
Systematic review and meta-analysis: diagnostic value of different ultrasound for benign and malignant thyroid nodules
Introduction
The thyroid is often included in routine clinical health examinations, and the detection rate of thyroid diseases is increasing each year, with thyroid nodules being the most prevalent thyroid diseases. In addition, there is an increasing incidence of thyroid nodules in younger patients. Thyroid disease is 8 times more common in women compared to men (1-3). Thyroid lesions are mostly benign, presenting as nodular goiter and thyroid tumors. However, a small number of thyroid nodules are malignant lesions, with the majority being thyroid cancers. In adults, thyroid nodules occur about 45–56% of the time, of which 5.0–6.5% are thyroid cancers. Thyroid carcinoma can be classified into four types according to pathological features, namely, follicular carcinoma, papillary carcinoma, medullary carcinoma, and undifferentiated carcinoma (4-7). Thyroid nodules are usually insidious, with no obvious clinical symptoms and can be easily missed. Clinical scientific examination is particularly important for effective diagnosis. In addition, there are significant differences in surgical methods, resection scope, and postoperative management between benign and malignant thyroid nodules. Accurate preoperative assessment of benign and malignant nodules can provide accurate guidance for treatment. Therefore, in clinical practice, the differential diagnosis of benign and malignant thyroid nodules has become a widespread concern (8,9).
Clinically, there are a variety of methods to distinguish between benign and malignant thyroid nodules, including ultrasound, computer scanning technology, and nuclide scanning. Conventional ultrasound examination has many advantages, such as safety, low costs, simple to use, repeatable, and no radioactivity, and thus, has been widely used in clinical practice (10-12). Conventional ultrasound images of thyroid malignant nodules are characterized by irregular shape, unclear edge, non-uniformity, microcalcification, low echo, and aspect ratio greater than 1 (13). However, conventional ultrasound has great limitations in the diagnosis of clinically complicated benign and malignant thyroid nodules. For example, it is often difficult to determine small thyroid cancers, multiple nodules, and benign and malignant nodules using conventional ultrasound images. When thyroid glial cysts are accompanied by intracystic bleeding, or if benign nodules calcify or automate hematoma, or when thyroid cystic solid nodules develop into “zombie nodules”, conventional ultrasound images often cannot correctly diagnose the condition. In addition, ultrasound features of some thyroid nodules are not obvious. In such cases, conventional ultrasound techniques do not meet clinical needs (14-16).
Contrast-enhanced ultrasound (CEUS) is a new ultrasonic diagnostic technology, which has a great application prospect in the diagnosis of benign and malignant thyroid nodules (17,18). Unlike conventional ultrasound, CEUS can reveal not only morphological changes, but also functional features of the thyroid (19). CEUS makes use of the gas-liquid interface generated by contrast agent microbubbles to enhance doppler signals to fully display the specific characteristics of tumor blood vessels. Furthermore, the distribution of the contrast agent can be observed in various thyroid lesions in real time (20,21). Among all organs in the human body, the thyroid is one of the endocrine organs with abundant blood perfusion. When patients undergo CEUS examination, the normal thyroid gland parenchyma shows a uniform enhancement pattern (22). When thyroid nodules appear, the difference between the CEUS mode and normal thyroid parenchyma is enhanced. During the growth and formation of benign and malignant thyroid nodules, abundant new blood vessels are formed, which can be distinguished from the blood perfusion pattern of normal thyroid tissues. In addition, compared with benign thyroid nodules, the degree of differentiation of newly formed vascular endothelial cells in malignant nodules is lower, the connections between cells are not as tight, and the terminals of the blood vessels are open. Therefore, CEUS can be used to differentiate between benign and malignant thyroid nodules according to their various pathological features (23-25). However, some studies pointed out that partial volume effect is enhanced due to the occurrence of small nodules in CEUS diagnosis of thyroid nodules. The false-negative diagnosis was caused by the absence of prominent neovascularization in micropapillary carcinoma and the degeneration that may reduce the vascular density of nodules resulting in the loss of contrast media (26,27). At the same time, some malignant nodules (medullary carcinoma and lymphoma) have equal or high enhancement and are often confused with benign nodules. Inflammatory nodules cause damage to surrounding tissues and blood vessels, resulting in low enhancement of nodular fibrosis. Nodules accompanied by Hashimoto are mainly fibrotic, with massive destruction of blood vessels and low enhancement of CEUS. The influence of the above factors will lead to false positive results of thyroid nodules diagnosed by EUS. Therefore, at present, the value of CEUS in the diagnosis of benign and malignant thyroid nodules is still controversial, and whether its diagnostic value is superior to conventional ultrasound needs to be verified by many samples.
