in accordance with the MOOSE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-297/rc).
Methods
Search strategy
PubMed, Embase, Cochrane Library, and Web of Science databases were used to search for articles published up to September 14, 2020. The search terms from PubMed included “Breast Neoplasms” OR “Breast Neoplasm” OR “Neoplasm, Breast” OR “Breast Tumors” OR “Breast Tumor” OR “Tumor, Breast” OR “Tumors, Breast” OR “Neoplasms, Breast” OR “Breast Cancer” OR “Cancer, Breast” OR “Mammary Cancer” OR “Cancer, Mammary” OR “Cancers, Mammary” OR “Mammary Cancers” OR “Malignant Neoplasm of Breast” OR “Breast Malignant Neoplasm” OR “Breast Malignant Neoplasms” OR “Malignant Tumor of Breast” OR “Breast Malignant Tumor” OR “Breast Malignant Tumors” OR “Cancer of Breast” OR “Cancer of the Breast” OR “Mammary Carcinoma, Human” OR “Carcinoma, Human Mammary” OR “Carcinomas, Human Mammary” OR “Human Mammary Carcinomas” OR “Mammary Carcinomas, Human” OR “Human Mammary Carcinoma” OR “Mammary Neoplasms, Human” OR “Human Mammary Neoplasm” OR “Human Mammary Neoplasms” OR “Neoplasm, Human Mammary” OR “Neoplasms, Human Mammary” OR “Mammary Neoplasm, Human” OR “Breast Carcinoma” OR “Breast Carcinomas” OR “Carcinoma, Breast” OR “Carcinomas, Breast” AND “Fat Autografting” OR “Fat Grafting” OR “Fat Autograft” OR “Fat Graft” OR “Fat Transplantation” OR “Fat Injection” OR “Autologous Fat” OR “Lipostructuring” OR “Lipotransfer” OR “Lipomodelling” OR “Lipomodeling” OR “Autologous Fat Transplantation” OR “Autologous fat transfer” OR “AFT” OR “Fat Transfer”.
Inclusion and exclusion criteria
Inclusion criteria were: (I) populations: patients diagnosed with breast cancer; (II) interventions: patients undergoing AFT after breast cancer surgery as the experimental group; (III) comparators: patients who did not receive AFT after breast cancer surgery as the control group; (IV) outcomes: LR rate, regional recurrence (RRR) rate, LRR rate, distant metastasis rate, systemic recurrence (SR) rate, and total death rate; (V) study design: cohort studies; (VI) studies published in English; (VII) the most recent study of an author.
Exclusion criteria were: (I) animal experiments and pharmacological or pharmacokinetic studies; (II) women with a history of breast cancer and surgical management; (III) reviews, meta-analyses, case reports, conference abstracts, or letters; (IV) interventions other than AFT during treatment; (V) outcomes not relevant to AFT; (VI) literature published repeatedly or without complete data.
Data extraction and quality appraisal
Two researchers screened the articles independently, and a third researcher participated in the extraction of data if there was disagreement between them. Information extracted in the present study included the first author, year, country, total number of patients, age, type of surgery, outcomes, and quality assessment scores.
The modified Newcastle-Ottawa Scale (NOS) was employed for evaluating article quality (15). Three major separate items contributed to the overall NOS quality assessment tool: patient selection, comparability of the treatment and observation groups, and outcome assessment. The scale has 10 points, with 1–4 considered to be low-quality articles and 5–10 high-quality articles.
Statistical analysis
STATA 15 software (Stata Corporation, USA) was used for data analysis. Relative risk (RR) was used as the efficacy statistic indicator, and effect size was described as 95% confidence intervals (CIs). The heterogeneity among the articles was explored using the I2 test. When I2≥50%, the random effects model was employed; otherwise, the fixed effect model was used. Sensitivity analysis was performed for all outcomes. The potential bias in studies was evaluated using a comparison-adjusted funnel plot, which serves as an intuitive visual instrument for detecting the presence of any dominant types of potential bias, such as publication bias, selective reporting, or other biases. Egger’s test was performed to determine whether P values were less than 0.05. P<0.05 was considered statistically significant.
