
Influence of ovarian-sparing surgery and ovariectomy on prognosis in early cervical adenocarcinoma: a systematic review and meta-analysis
Introduction
Cervical cancer is the most common malignant tumor of the female reproductive system. The pathological types of cervical cancer include squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma, of which squamous cell carcinoma is the most common, followed by adenocarcinoma (1,2). In recent years, the incidence rate of cervical cancer has decreased, which may be related to the extensive implementation of cytology screening (1,3,4). However, the incidence rate of cervical adenocarcinoma is increasing, especially in women under 40 years old (5,6). Patients with early cervical cancer generally undergo radical hysterectomy and pelvic lymphadenectomy (7,8).
Ovarian preservation and ovariectomy have always been controversial for early cervical adenocarcinoma patients (9-11). The main issues are the risk of ovarian metastasis, the poor prognosis of such patients, and the need to preserve ovarian function in young women. Bilateral ovariectomy can eliminate the risk of occult ovarian cancer. However, an ovariectomy leads to a decrease in the estrogen levels of patients, causes a series of problems, such as mood disorder, vaginal dryness and atrophy, night sweats, anxiety, depression, sleep disorder, cardiovascular disease, water, and electrolyte metabolism disorder, and reduces the quality of life of patients (12-14).
Recent studies have shown that the incidence of ovarian metastasis in early cervical adenocarcinoma is low, ranging from 0–10% (15,16). Radical hysterectomy + pelvic lymph node dissection is the standard surgical procedure for cervical adenocarcinoma in Federation of Gynecology and Obstetrics (FIGO) stages IA to IIA, but there is no consensus on whether to preserve the ovaries (17). Bilateral oophorectomy is currently preferred. A bilateral ovariectomy can produce a good therapeutic effect (17). However, one study also pointed out that the effect of ovarian-sparing surgery and oophorectomy on the prognosis of cervical adenocarcinoma is similar (15). Another study has noted that ovarian preservation may improve the prognosis of patients with cervical adenocarcinoma (18). Most of the studies were retrospective single-center studies with small sample sizes, and no reliable conclusions could be drawn. Thus, we sought to conduct a meta-analysis to clarify the effect of ovarian preservation on the prognosis of patients with early cervical adenocarcinoma. We present the following article in accordance with the MOOSE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-310/rc).
Methods
Literature download
A literature search was conducted of the PubMed, Excerpta Medica Database, Medline, CENTRAL, and China National Knowledge Infrastructure databases using the following keywords: (cervical adenocarcinoma OR adenocarcinoma of cervical) AND (ovarian preservation OR ovarian retention) AND prognosis. There were no restrictions on the document language. The retrieval date was April 3, 2022.
Literature screening
To be eligible for inclusion in the meta-analysis, the studies had to meet the following inclusion criteria: (I) comprise patients with cervical adenocarcinoma; (II) comprise an experimental group of patients treated with radical surgery and preserved ovaries, and a control group of patients treated with radical surgery and oophorectomy; the patients in the 2 groups could be treated with or without postoperative adjuvant radiotherapy and chemotherapy; (III) be a case-control study or cohort study; (IV) the observation result is the patient’s survival, including at least one of overall survival (OS), progression free survival (PFS) and disease-specific survival (DSS); (V) contain a description of the article results, including the hazard ratios (HRs) and 95% confidence intervals (CIs), or data from which these could be calculated.
Studies were excluded from the meta-analysis if they met any of the following exclusion criteria: (I) was a repeated report or case report; (II) comprised patients with cervical cancer, but the pathological type could not be distinguished; (III) included no control group; and/or (IV) the required data were unavailable, and the author of the article could not be contacted to supplement the data.