A meta-analysis was performed on the published literature examining the use of conventional ultrasound and CEUS for the diagnosis of thyroid nodules. The diagnosis and prediction ability of the two ultrasonic diagnosis methods for thyroid malignant nodules was systematically evaluated and compared. We present the following article in accordance with the PRISMA-DTA reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-254/rc).
Methods
Literature searching
Relevant literatures published in PubMed, MEDLINE, EBSCO, Science Direct, Cochrane Library, and CNKI were identified using the following keywords: “contrast enhanced ultrasound”, “conventional ultrasound”, “diagnosis”, “thyroid nodules”, “benign”, and “malignant”. Relevant literatures published between January 2000 and January 2022 were manually searched in professional journals to avoid omissions, and the literatures were searched for human subjects.
In the retrieval process, the combination of subject words and free words was used several times to obtain references that can be included. The search engines were then used to track down each document. The quality of the included literature was assessed using RevMan 5.3 provided by the Cochrane Collaboration.
Literature inclusion and exclusion criteria
The following inclusion criteria were applied: (I) ambiguous diagnostic study on benign and malignant thyroid nodules; (II) histopathological diagnosis by surgery or acupuncture was used as the gold standard; (III) patients were adults older than 18 years; (IV) the true positive, false positive, false negative, and true negative values of diagnosis could be obtained directly or indirectly in the study; (V) the sample size in the study was more than 30 cases.
The following exclusion criteria were applied: (I) case reports, reviews, meetings, letters, etc.; (II) histopathological features were not used as the gold standard in the study; (III) literatures with less than 30 subjects; and (IV) literatures with insufficient data to determine the diagnostic indicators.
Data extraction
Two professionals used a unified Microsoft Excel (Microsoft, USA) spreadsheet to independently conduct literature screening and data extraction. The results were cross-checked and any disagreements were resolved through discussion. The extracted data included the following: (I) the general information of included studies such as title, first author, and publication year; (II) the basic characteristics of the research study, such as sample size and detection method; (III) the test results of the diagnostic indicators; and (IV) the detection rate of thyroid malignant nodules in each study and the data (sensitivity and specificity) used to determine the accuracy of the test.
Literature evaluation criteria
The Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS) was adopted to evaluate the included studies. For each included literature, the quality evaluation was carried out and rated as “yes”, “no”, or “unclear”. Meeting this criterion was “yes”, not meeting or not mentioned was “no”, failure to acquire information from the literature was “unclear”. The studies were rated based on their risk of bias and applicability to the research questions. Applicability and bias risk were considered “low”, “high”, or “unclear”. If there was a dispute, it should be resolved through discussion.
Statistical analysis
RevMan 5.3 (Cochrane, USA) and Stata Corp (Stata Corp, USA) were used for statistical analyses. The risk bias of the included references was assessed using the bias risk assessment map. The sensitivity and specificity of different ultrasonic diagnosis methods were analyzed, and the sensitivity and specificity were quantitatively combined to draw the combined summary receiver operating characteristic (SROC) curve. The sensitivity and specificity of conventional ultrasound and the CEUS detection methods were calculated and compared and expressed with a 95% confidence interval (CI). Heterogeneity was assessed across studies using the Q test and heterogeneity test (I2). If heterogeneity existed, the random-effects model was used for meta-analysis. If heterogeneity did not exist, the fixed-effects model was used for meta-analysis. Deek’s method was used to evaluate publication bias. Funnel plots of different diagnostic indicators were used to test potential publication bias, and sensitivity analysis was performed. The forest map was output by the software, which extracted 95% CI and P of the results to judge the results of meta-analysis. P<0.05 was considered statistically significant.