Results
Literature search and study characteristics
The literature selection process is shown in Figure 1. Initially, 1,614 studies were identified in the electronic search. After duplications were removed, 1,230 articles remained, among which 1,189 were excluded based on the inclusion and exclusion criteria. The titles and abstracts were then screened for 41 studies. Ultimately, 22 cohort studies (16-37) were included in the study based on the full text (Figure 1).
In total, 9,971 patients treated for breast cancer were included in this meta-analysis. Of those patients, 3,622 patients underwent AFT (experimental group), and 6,349 patients did not undergo AFT (control group). The results of the upgraded NOS indicated that the 22 articles were all regarded as high quality (Table 1).
Table 1
| Author | Year | Country | Group | Total patients | Age [range or ± SD] | Type of surgery | Histology | Outcomes | NOS scores | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mastectomy | BCS | Quadrantectomy | Invasive | In situ | |||||||||
| Petit | 2012 | Italy | AFT | 321 | 45 [22–71] | 196 | 125 | 284 | 37 | ABCDF | 6 | ||
| NFT | 642 | 46 [26–69] | 392 | 250 | 568 | 74 | |||||||
| Seth | 2012 | USA | AFT | 90 | 49.4±8.8 | 90 | 50 | B | 6 | ||||
| NFT | 1,112 | 48.0±10.6 | 1,112 | 587 | |||||||||
| Petit | 2013 | Italy | AFT | 59 | 49 [33–65] | 47 | 12 | B | 7 | ||||
| NFT | 118 | 50 [29–72] | 94 | 24 | |||||||||
| Kim | 2014 | Korea | AFT | 102 | 46.3 [22–63] | 102 | 42 | A | 5 | ||||
| NFT | 449 | 449 | |||||||||||
| Gale | 2015 | USA | AFT | 211 | 52.2 [30–76] | 176 | 35 | 184 | 27 | ABCDF | 7 | ||
| NFT | 422 | 52.7 [30–72] | 358 | 64 | 368 | 54 | |||||||
| Laporta | 2015 | Italy | AFT | 20 | 44.8 [35–57] | 20 | B | 6 | |||||
| NFT | 20 | 44.95 [35–59] | 20 | ||||||||||
| Masia | 2015 | Italy | AFT | 107 | 49.19 [31–65] | 107 | 0 | 75 | 16 | B | 6 | ||
| NFT | 107 | 48.98 [31–71] | 107 | 0 | 72 | 14 | |||||||
| Pinell-White | 2015 | USA | AFT | 51 | 49.6 [32–68] | 51 | 0 | A | 5 | ||||
| NFT | 51 | 48.9 [32–66] | 51 | 0 | |||||||||
| Mestak | 2016 | Czech | AFT | 32 | 53 [39–67] | 0 | 32 | 24 | 4 | AD | 6 | ||
| NFT | 45 | 64 [37–84] | 0 | 45 | 41 | 3 | |||||||
| Kronowitz | 2016 | USA | AFT | 719 | 47.7±9.6 | 639 | 79 | 552 | 108 | AE | 7 | ||
| NFT | 670 | 46.5±10.5 | 591 | 73 | 548 | 61 | |||||||
| Cohen | 2017 | USA | AFT | 414 | 52.6±11.1 | 414 | 319 | 83 | BD | 7 | |||
| NFT | 162 | 47.8±8.7 | 162 | 111 | 51 | ||||||||
| Fertsch | 2017 | Germany | AFT | 100 | 49.6 | 100 | 0 | 73 | 9 | A | 7 | ||
| NFT | 100 | 50.7 | 100 | 0 | 73 | 9 | |||||||
| Khan | 2017 | UK | AFT | 35 | 49 [35–70] | 0 | 35 | B | 5 | ||||
| NFT | 64 | 54 [36–73] | 0 | 64 | |||||||||
| Petit | 2017 | Italy | AFT | 322 | 0 | 322 | 322 | BCDF | 6 | ||||