Data extraction
In this study, 2 researchers jointly extracted the data from the articles included in the analysis, including the author, title, publication time, research type, number of researchers, number of patients with ovarian preservation, and number of patients with ovariectomy, and the survival data of the patients with ovarian preservation and ovariectomy, including OS, PFS, and DSS. If the data could not be obtained from the articles, the author was contacted, and the data were requested. If there were different opinions in relation to the data extraction, the 2 researchers discussed the issue and reached an agreement.
Literature quality evaluation
In this study, 2 researchers used the Newcastle-Ottawa Scale (NOS) to evaluate the quality of the articles included in the study, including the selectivity and comparability of the research methods, exposure factors, and outcomes. In cases of inconsistency in the judgment results of the article quality, the 2 researchers reached an agreement after engaging in a discussion.
Statistical method
This study used Cochrane software RevMan5.3 for the statistical analysis of the data. This study obtained HR values and 95% CI calculated from univariate cyclooxygenase (COX) analyses in the literature. HR values and 95% CIs were used to describe the effect quantity. The Chi-square test was used for the heterogeneity test. When the I2 corrected by degrees of freedom was >50% or P<0.1, the results indicated that there was heterogeneity among the published articles, and a random-effects model was used. A subgroup analysis was used to explore the causes of heterogeneity. If the source of heterogeneity could not be found, we could only describe the article results without merging. When the I2 corrected by degrees of freedom was ≤50% and P≥0.1, the results indicated that there was no heterogeneity among the published articles, and the fixed-effects model was used. A funnel plot and Egger test were used to evaluate the publication bias. If the scattered points were distributed within the confidence interval and were roughly symmetrical, the results indicated that there was no publication bias. Conversely, if the scatter was biased to 1 side, the results indicated that there was publication bias. In Egger’s test, P<0.05 indicates that there is publication bias, and P>0.05 indicates that there is no publication bias. A bilateral P value <0.05 was considered statistically significant.
Results
Characteristics of the included articles
A total of 1,034 articles were retrieved in the above database. According to the screening criteria, 1,029 articles were excluded, and a total of 5 articles were included in the study (15,18-21). Figure 1 shows a flow chart of the article screening. The 5 articles were all retrospective analyses, published in English. There were 3,467 patients with stage IA–IIB cervical adenocarcinoma, including 995 patients with ovarian preservation and 1895 patients with ovariectomy. The basic information of the articles and the NOS scores are set out in Table 1.
Table 1
| Author | Year | Study type | NOS | No. of patients | OP (–) | OP (+) |
|---|---|---|---|---|---|---|
| Chen (15) | 2016 | Cohort study | 8 | 159 | 126 | 33 |
| Hu (21) | 2017 | Cohort study | 8 | 105 | 86 | 19 |
| Lyu (20) | 2014 | Cohort study | 7 | 1,639 | 485 | 577 |
| Theplib (19) | 2020 | Cohort study | 7 | 196 | 108 | 88 |
| Xu (18) | 2018 | Cohort study | 7 | 1,368 | 1,090 | 278 |
NOS, Newcastle-Ottawa scale; OP, ovarian preservation.
Comparison of OS between the ovarian preservation and ovariectomy groups
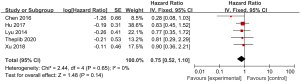
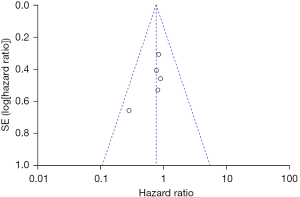
A total of 5 studies examining the correlation between ovarian retention and resection and OS in patients with stage IA–IIB cervical adenocarcinoma were included in our meta-analysis. The heterogeneity test showed that there was no heterogeneity among the 5 studies (χ2=2.44, P=0.65, I2=0%). Thus, the fixed-effects model was used for consolidation. No significant difference was found in the 5-year OS between the patients with stage IA–IIB cervical adenocarcinoma who retained their ovaries and the patients who underwent an ovariectomy (HR =0.75, 95% CI: 0.52–1.10, Z=1.48, P=0.14; see Figure 2). The funnel chart showed that the scatter points were distributed within the confidence interval, which was roughly symmetrical, and there was no publication bias (see Figure 3).