Results
Search results and basic information of the documents
A total of 178 articles were obtained through database retrieval (n=134) and manual journal retrieval (n=44). There were 2 repeated papers, 43 unqualified papers, and 8 papers which were excluded for other reasons. From the remaining 81 papers, a reading of the abstracts and titles resulted in the elimination of 12 articles. A further 43 research reports and review articles were eliminated, resulting in 26 articles from databases and 44 articles from manual journal retrieval remaining. All the remaining papers were read in full text, and 12 literatures from database retrieval and 5 articles from manual journal retrieval with incorrect research types were excluded. Another 3 articles from database retrieval and 3 articles from manual journal retrieval were excluded as the required diagnostic results were incomplete or unavailable. The research object was not thyroid nodules in 2 articles from database retrieval and 1 article from manual journal retrieval were also excluded. Finally, 11 articles (28-38) were included in this meta-analysis. Figure 1 shows a flow chart of literature retrieval process.
The basic information of the literatures was extracted by reading the contents of the publications. In the 11 included literatures, there were 1,378 thyroid nodules in total, including 535 malignant thyroid nodules and 843 benign thyroid nodules. The sample sizes of the 11 included studies ranged from 77 to 291. The 11 articles described in detail the diagnostic indicators of thyroid malignant nodules by conventional ultrasound and CEUS, with the histopathological diagnosis obtained by surgery or acupuncture as the gold standard. The quality evaluation of the 11 included papers showed that 8 articles were grade A (72.72%), 1 was grade B (9.10%), and 2 were grade C (18.18%). Table 1 shows the basic characteristics of the included literatures. The risk bias evaluation chart of references was drawn using RevMan 5.3 (Figure 2). Figure 3 is a summary of risk bias in the references.
Table 1
| First author | Publication year | Number of nodules | Number of malignant nodules | Number of benign nodules | Diagnostic method |
|---|---|---|---|---|---|
| Deng (28) | 2014 | 175 | 56 | 119 | CEUS |
| Jin (29) | 2017 | 74 | 34 | 41 | CEUS |
| Li (30) | 2015 | 80 | 50 | 30 | CEUS |
| Liu (31) | 2019 | 131 | 72 | 59 | Conventional ultrasound |
| Li (32) | 2015 | 103 | 53 | 50 | Conventional ultrasound |
| Shuzhen (33) | 2012 | 291 | 66 | 225 | Conventional ultrasound |
| Wang (34) | 2019 | 102 | 27 | 75 | Conventional ultrasound |
| Wu (35) | 2016 | 96 | 4 | 92 | CEUS |
| Zhang (36) | 2010 | 104 | 51 | 53 | CEUS |
| Zhao (37) | 2019 | 117 | 57 | 60 | CEUS |
| Zhao (38) | 2015 | 102 | 63 | 39 | Conventional ultrasound |
CEUS, contrast-enhanced ultrasound.
Heterogeneity evaluation
The heterogeneity of the two diagnostic methods in the included literature was evaluated. The heterogeneity results of CEUS diagnosis showed that there was no heterogeneity in sensitivity and heterogeneity in specificity among the studies (I2=0, 64.96%). The heterogeneity of conventional ultrasound diagnosis showed that there was heterogeneity in sensitivity and specificity among studies (I2=70.64, 76.39%). There was high heterogeneity between the data of the two examination methods, so the random effects model was used for analysis and constructing the SROC curve.