| NFT | 322 | 0 | 322 | 322 | |||||||||
| Silva-Vergara | 2017 | Spain | AFT | 205 | 49.1 [23–72] | 147 | 58 | 161 | 44 | BCDF | 7 | ||
| NFT | 410 | 49.7 [24–72] | 286 | 124 | 335 | 75 | |||||||
| Stumpf | 2017 | Brazil | AFT | 27 | 53.6±10.9 | 0 | 27 | 27 | 0 | BE | 6 | ||
| NFT | 167 | 56.4±12.0 | 0 | 167 | 167 | 0 | |||||||
| Calabrese | 2018 | Italy | AFT | 64 | 50.3 [33–69] | 64 | 0 | 23 | AE | 7 | |||
| NFT | 64 | 47.7 [33–60] | 64 | 0 | 25 | ||||||||
| Krastev | 2019 | Netherlands | AFT | 300 | 48.1 [9.0] | 161 | 139 | 261 | 39 | ADF | 6 | ||
| NFG | 300 | 49.4 [8.4] | 150 | 150 | 260 | 40 | |||||||
| Sorrentino | 2019 | Italy | AFT | 233 | 49.4 [±9.0] | 179 | 54 | 207 | 26 | ADF | 6 | ||
| NFT | 597 | 50.7 [±8.9] | 53 | 444 | 535 | 62 | |||||||
| Hanson | 2020 | USA | AFT | 72 | 53 [46.0–61.0] | A | 7 | ||||||
| NFT | 72 | 54 [46.5–64.0] | |||||||||||
| Stumpf | 2020 | Brazil | AFT | 65 | 53 [46.0–61.0] | 0 | 65 | 65 | BCD | 6 | |||
| NFT | 255 | 54 [46.5–64.0] | 0 | 255 | 255 | ||||||||
| Vyas | 2020 | USA | 73 | 48.6±8.8 | A | 6 | |||||||
| 200 | 50.2±9.2 | ||||||||||||
AFT, autologous fat transfer; NFT, non-autologous fat transfer; BCS, breast-conserving surgery; A, locoregional recurrence rate; B, local recurrence rate; C, regional recurrence rate; D, distant metastasis rate; E, systemic recurrence rate; F, total death rate; NOS, Newcastle-Ottawa Scale.
Overall results of the meta-analysis
LR rate
The LR rate (%) of breast cancer patients was analyzed in 12 studies. No significant heterogeneity was detected after merging studies (I2=0.0%). The fixed effect model demonstrated that the rate of LR in the AFT group was lower than that in the non-AFT group (RR: 0.92, 95% CI: 0.70–1.19). However, the difference was not statistically significant (P=0.514; Table 2, Figure 2).
Table 2
| Characteristics | RR (95% CI) | P value | I2 |
|---|---|---|---|
| LR rate | |||
| Overall | 0.92 (0.70–1.19) | 0.514 | 0.0 |
| Sensitivity analysis | 0.92 (0.70–1.19) | ||
| Publication bias | t=1.04 | 0.310 | |
| RRR rate | |||
| Overall | 1.17 (0.77–1.79) | 0.451 | 0.4 |
| Sensitivity analysis | 1.17 (0.77–1.79) | ||
| LRR rate | |||
| Overall | 0.79 (0.62–1.01) | 0.056 | 0.0 |
| Sensitivity analysis | 0.79 (0.62–1.01) | ||
| Publication bias | t=1.08 | 0.315 | |
| Distant metastasis rate | |||
| Overall | 1.13 (0.91–1.42) | 0.248 | 0.0 |
| Sensitivity analysis | 1.13 (0.91–1.42) | ||
| Publication bias | t=1.27 | 0.225 | |
| SR rate | |||
| Overall | 0.67 (0.49–0.92) | 0.012 | 0.0 |
| Sensitivity analysis | 0.67 (0.49–0.92) | ||
| Total death rate | |||
| Overall | 0.75 (0.54–1.05) | 0.096 | 0.0 |
| Sensitivity analysis | 0.75 (0.54–1.05) |
LR, local recurrence; RRR, regional recurrence; LRR, locoregional recurrence; SR, systemic recurrence; RR, relative ratio.