Comparison of PFS between the ovarian preservation and ovariectomy groups
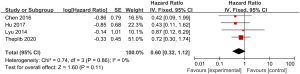
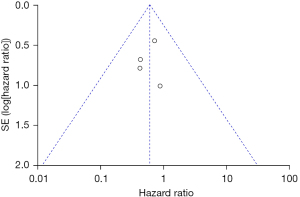
A total of 4 studies examining the correlation between ovarian retention and resection and PFS in patients with stage IA–IIB cervical adenocarcinoma were included in our meta-analysis. The heterogeneity test showed that there was no heterogeneity among the 4 studies (χ2=0.74, P=0.86, I2=0%). Thus, the fixed-effects model was used for consolidation. The analysis results revealed no significant difference in the 5-year PFS between ovarian-preserved and ovariectomized patients with stage IA–IIB cervical adenocarcinoma (HR =0.60, 95% CI: 0.32–1.12, Z=1.60, P=0.11; see Figure 4). The funnel diagram showed that the scatter points were distributed within the confidence interval, which was roughly symmetrical, and there was no publication bias (see Figure 5).
Comparison of DFS between the ovarian preservation and ovariectomy groups
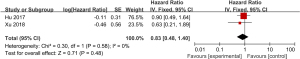
A total of 2 studies examining the correlation between ovarian retention and resection and DFS in patients with stage IA–IIB cervical adenocarcinoma were included in our meta-analysis. The heterogeneity test showed that there was no heterogeneity between the 2 studies (χ2=0.30, P=0.58, I2=0%). Thus, the fixed-effects model was used for consolidation. There was no significant difference in the 5-year DFS between the ovarian-preserved and ovariectomized patients with stage IA–IIB cervical adenocarcinoma (HR =0.83, 95% CI: 0.48–1.40, Z=0.71, P=0.48; see Figure 6).
Discussion
Our meta-analysis results revealed that, in the patients of cervical adenocarcinoma stage IA-IIB undergoing radical surgery, there is no difference in the OS, PFS, and DFS between ovarian preservation and ovariectomy. Ovarian preservation did not affect the survival time of patients. Our results are consistent with the findings of some of the studies we included in our analysis (20-22). In a retrospective paired study of 300 patients undergoing radical hysterectomy for cervical cancer (22), the 5-year survival rate was 98% in the ovarian preservation group and 97% in the ovariectomy group, and the difference between the 2 groups was not significant; however, the study did not distinguish between the pathological types of cervical cancer and was thus not included in the present study. Hu et al. (21) showed that among patients with stage IA–IIB cervical adenocarcinoma undergoing radical uterine and pelvic lymph node dissection, the 5-year OS rate of patients with bilateral fallopian tubes and ovaries was 88.6%. Conversely, patients with ovaries demonstrated a 100% 5-year survival rate, and no metastasis or recurrence was observed. Additionally, there was no significant difference between the 2 groups in that study. However, Hu et al. (21) suggested that ovarian preservation should be considered in the surgical treatment of patients with early premenopausal cervical adenocarcinoma, as ovariectomy may reduce patients’ quality of life. Lyu et al. (20) found no significant difference in the OS and DFS between patients in the ovarian preservation group and patients in the ovariectomy group.
Additionally, the preservation (or non-preservation) of the ovary does not affect the efficacy of chemotherapy. Theplib et al. (19) showed that for patients with stage IA–B1 adenocarcinoma undergoing radical surgery, there was no significant difference between ovarian preservation and ovariectomy in terms of OS and PFS, and ovarian preservation was safe. However, that study also stated that the 15-year OS of patients with ovarian preservation was better than that of patients with ovariectomy, which needs to be confirmed by further research. Xu et al. (18) showed that among patients with T1N0M0 cervical adenocarcinoma aged 45 years and below who received surgical treatment, the OS and DFS of the ovarian-preserved patients were better than those of the ovariectomized patients. These results differed from other research results and could not be further supported. Our analysis suggests that the study by Xu et al. (18) may be related to the limitations, such as the age and the tumor-node-metastasis (TNM) stage, of the subjects in this study.