Meta-analysis of CEUS diagnosis
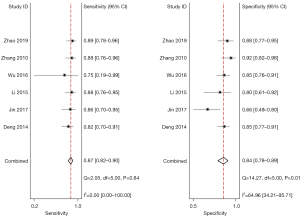
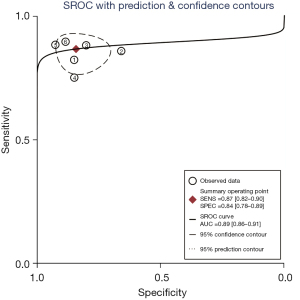
A total of 6 included articles (28-30,35-37) analyzed the diagnostic results of CEUS. Figure 4 is a forest plot of the sensitivity and specificity of the individual CEUS studies and the aggregate of the studies. The diagnostic sensitivity of CEUS was tested for heterogeneity and no heterogeneity was detected among the study groups [Q=2.05, degree of freedom (df) =5.00, I2=0.00%, P=0.84]. The combined sensitivity was 0.87, 95% CI: 0.82 to 0.90. The lowest sensitivity was 0.75, 95% CI: 0.19 to 0.99, and the highest sensitivity was 0.89, 95% CI: 0.78 to 0.96. Heterogeneity testing on the diagnostic specificity of CEUS of the 6 included literatures indicated that there was heterogeneity among the different study groups (Q=14.27, df =5.00, I2=64.96%, P=0.01). The combined specificity was 0.84, 95% CI: 0.78 to 0.89. The lowest specificity was 0.66, 95% CI: 0.49 to 0.80, and the highest specificity was 0.92, 95% CI: 0.82 to 0.98. Figure 5 shows the SROC curve of CEUS. The closer the SROC curve is to the upper left corner of the image, the greater the area under the SROC curve and the higher the diagnostic accuracy. The CEUS diagnosis results demonstrated that the proportion of false negatives and false positives was low, and the diagnostic accuracy was relatively high.
Meta-analysis of conventional ultrasound diagnosis
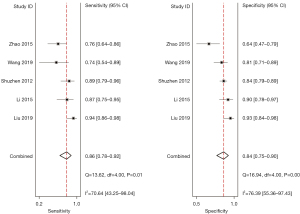
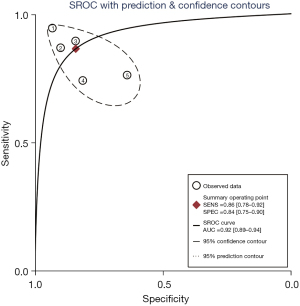
A total of 5 included articles (31-34,38) analyzed the diagnostic results of conventional ultrasound. Figure 6 shows a forest plot of the sensitivity and specificity of individual and aggregate studies of conventional ultrasound. The heterogeneity test on the sensitivity of 5 conventional ultrasound diagnosis articles showed that there was heterogeneity among different study groups (Q=13.62, df =4.00, I2=70.64%, P=0.01). The combined sensitivity was 0.86, 95% CI: 0.78 to 0.92. The lowest sensitivity was 0.74, 95% CI: 0.54 to 0.89, and the highest sensitivity was 0.94, 95% CI: 0.86 to 0.98. Heterogeneity testing for the specificity of conventional ultrasound diagnosis in the 5 included literatures indicated that there was heterogeneity among different study groups (Q=16.94, df =4.00, I2=76.39%, P=0.00). The combined specificity was 0.84, 95% CI: 0.75 to 0.90. The lowest specificity was 0.64, 95% CI: 0.47 to 0.79, and the highest specificity was 0.93, 95% CI: 0.84 to 0.98. Figure 7 shows the SROC curve of conventional ultrasound. The closer the SROC curve is to the upper left corner of the image, the greater the area under the SROC curve and the higher the diagnostic accuracy. The conventional ultrasonic diagnosis results showed that the proportion of false negative and false positive was low, and the diagnostic accuracy was relatively high.
Sensitivity analysis
Sensitivity analysis was conducted by changing the analysis model. Meta-analysis results showed that there was no significant change in the summary results of different analysis models, indicating that the included literatures had good stability.