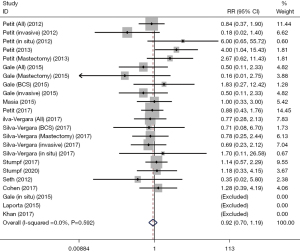
RRR rate
The rates of RRR (%) were identified in 5 cohort studies. Analysis of the fixed effect model showed no difference between the RRR rate of the patients who underwent AFT and those who did not receive AFT (RR: 1.17, 95% CI: 0.77–1.79, P=0.451; Table 2, Figure 3).

LRR rate
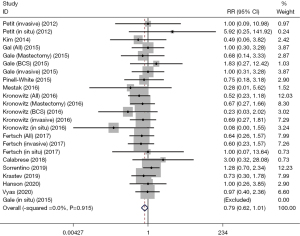
Twelve cohort studies reported the LRR rate. The fixed effect model showed that the AFT group had a relatively lower LRR rate compared with the control group (RR: 0.79, 95% CI: 0.62–1.01, P=0.056; Table 2, Figure 4).
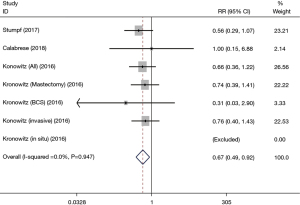
SR rate
The SR rate was included in 3 studies. The results indicated that the SR rate in patients undergoing AFT was lower than in those who did not receive AFT (RR: 0.67, 95% CI: 0.49–0.92, P=0.012; I2=0.0%; Table 2, Figure 5).
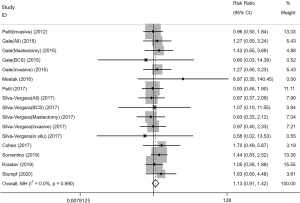
Distant metastasis rate
In total, 9 cohort studies investigated distant metastasis rate. The pooled RR showed no difference in the rate of distant metastasis between breast cancer patients who received AFT and those who did not (RR: 1.13, 95% CI: 0.91–1.42, P=0.248; I2=0.0%; Table 2, Figure 6).
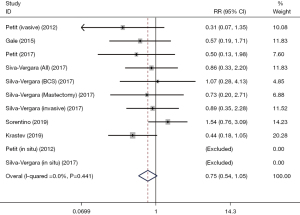
Total death rate
Six studies reported the total death rate. Our analysis found that there was no significant difference in total death rate between patients who underwent breast cancer surgery with AFT and those without AFT (RR: 0.75, 95% CI: 0.54–1.05, P=0.096; I2=0.0%; Table 2, Figure 7).
Sensitivity analysis and publication bias assessment
In the current meta-analysis, sensitivity analysis was used to evaluate the robustness and reliability of pooled results. The outcome of sensitivity analysis showed that the removal of each study did not markedly affect the overall RRs, and the results of this meta-analysis were reliable and steady.
The publication bias of our study was evaluated using Egger’s test, which showed that there was no publication bias in LR rate (t=1.04, P=0.310), LRR (t=1.08, P=0.295), and distant metastasis (t=1.27, P=0.225; Table 2).
Discussion
In the present study, we performed a comprehensive assessment of the oncological safety of AFT in terms of the rate of recurrence, metastasis, and total deaths in 9,971 postoperative breast cancer patients. Compared with the controls who did not receive AFT, our study found that there was no increased risk of LR, RRR, LRR, distant metastasis, and total deaths in breast cancer patients after AFT. However, a decreased risk of SR rate was observed in breast cancer patients receiving AFT. The results of this study confirmed that AFT could be conducted safely in breast reconstruction following breast cancer surgery.
