Management dilemma of thyroid nodules in patients with malignant struma ovarii
Introduction
Struma ovarii is a rare type of ovarian teratoma occurring in females at all ages, most often between the fifth and sixth decade of life. They are comprised of at least 50% thyroid tissue. Though typically benign, malignancy occurs in 5–10% of reported cases, with 70% of malignant cases diagnosed as papillary carcinoma (1-3). Struma ovarii typically presents as a pelvic mass, often found incidentally on pelvic imaging (4). Common symptoms at initial presentation include abdominal pain, abnormal vaginal bleeding, and menstrual cycle abnormalities (5). Thyroid dysregulation and hyperthyroidism are rare presenting symptoms of struma ovarii, only occurring in 5–8% of cases (6,7).
Historically, the treatment of choice has been surgical removal of the tumor to rule out ovarian carcinoma (8). Surgical options include a fertility-conserving approach or radical bilateral salpingo-oophorectomy (9). Some physicians elect for a more aggressive approach with a radical total hysterectomy and bilateral or unilateral salpingo-oophorectomy (10,11). For benign unilateral disease, unilateral oophorectomy is the most common treatment of choice (8). However, for struma ovarii with high-grade malignancy or distant metastasis, there is no consensus for which gynecologic surgical procedure is recommended (5). Management of patients with thyroid nodules following gynecologic surgery also remains controversial. Thyroid follow-up and further treatment options are guided by characteristics of the tumor; malignant or benign, metastatic or non-metastatic. Total thyroidectomy followed by radioiodine therapy is proposed as the treatment of choice for high-grade malignant struma ovarii and for overtly metastatic disease (8). However, there are a limited number of case reports documenting treatment-specific outcomes following thyroid surgery and management recommendations remain controversial and variable.
Case presentation
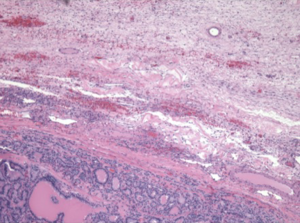
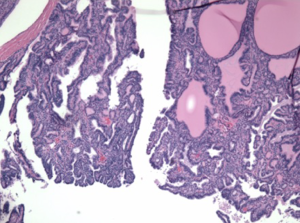
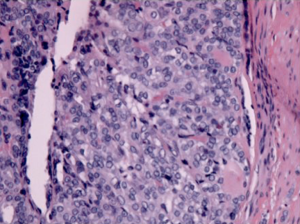
A 49-year-old African American female presented to her gynecologist with severe lower abdominal pain. Pelvic ultrasound revealed several bilateral ovarian cyst-like masses. She was scheduled for bilateral oophorectomy due to associated significant pain. A suspicious tumor arising from the left ovary was noted intraoperatively and a bilateral salpingo-oophorectomy was subsequently performed. Surgical pathology revealed papillary thyroid carcinoma arising from struma ovarii and serous cystadenomas with squamous metaplasia within the left ovary. The histological image in Figure 1 demonstrates ovarian stroma surrounding the teratoma composed of thyroid follicles of varying shapes and sizes. Papillary architecture of the tumor is shown in Figure 2. The image in Figure 3 demonstrates nuclear crowding and central clearing, nuclear grooves and nuclear atypia (12). The right ovary and tube revealed normal ovarian tissue.
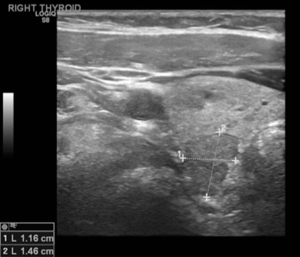
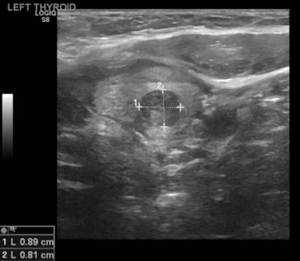
Her endocrinologist identified multiple suspicious nodules on thyroid ultrasound examination postoperatively and referred the patient to our endocrine surgery clinic. On presentation to our clinic, the patient complained of significant dysphagia. The ultrasound examination revealed a goiter with multiple nodules and the most suspicious solid nodule on the right lobe measured 1.7×1.1×1.0 cm3, and contained microcalcifications (Figure 4). In the left lobe there was a 1.0×0.8×0.8 cm3 suspicious solid, hypoechoic nodule with stiffness on the elastography (Figure 5) (13). There was no suspicious lymphadenopathy seen on bilateral central, lateral or posterior compartments. Fine needle aspiration biopsy of these suspicious nodules indicated benign findings. Laboratory evaluation revealed normal thyroid stimulating hormone (1.41 mIU/L), T4, free T4 levels (6.6 mcg/dL and 1.1 ng/L respectively).