Some studies have examined ovarian outcomes for ovarian-preserved patients with early cervical adenocarcinoma (21,23-25). In one study by Jiao et al., no ovarian metastasis was found in the short-term follow-up period of 23 months (23). Additionally, Hu et al. did not observe ovarian metastasis in patients with ovarian-preserved cervical adenocarcinoma with a follow-up time of 2 to 71 months (21). Zhou et al. (24) suggested that cervical adenocarcinoma was more prone to ovarian metastasis than squamous cell carcinoma, but that ovarian metastasis of cervical adenocarcinoma did not affect the prognosis of patients. Matsuo et al. (25) observed ovarian outcomes after ovarian preservation in 4,368 patients with early cervical cancer, and found that the incidence of ovarian metastasis after ovarian preservation was <1%, but that the incidence of ovarian metastasis increased threefold among patients with adenocarcinoma or adenosquamous cell carcinoma. Age and postoperative radiotherapy may be risk factors for postoperative ovarian metastasis. The incidence of ovarian metastasis in cervical adenocarcinoma after ovarian preservation is low. Other studies have explored the risk factors of ovarian metastasis after ovarian preservation in cervical adenocarcinoma. According to Zhou et al. (24), uterine body involvement, endometrial involvement, and vaginal infiltration are independently related to ovarian metastasis. Hu et al. (21) are of the view that the International Federation of Gynecology and Obstetrics (FIGO) stage is an independent risk factor for ovarian metastasis in patients with cervical adenocarcinoma.
In conclusion, ovarian preservation is safe in stage IA–IIB cervical adenocarcinoma patients. However, all of the regression studies included in this study are still in need of high-quality randomized controlled trials to confirm this conclusion.
Acknowledgments
Funding: This research was supported by a grant from the Shanghai Health System Talent Training Program (No. 2018YQ60 to J Nie).
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-310/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-310/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet 2019;393:169-82. [Crossref] [PubMed]
- Xie X, Song K, Cui B, et al. A comparison of the prognosis between adenocarcinoma and squamous cell carcinoma in stage IB-IIA cervical cancer. Int J Clin Oncol 2018;23:522-31. [Crossref] [PubMed]
- Eun TJ, Perkins RB. Screening for Cervical Cancer. Med Clin North Am 2020;104:1063-78. [Crossref] [PubMed]
- Ngo-Metzger Q, Adsul P. Screening for Cervical Cancer. Am Fam Physician 2019;99:253-4. [PubMed]
- Zhu HT, Xia R, Zhang X. Clinical characteristics and prognostic factors of cervical adenocarcinoma. Asian J Surg 2021;44:1283-5. [Crossref] [PubMed]
- Wang M, Yuan B, Zhou ZH, et al. Clinicopathological characteristics and prognostic factors of cervical adenocarcinoma. Sci Rep 2021;11:7506. [Crossref] [PubMed]
- Hill EK. Updates in Cervical Cancer Treatment. Clin Obstet Gynecol 2020;63:3-11. [Crossref] [PubMed]
- Liang BQ, Zhou SG, Liu JH, et al. Clinicopathologic features and outcome of cervical cancer: implications for treatment. Eur Rev Med Pharmacol Sci 2021;25:696-709. [PubMed]
- Hu J, Zheng PZ, Zhu LR. Comparison of clinical pathological characteristics in ovarian preserving patients with stage IB1 cervical adenocarcinoma and squamous cell carcinoma. Beijing Da Xue Xue Bao Yi Xue Ban 2016;48:783-7. [PubMed]