The primary concern with the application of AFT in breast reconstruction is that it might directly or indirectly affect the rate of tumor recurrences. Our meta-analysis evaluating the oncological safety of AFT with a large sample size revealed that no significant differences were observed between the 2 groups regarding the rate of LR, RR, and LRR, and the AFT group displayed a lower SR. A retrospective review demonstrated that AFT did not increase the rate of LRR following breast reconstruction operations combined with improved radiographic imaging (18). Our results were also consistent with the LR rate in studies by Rigotti et al. (0.43%) (38) and Masia et al. (4%) (21) of patients undergoing AFT. These studies involved lengthy follow-up after AFT, but they did not include a control group of patients (21,38,39). A study conducted by the Nottingham Breast Institute found no evidence that AFT increased the risk of carcinoma in women who had formerly been treated for breast cancer. In contrast to controls, the LRR was slightly higher in the AFT group but not significantly (2.1% versus 1.1%, P>0.05). No significant additional tumor events were found in patients with AFT compared with controls in terms of LRR and RRR (16). These results supported our findings. In addition, we discovered a relatively low risk of SR in women diagnosed with breast cancer who underwent AFT surgery. The possible reason is that aesthetic breast augmentation brings less trauma for women in pursuit of beauty.
Another major concern regarding the safety of AFT is the rate of distant metastasis after breast cancer surgery. In the present meta-analysis, there was no significant difference in distant metastasis between the AFT group and non-AFT group. Similarly, another study showed no remote metastasis was documented during the follow-up period (40). Only 6 studies included total deaths, and no prominent findings were found concerning the rate of distant metastasis because there was no significant difference between the 2 groups. A large prospective, randomized, multicenter clinical research is still needed to clearly evaluate the safety of AFT in a cancer setting.
AFT techniques are promoted, to a certain extent, to women seeking aesthetic breast augmentation in an oncologically safe way. Compared with other breast cancer surgeries, the benefits of AFT include low incidence of complications, easy access to donor sites, low morbidity, and the fact that it can be performed in an outpatient setting. Fat grafts are obtained by sucking accumulated fat from the abdomen, thighs, and other parts of the body, and thus breast cancer patients have less trauma, no obvious immune rejection response, natural feel, and postoperative morphological improvement. Low donor-site morbidity and improved cosmetic results are the main advantages of AFT, and these reasons make it easier for women who have received breast cancer surgery, and even doctors, to opt for the AFT procedure (41,42).
Our study had several strengths. Firstly, we searched multiple databases and collected as much literature as possible for inclusion in this meta-analysis. Secondly, we selected high-quality literature for analysis to enhance the persuasiveness of our findings. Finally, publication bias of the included studies was synthetically evaluated via Egger’s test and funnel plots. However, there were several limitations that should be noted. First, corresponding factors influencing RR, LR, LRR, and SR were not reported in the included studies, such as tumor size and stage, surgical modalities (breast conservative operation or mastectomy), cancer histology types (in situ or infiltrating cancers), and postoperative radiotherapy. Second, further imaging in patients should be added in future studies. Last, our meta-analysis comprised only publications in English, which may cause language bias. Considering the above limitations, the findings of our study should be interpreted with caution.
Conclusions