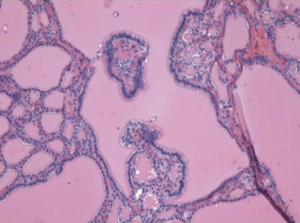
Given the patient’s compressive symptoms and concern that the ovarian pathology could represent metastatic primary thyroid carcinoma rather than isolated struma ovarii, total thyroidectomy or conservative follow-up were both presented as management options to the patient. After extensive counseling, the patient elected to undergo surgical intervention (14). Total thyroidectomy was uneventful and the patient was discharged home after an overnight stay. Postoperative histological analysis revealed benign thyroid nodular hyperplasia, as shown in Figure 6. Due to benign pathology, the patient did not undergo postoperative radioiodine ablation.
Discussion
There is no current consensus for the treatment and follow-up for women diagnosed with malignant struma ovarii (15). Gynecologic surgical options vary from cystectomy, unilateral or bilateral salpingo-oophorectomy, with or without a total hysterectomy depending on concern for metastasis or high grade malignancy. Management of suspicious thyroid nodules in these patients is also variable, and ranges from regular follow-up with ultrasound and thyroglobulin levels to total thyroidectomy with or without radioiodine ablation therapy postoperatively (10,11).
Current treatment recommendations are based mostly on case reports. The rarity of the condition results in a lack of evidence-based recommendations for conservative monitoring and/or thyroidectomy and radioactive iodine therapy (10,11). Some authors support a more aggressive approach, with total thyroidectomy and radioactive iodine therapy. Interestingly, radioactive iodine therapy is recommended by these authors despite benign findings on thyroid pathology with the hope to decrease local and distant recurrence and to allow the use of thyroglobulin levels as an easy way to monitor for recurrence (8,16,17). They found this particularly necessary because their review reported a 27% incidence rate of metastasis, although previous reports found the incidence of metastasis to be about 5% (16).
These cases all required a treatment decision to be made regarding thyroid surgery and need for postoperative radio-iodine therapy after gynecologic surgery. Management by a multidisciplinary team, including a thyroid surgeon is recommended for cases of struma ovarii. However, due to the rarity, the need for thyroidectomy and radio-iodine therapy is still controversial and must be considered on an individualized basis.
Our patient presented with histological evidence of malignant struma ovarii in her left ovary, status post bilateral salpingo-oophorectomy. The patient also has multiple suspicious thyroid nodules and compressive symptoms due to her goiter. Eventually our patient elected to proceed with total thyroidectomy instead of conservative follow-up. Fortunately for the patient, the pathology revealed thyroid hyperplasia, and no evidence of malignancy.
According to Devaney et al., a diagnosis of malignant struma ovarii requires histological characteristics similar to malignant thyroid carcinoma. Malignant features include cytological atypia, ‘ground-glass’ nuclei, increased mitotic activity and/or evidence of vascular invasion (18). The tumor displayed areas of cytological atypia with nuclear clearing and nuclear grooves. Prognosis for malignant struma ovarii based on 88 cases, determined by Robboy et al., shows 10- and 25-year survival to be 89% and 84% respectively (19). However, the limited research done on this rare tumor means there is still significant uncertainty regarding the management and prognosis.
A multi-institutional analysis is needed in order to determine a treatment algorithm for struma ovarii patients in regards to treatment options including conservative versus surgical treatment.