Discussion
In recent years, the incidence of thyroid nodules has shown an increasing trend, with the age of onset being younger. The incidence of thyroid nodules is about 45–56%, of which about 5.0–6.5% are malignant thyroid nodules. Thyroid cancer can be classified into four types according to pathological features, namely, follicular carcinoma, papillary carcinoma, medullary carcinoma, and undifferentiated carcinoma (39). For benign and malignant thyroid nodules, there are significant differences in surgical methods, resection scope, and postoperative management during treatment. Accurate preoperative assessment of benign and malignant thyroid nodules can provide accurate guidance for treatment. Therefore, it is very important to use accurate and effective diagnostic methods for the follow-up treatment of thyroid nodules.
Conventional ultrasound, as the most basic examination method, has many advantages. In the Guidelines for the Diagnosis and Treatment of Thyroid Nodules and differentiated Thyroid Cancer, it is recommended that all patients with thyroid nodules be diagnosed by routine ultrasound. Conventional ultrasound images of thyroid malignant nodules are characterized by irregular shape, unclear edge, non-uniformity, microcalcification, low echo, aspect ratio greater than 1, etc. This diagnostic method has been widely used in clinical practice. However, for multiple nodules and benign and malignant nodules, conventional ultrasound examination has certain limitations, often resulting in missed diagnosis and misdiagnosis (40-42). CEUS, as a new ultrasonic diagnosis technology, has gradually become more popular. The degree of differentiation of newly formed vascular endothelial cells in malignant nodules is generally low. Furthermore, the connection between cells is not tight, which is manifested by the open end of blood vessels and other features. CEUS can detect these pathologies for diagnosis. However, to date, there is no unified evaluation standard for CEUS. It has been reported that this new ultrasonic diagnostic method has high sensitivity and specificity. Therefore, the current published literatures regarding conventional ultrasound and CEUS diagnosis of thyroid nodules were included in this meta-analysis. The diagnosis and prediction ability of these two ultrasonic diagnostic methods for thyroid malignant nodules were systematically evaluated and compared through meta-analysis.
A total of 11 literatures were included, including 5 conventional ultrasound studies. The diagnostic sensitivity of conventional ultrasound was tested for heterogeneity and the results indicated that there was heterogeneity among different study groups. The combined sensitivity was 0.86, 95% CI: 0.78 to 0.92. The lowest sensitivity was 0.74, 95% CI: 0.54 to 0.89, and the highest sensitivity was 0.94, 95% CI: 0.86 to 0.98. Heterogeneity tests for the specificity of conventional ultrasound diagnosis in the 5 included literatures showed that there was heterogeneity among the different study groups. The combined specificity was 0.84, 95% CI: 0.75 to 0.90. The lowest specificity was 0.64, 95% CI: 0.47 to 0.79, and the highest specificity was 0.93, 95% CI: 0.84 to 0.98. The heterogeneity tests for diagnostic sensitivity of CEUS revealed that there was no heterogeneity among the study groups. The combined sensitivity was 0.87, 95% CI: 0.82 to 0.90. The lowest sensitivity was 0.75, 95% CI: 0.19 to 0.99, and the highest sensitivity was 0.89, 95% CI: 0.78 to 0.96. Heterogeneity tests on the diagnostic specificity of CEUS of the 6 included literatures indicated that there was heterogeneity among different study groups. The combined specificity was 0.84, 95% CI: 0.78 to 0.89. The lowest specificity was 0.66, 95% CI: 0.49 to 0.80, and the highest specificity was 0.92, 95% CI: 0.82, to 0.98. Comparison of the specificity and sensitivity of the two types of thyroid nodule ultrasound revealed that the combined specificity of CEUS studies was similar to that of conventional ultrasound, while its sensitivity was slightly higher than that of conventional ultrasound. In addition, the area under the SROC curve reflected the diagnostic value of the method. The greater the area under the curve, the higher the diagnostic value. In this study, the area under the curve of the two-thyroid ultrasound diagnostic methods was greater than 0.7, indicating that both had high diagnostic value. The area under the curve of conventional ultrasound was more than 0.9, indicating that it had very high diagnostic value. This was not consistent with the study by Radzina et al. (2021) (43), and the reasons for this may be as follows: there were few cases of multi-source nodules in the included studies; and thyroid nodules were typical.