This analysis found that there was no increased risk of LR, RR, LRR, distant metastasis, and total deaths in patients receiving AFT, providing valuable evidence-based support for the oncological safety of AFT. Overall, for breast cancer patients, AFT appeared to be a safe procedure. Further research with follow-up and oncological series are needed to validate the findings of this meta-analysis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-297/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-297/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doornaert M, Colle J, De Maere E, et al. Autologous fat grafting: Latest insights. Ann Med Surg (Lond) 2019;37:47-53. [Crossref] [PubMed]
- American Society of Plastic Surgeons. 2018 Plastic surgery statistics report. [Accessibility verified May 26, 2021]. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf/
- Osswald R, Boss A, Lindenblatt N, et al. Does lipofilling after oncologic breast surgery increase the amount of suspicious imaging and required biopsies?-A systematic meta-analysis. Breast J 2020;26:847-59. [Crossref] [PubMed]
- Chiu WK, Fracol M, Feld LN, et al. A Comparison of Fat Graft Processing Techniques: Outcomes in 1,158 Procedures in Prosthetic Breast Reconstructions. Plast Reconstr Surg Glob Open 2019;7:e2276. [Crossref] [PubMed]
- Garza R 3rd, Ochoa O, Chrysopoulo M. Post-mastectomy Breast Reconstruction with Autologous Tissue: Current Methods and Techniques. Plast Reconstr Surg Glob Open 2021;9:e3433. [Crossref] [PubMed]
- Tayeh S, Muktar S, Wazir U, et al. Is Autologous Fat Grafting an Oncologically Safe Procedure following Breast Conserving Surgery for Breast Cancer? A Comprehensive Review. J Invest Surg 2022;35:390-9. [Crossref] [PubMed]
- Waked K, Colle J, Doornaert M, et al. Systematic review: The oncological safety of adipose fat transfer after breast cancer surgery. Breast 2017;31:128-36. [Crossref] [PubMed]
- Krastev TK, Schop SJ, Hommes J, et al. Meta-analysis of the oncological safety of autologous fat transfer after breast cancer. Br J Surg 2018;105:1082-97. [Crossref] [PubMed]
- Groen JW, Negenborn VL, Twisk DJWR, et al. Autologous fat grafting in onco-plastic breast reconstruction: A systematic review on oncological and radiological safety, complications, volume retention and patient/surgeon satisfaction. J Plast Reconstr Aesthet Surg 2016;69:742-64. [Crossref] [PubMed]
- Berti M, Goupille C, Doucet M, et al. Oncological Safety of Autologous Fat Grafting in Breast Reconstruction after Mastectomy for cancer: A case-control study. J Gynecol Obstet Hum Reprod 2022;51:102257. [Crossref] [PubMed]
- Chung JH, Kim KJ, Jung SP, et al. Analysis of oncological safety of autologous fat grafting after immediate breast reconstruction. Gland Surg 2021;10:584-94. [Crossref] [PubMed]
- Kempa S, Brix E, Heine N, et al. Autologous fat grafting for breast reconstruction after breast cancer: a 12-year experience. Arch Gynecol Obstet 2022;305:921-7. [Crossref] [PubMed]
- Tukiama R, Vieira RAC, Facina G, et al. Oncologic Safety of Autologous Fat Grafting after Breast Cancer Surgical Treatment: A Matched Cohort Study. Plast Reconstr Surg 2021;148:11-20. [Crossref] [PubMed]
- Hao Y, Shan G, Nan K. Establishment of apoptotic regulatory network for genetic markers of colorectal cancer. Saudi J Biol Sci 2017;24:466-76. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Gale KL, Rakha EA, Ball G, et al. A case-controlled study of the oncologic safety of fat grafting. Plast Reconstr Surg 2015;135:1263-75. [Crossref] [PubMed]
- Petit JY, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol 2012;23:582-8. [Crossref] [PubMed]
- Seth AK, Hirsch EM, Kim JYS, et al. Long-term outcomes following fat grafting in prosthetic breast reconstruction: a comparative analysis. Plast Reconstr Surg 2012;130:984-90. [Crossref] [PubMed]
- Petit JY, Rietjens M, Botteri E, et al. Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol 2013;24:1479-84. [Crossref] [PubMed]
- Laporta R, Longo B, Sorotos M, et al. Breast Reconstruction with Delayed Fat-Graft-Augmented DIEP Flap in Patients with Insufficient Donor-Site Volume. Aesthetic Plast Surg 2015;39:339-49. [Crossref] [PubMed]
- Masia J, Bordoni D, Pons G, et al. Oncological safety of breast cancer patients undergoing free-flap reconstruction and lipofilling. Eur J Surg Oncol 2015;41:612-6. [Crossref] [PubMed]