- Ashton KA, Scurry J, Tabrizi SN, et al. The problem of late ovarian metastases from primary cervical adenocarcinoma. Gynecol Oncol Rep 2015;13:23-5. [Crossref] [PubMed]
- Abu-Sinn D, Jamison J, Evans M, et al. Pelvic and Ovarian Recurrence of Small HPV-associated Cervical Adenocarcinoma With Transformation to Neuroendocrine Carcinoma. Int J Gynecol Pathol 2021;40:541-8. [Crossref] [PubMed]
- Matsuo K, Machida H, Shoupe D, et al. Ovarian Conservation and Overall Survival in Young Women With Early-Stage Cervical Cancer. Obstet Gynecol 2017;129:139-51. [Crossref] [PubMed]
- Jacoby VL. Hysterectomy controversies: ovarian and cervical preservation. Clin Obstet Gynecol 2014;57:95-105. [Crossref] [PubMed]
- Mandelbaum RS, Chen L, Shoupe D, et al. Patterns of utilization and outcome of ovarian conservation for young women with minimal-risk endometrial cancer. Gynecol Oncol 2019;154:45-52. [Crossref] [PubMed]
- Chen J, Wang R, Zhang B, et al. Safety of ovarian preservation in women with stage I and II cervical adenocarcinoma: a retrospective study and meta-analysis. Am J Obstet Gynecol 2016;215:460.e1-13. [Crossref] [PubMed]
- Fan Y, Wang MY, Mu Y, et al. Ovarian metastasis in women with cervical carcinoma in stages IA to IIB: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e21146. [Crossref] [PubMed]
- Al-Kalbani M, McVeigh G, Nagar H, et al. Do FIGO stage IA and small (≤2 cm) IB1 cervical adenocarcinomas have a good prognosis and warrant less radical surgery? Int J Gynecol Cancer 2012;22:291-5. [Crossref] [PubMed]
- Xu HY, Tang X, Ding J, et al. Ovarian conservation is associated with better survival in young patients with T1N0M0 cervical adenocarcinoma: a population-based study. Arch Gynecol Obstet 2018;297:775-84. [Crossref] [PubMed]
- Theplib A, Hanprasertpong J, Leetanaporn K. Safety and Prognostic Impacts of Ovarian Preservation during Radical Hysterectomy for Early-Stage Adenocarcinoma and Adenosquamous Cervical Cancer. Biomed Res Int 2020;2020:5791381. [Crossref] [PubMed]
- Lyu J, Sun T, Tan X. Ovarian preservation in young patients with stage I cervical adenocarcinoma: a surveillance, epidemiology, and end results study. Int J Gynecol Cancer 2014;24:1513-20. [Crossref] [PubMed]
- Hu J, Jiao X, Yang Z, et al. Should ovaries be removed or not in early-stage cervical adenocarcinoma: a multicenter retrospective study of 105 patients. J Obstet Gynaecol 2017;37:1065-9. [Crossref] [PubMed]
- Windbichler GH, Müller-Holzner E, Nicolussi-Leck G, et al. Ovarian preservation in the surgical treatment of cervical carcinoma. Am J Obstet Gynecol 1999;180:963-9. [Crossref] [PubMed]
- Jiao XB, Hu J, Zhu LR. The Safety of Ovarian Preservation in Early-Stage Adenocarcinoma Compared With Squamous Cell Carcinoma of Uterine Cervix: A Systematic Review and Meta-Analysis of Observational Studies. Int J Gynecol Cancer 2016;26:1510-4. [Crossref] [PubMed]
- Zhou J, Chen Y, Zhang P, et al. Ovarian preservation in adenocarcinoma of the uterine cervix. J Ovarian Res 2017;10:48. [Crossref] [PubMed]
- Matsuo K, Machida H, Horowitz MP, et al. Risk of metachronous ovarian cancer after ovarian conservation in young women with stage I cervical cancer. Am J Obstet Gynecol 2017;217:580.e1-10. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