In summary, the ability of conventional ultrasound and CEUS to diagnose benign and malignant thyroid nodules was evaluated in this meta-analysis, providing evidence-based recommendations for clinical practice guidelines. In clinical studies, conventional ultrasound is utilized as the basis of diagnosis, and CEUS is performed to accurately evaluate complex situations, providing a more accurate reference for subsequent treatments.
Conclusions
In this meta-analysis, literatures related to the diagnosis of benign and malignant thyroid nodules by conventional ultrasound and CEUS were evaluated with the aim of determining the accuracy of different ultrasound methods in the diagnosis of benign and malignant thyroid nodules. The meta-analysis results confirmed that the combined specificity of CEUS studies was similar to that of conventional ultrasound, while its sensitivity was slightly higher than that of conventional ultrasound. However, the potential of CEUS in the differential diagnosis of benign and malignant thyroid gland requires the development of a unified diagnostic criteria. At the same time, more samples and higher quality studies are needed for further in-depth investigation to provide more accurate and effective basis for clinical practice.
Acknowledgments
Funding: The study was supported by Sichuan Provincial Science and Technology Plan Project (No. 2021YJ0204).
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-254/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-254/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rehman HAU, Lin CY, Su SF. Deep Learning Based Fast Screening Approach on Ultrasound Images for Thyroid Nodules Diagnosis. Diagnostics (Basel) 2021;11:2209. [Crossref] [PubMed]
- Reverter JL, Ferrer-Estopiñan L, Vázquez F, et al. Reliability of a computer-aided system in the evaluation of indeterminate ultrasound images of thyroid nodules. Eur Thyroid J 2022;11:210023. [Crossref] [PubMed]
- Sun J, Li C, Lu Z, et al. TNSNet: Thyroid nodule segmentation in ultrasound imaging using soft shape supervision. Comput Methods Programs Biomed 2022;215:106600. [Crossref] [PubMed]
- Agyekum EA, Fu JH, Xu FJ, et al. Ultrasound-Guided Thermal Ablation of Thyroid Nodules: Technicalities Progress and Clinical Applications, Especially in Malignant Thyroid Nodules. Front Oncol 2021;11:761005. [Crossref] [PubMed]
- Alshahrani AS, Alamri AS, Balkhoyor AH, et al. The Prediction of Malignancy Risk in Thyroid Nodules Classified as Bethesda System Category III (AUS/FLUS) and the Role of Ultrasound Finding for Prediction of Malignancy Risk. Cureus 2021;13:e17924. [Crossref] [PubMed]
- Boers T, Braak SJ, Versluis M, et al. Matrix 3D ultrasound-assisted thyroid nodule volume estimation and radiofrequency ablation: a phantom study. Eur Radiol Exp 2021;5:31. [Crossref] [PubMed]
- Cancela E, Penna G, Costa CT, Pires MC, et al. Are the anatomical, clinical, and ultrasound characteristics of thyroid nodules with Bethesda III or IV cytology and ACR TI-RADS 3, 4, or 5 able to refine the indications for molecular diagnostic tests? Arch Endocrinol Metab 2021;65:625-31. [PubMed]
- Cao H, Fan Q, Zhuo S, et al. The Value of Chinese Thyroid Imaging Report and Data System Combined With Contrast-Enhanced Ultrasound Scoring in Differential Diagnosis of Benign and Malignant Thyroid Nodules. J Ultrasound Med 2022;47:1753-61. [Crossref] [PubMed]