We present this case papillary thyroid carcinoma in struma ovarii and feel it is important to emphasize the difficulties in management dilemma of this rare condition. There is a paucity of data in the literature regarding the optimal surgical management and appropriate thyroid follow-up modality for such patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The authors de-identified the patient. The authors didn’t include any personal pictures of the patient and they did not include any information regarding the patient’s demographics.
References
- Dunzendorfer T, deLas Morenas A, Kalir T, et al. Struma ovarii and hyperthyroidism. Thyroid 1999;9:499-502. [Crossref] [PubMed]
- Makani S, Kim W, Gaba AR. Struma Ovarii with a focus of papillary thyroid cancer: a case report and review of the literature. Gynecol Oncol 2004;94:835-9. [Crossref] [PubMed]
- Salman WD, Singh M, Twaij Z. A case of papillary thyroid carcinoma in struma ovarii and review of the literature. Patholog Res Int 2010;2010:352476.
- Yoo SC, Chang KH, Lyu MO, et al. Clinical characteristics of struma ovarii. J Gynecol Oncol 2008;19:135-8. [Crossref] [PubMed]
- Brusca N, Del Duca SC, Salvatori R, et al. A case report of thyroid carcinoma confined to ovary and concurrently occult in the thyroid: is conservative treatment always advised? Int J Endocrinol Metab 2015;13:e18220. [PubMed]
- Rosenblum NG. Malignant struma ovarii. Gynecol Oncol 1989;32:224-7. [Crossref] [PubMed]
- Alvarez DM, Lee V, Bhatt S, et al. Struma ovarii with papillary thyroid carcinoma. J Clin Imaging Sci 2011;1:44. [Crossref] [PubMed]
- DeSimone CP, Lele SM, Modesitt SC. Malignant struma ovarii: a case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol Oncol 2003;89:543-8. [Crossref] [PubMed]
- Wee JY, Li X, Chern BS, et al. Struma ovarii: management and follow-up of a rare ovarian tumour. Singapore Med J 2015;56:35-9. [Crossref] [PubMed]
- Gunasekaran S, Kopecka E, Maung KH, et al. Struma ovarii and the thyroid surgeon. J Laryngol Otol 2012;126:858-60. [Crossref] [PubMed]
- Luo JR, Xie CB, Li ZH. Treatment for malignant struma ovarii in the eyes of thyroid surgeons: a case report and study of Chinese cases reported in the literature. Medicine (Baltimore) 2014;93:e147. [Crossref] [PubMed]
- Young RH. New and unusual aspects of ovarian germ cell tumors. Am J Surg Pathol 1993;17:1210-24. [Crossref] [PubMed]
- Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol 2009;193:207-13. [Crossref] [PubMed]
- Leite I, Cunha TM, Figueiredo JP, et al. Papillary carcinoma arising in struma ovarii versus ovarian metastasis from primary thyroid carcinoma: a case report and review of the literature. J Radiol Case Rep 2013;7:24-33. [Crossref] [PubMed]
- Marti JL, Clark VE, Harper H, et al. Optimal surgical management of well-differentiated thyroid cancer arising in struma ovarii: a series of 4 patients and a review of 53 reported cases. Thyroid 2012;22:400-6. [Crossref] [PubMed]
- Jean S, Tanyi JL, Montone K, et al. Papillary thyroid cancer arising in struma ovarii. J Obstet Gynaecol 2012;32:222-6. [Crossref] [PubMed]
- Hatami M, Breining D, Owers RL, et al. Malignant struma ovarii--a case report and review of the literature. Gynecol Obstet Invest 2008;65:104-7. [Crossref] [PubMed]
- Devaney K, Snyder R, Norris HJ, et al. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynecol Pathol 1993;12:333-43. [Crossref] [PubMed]
- Robboy SJ, Shaco-Levy R, Peng RY, et al. Malignant struma ovarii: an analysis of 88 cases, including 27 with extraovarian spread. Int J Gynecol Pathol 2009;28:405-22. [Crossref] [PubMed]