- Cohen O, Lam G, Karp N, et al. Determining the Oncologic Safety of Autologous Fat Grafting as a Reconstructive Modality: An Institutional Review of Breast Cancer Recurrence Rates and Surgical Outcomes. Plast Reconstr Surg 2017;140:382e-92e. [Crossref] [PubMed]
- Petit JY, Maisonneuve P, Rotmensz N, et al. Fat Grafting after Invasive Breast Cancer: A Matched Case-Control Study. Plast Reconstr Surg 2017;139:1292-6. [Crossref] [PubMed]
- Silva-Vergara C, Fontdevila J, Weshahy O, et al. Breast Cancer Recurrence Is not Increased With Lipofilling Reconstruction: A Case-Controlled Study. Ann Plast Surg 2017;79:243-8. [Crossref] [PubMed]
- Khan LR, Raine CR, Dixon JM. Immediate lipofilling in breast conserving surgery. Eur J Surg Oncol 2017;43:1402-8. [Crossref] [PubMed]
- Stumpf CC, Biazus JV, Zucatto FSÂE, et al. Immediate reconstruction with autologous fat grafting: influence in breast cancerregional recurrence. Rev Col Bras Cir 2017;44:179-86. [Crossref] [PubMed]
- Stumpf CC, Zucatto ÂE, Cavalheiro JAC, et al. Oncologic safety of immediate autologous fat grafting for reconstruction in breast-conserving surgery. Breast Cancer Res Treat 2020;180:301-9. [Crossref] [PubMed]
- Calabrese C, Kothari A, Badylak S, et al. Oncological safety of stromal vascular fraction enriched fat grafting in two-stage breast reconstruction after nipple sparing mastectomy: long-term results of a prospective study. Eur Rev Med Pharmacol Sci 2018;22:4768-77. [PubMed]
- Fertsch S, Hagouan M, Munder B, et al. Increased risk of recurrence associated with certain risk factors in breast cancer patients after DIEP-flap reconstruction and lipofilling-a matched cohort study with 200 patients. Gland Surg 2017;6:315-23. [Crossref] [PubMed]
- Hanson SE, Kapur SK, Garvey PB, et al. Oncologic Safety and Surveillance of Autologous Fat Grafting following Breast Conservation Therapy. Plast Reconstr Surg 2020;146:215-25. [Crossref] [PubMed]
- Kim HY, Jung BK, Lew DH, et al. Autologous Fat Graft in the Reconstructed Breast: Fat Absorption Rate and Safety based on Sonographic Identification. Arch Plast Surg 2014;41:740-7. [Crossref] [PubMed]
- Krastev T, van Turnhout A, Vriens E, et al. Long-term Follow-up of Autologous Fat Transfer vs Conventional Breast Reconstruction and Association With Cancer Relapse in Patients With Breast Cancer. JAMA Surg 2019;154:56-63. [Crossref] [PubMed]
- Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the Breast Does Not Increase the Risk of Recurrence of Breast Cancer: A Matched Controlled Study. Plast Reconstr Surg 2016;137:385-93. [Crossref] [PubMed]
- Mestak O, Hromadkova V, Fajfrova M, et al. Evaluation of Oncological Safety of Fat Grafting After Breast-Conserving Therapy: A Prospective Study. Ann Surg Oncol 2016;23:776-81. [Crossref] [PubMed]
- Pinell-White XA, Etra J, Newell M, et al. Radiographic Implications of Fat Grafting to the Reconstructed Breast. Breast J 2015;21:520-5. [Crossref] [PubMed]
- Sorrentino L, Regolo L, Scoccia E, et al. Autologous fat transfer after breast cancer surgery: An exact-matching study on the long-term oncological safety. Eur J Surg Oncol 2019;45:1827-34. [Crossref] [PubMed]
- Vyas KS, DeCoster RC, Burns JC, et al. Autologous Fat Grafting Does Not Increase Risk of Oncologic Recurrence in the Reconstructed Breast. Ann Plast Surg 2020;84:S405-10. [Crossref] [PubMed]
- Rigotti G, Marchi A, Stringhini P, et al. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast Surg 2010;34:475-80. [Crossref] [PubMed]
- Riggio E, Bordoni D, Nava MB. Oncologic surveillance of breast cancer patients after lipofilling. Aesthetic Plast Surg 2013;37:728-35. [Crossref] [PubMed]
- Zhang X, Cai L, Yin B, et al. Total breast reconstruction using large-volume condensed and viable fat grafting after mastectomy. J Plast Reconstr Aesthet Surg 2021;74:966-73. [Crossref] [PubMed]
- Panettiere P, Marchetti L, Accorsi D. The serial free fat transfer in irradiated prosthetic breast reconstructions. Aesthetic Plast Surg 2009;33:695-700. [Crossref] [PubMed]
- Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg 2013;24:1610-5. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)