- Ghai S, O’Brien C, Goldstein DP, et al. Ultrasound in active surveillance for low-risk papillary thyroid cancer: imaging considerations in case selection and disease surveillance. Insights Imaging 2021;12:130. [Crossref] [PubMed]
- Gild ML, Chan M, Gajera J, et al. Risk stratification of indeterminate thyroid nodules using ultrasound and machine learning algorithms. Clin Endocrinol (Oxf) 2022;96:646-52. [Crossref] [PubMed]
- Hou J, Li M, Peng X, et al. The effect of Hashimoto’s thyroiditis on the diagnostic efficacy of ultrasound-guided fine needle aspiration cytology for thyroid nodules ≥ 1 cm. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2021;35:807-12. [PubMed]
- Jiang S, Xie Q, Li N, et al. Modified Models for Predicting Malignancy Using Ultrasound Characters Have High Accuracy in Thyroid Nodules With Small Size. Front Mol Biosci 2021;8:752417. [Crossref] [PubMed]
- Kang S, Lee E, Chung CW, et al. A beneficial role of computer-aided diagnosis system for less experienced physicians in the diagnosis of thyroid nodule on ultrasound. Sci Rep 2021;11:20448. [Crossref] [PubMed]
- Li L, Chen X, Li P, et al. The Value of Ultrasound-Guided Fine-Needle Aspiration Cytology Combined with Puncture Feeling in the Diagnosis of Thyroid Nodules. Acta Cytol 2021;65:368-76. [Crossref] [PubMed]
- Liwen L, Xinguang Q. Safety and efficacy of ultrasound-guided radiofrequency ablation for benign nonfunctional thyroid nodules in children: a retrospective study of 62 patients with over 4 years of follow-up. Thyroid 2022; Epub ahead of print. [Crossref]
- Maddaloni E, Briganti SI, Crescenzi A, et al. Usefulness of Color Doppler Ultrasonography in the Risk Stratification of Thyroid Nodules. Eur Thyroid J 2021;10:339-44. [Crossref] [PubMed]
- Mao S, Zhao LP, Li XH, et al. The diagnostic performance of 2020 Chinese Ultrasound Thyroid Imaging Reporting and Data System in thyroid nodules. Zhonghua Yi Xue Za Zhi 2021;101:3748-53. [PubMed]
- Piccardo A, Fiz F, Bottoni G, et al. Facing Thyroid Nodules in Paediatric Patients Previously Treated with Radiotherapy for Non-Thyroidal Cancers: Are Adult Ultrasound Risk Stratification Systems Reliable? Cancers (Basel) 2021;13:4692. [Crossref] [PubMed]
- Saade-Lemus SM, Sridharan A, Smitthimedhin A, et al. Advanced Ultrasound Techniques for Differentiation of Benign Versus Malignant Thyroid Nodules: A Review. Ultrasound Q 2021;37:315-23. [Crossref] [PubMed]
- Scappaticcio L, Maiorino MI, Iorio S, et al. Exploring the Performance of Ultrasound Risk Stratification Systems in Thyroid Nodules of Pediatric Patients. Cancers (Basel) 2021;13:5304. [Crossref] [PubMed]
- Seifert P, Schenke S, Zimny M, et al. Diagnostic Performance of Kwak, EU, ACR, and Korean TIRADS as Well as ATA Guidelines for the Ultrasound Risk Stratification of Non-Autonomously Functioning Thyroid Nodules in a Region with Long History of Iodine Deficiency: A German Multicenter Trial. Cancers (Basel) 2021;13:4467. [Crossref] [PubMed]
- Sorrenti S, Dolcetti V, Fresilli D, et al. The Role of CEUS in the Evaluation of Thyroid Cancer: From Diagnosis to Local Staging. J Clin Med 2021;10:4559. [Crossref] [PubMed]
- Thedinger W, Raman E, Dhingra JK. Comparative Study of ACR TI-RADS and ATA 2015 for Ultrasound Risk Stratification of Thyroid Nodules. Otolaryngol Head Neck Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Trimboli P, Castellana M, Virili C, et al. Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: a systematic review and meta-analysis using histological standard of reference. Radiol Med 2020;125:406-15. [Crossref] [PubMed]
- Wan Q, Cao P, Liu J. Meta-Analysis of Contrast Enhanced Ultrasound in Judging Benign and Malignant Thyroid Tumors. Comput Math Methods Med 2021;2021:2577113. [Crossref] [PubMed]
- Zhang Y, Zhang X, Li J, et al. Contrast-enhanced ultrasound: a valuable modality for extracapsular extension assessment in papillary thyroid cancer. Eur Radiol 2021;31:4568-75. [Crossref] [PubMed]
- Zhan J, Ding H. Application of contrast-enhanced ultrasound for evaluation of thyroid nodules. Ultrasonography 2018;37:288-97. [Crossref] [PubMed]
- Deng J, Zhou P, Tian SM, et al. Comparison of diagnostic efficacy of contrast-enhanced ultrasound, acoustic radiation force impulse imaging, and their combined use in differentiating focal solid thyroid nodules. PLoS One 2014;9:e90674. [Crossref] [PubMed]
- Jin L, Xu C, Xie X, et al. An Algorithm of Image Heterogeneity with Contrast-Enhanced Ultrasound in Differential Diagnosis of Solid Thyroid Nodules. Ultrasound Med Biol 2017;43:104-10. [Crossref] [PubMed]
- Li F, Zhang J, Wang Y, et al. Clinical value of elasticity imaging and contrast-enhanced ultrasound in the diagnosis of papillary thyroid microcarcinoma. Oncol Lett 2015;10:1371-7. [Crossref] [PubMed]
- Liu C, Xie L, Kong W, et al. Prediction of suspicious thyroid nodule using artificial neural network based on radiofrequency ultrasound and conventional ultrasound: A preliminary study. Ultrasonics 2019;99:105951. [Crossref] [PubMed]
- Li WB, Zhang B, Zhu QL, et al. Comparison between Thin-Slice 3-D Volumetric Ultrasound and Conventional Ultrasound in the Differentiation of Benign and Malignant Thyroid Lesions. Ultrasound Med Biol 2015;41:3096-101. [Crossref] [PubMed]
- Shuzhen C. Comparison analysis between conventional ultrasonography and ultrasound elastography of thyroid nodules. Eur J Radiol 2012;81:1806-11. [Crossref] [PubMed]
- Wang J, Wei W, Guo R. Ultrasonic elastography and conventional ultrasound in the diagnosis of thyroid micro-nodules. Pak J Med Sci 2019;35:1526-31. [Crossref] [PubMed]
- Wu Q, Li Y, Wang Y. Diagnostic value of "absent" pattern in contrast-enhanced ultrasound for the differentiation of thyroid nodules. Clin Hemorheol Microcirc 2016;63:325-34. [Crossref] [PubMed]
- Zhang B, Jiang YX, Liu JB, et al. Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid 2010;20:51-7. [Crossref] [PubMed]
- Zhao H, Liu X, Lei B, et al. Diagnostic performance of thyroid imaging reporting and data system (TI-RADS) alone and in combination with contrast-enhanced ultrasonography for the characterization of thyroid nodules. Clin Hemorheol Microcirc 2019;72:95-106. [Crossref] [PubMed]
- Zhao RN, Zhang B, Yang X, et al. Logistic Regression Analysis of Contrast-Enhanced Ultrasound and Conventional Ultrasound Characteristics of Sub-centimeter Thyroid Nodules. Ultrasound Med Biol 2015;41:3102-8. [Crossref] [PubMed]
- Xia B, Yu B, Wang X, et al. Conspicuousness and recurrence related factors of ultrasound-guided microwave ablation in the treatment of benign thyroid nodules. BMC Surg 2021;21:317. [Crossref] [PubMed]
- Yan L, Li X, Xiao J, et al. Contrast-enhanced ultrasound is a reliable and reproducible assessment of necrotic ablated volume after radiofrequency ablation for benign thyroid nodules: a retrospective study. Int J Hyperthermia 2022;39:40-7. [Crossref] [PubMed]
- Yeste Fernández D, Vega Amenabar E, Coma Muñoz A, et al. Ultrasound criteria (EU-TIRADS) to identify thyroid nodule malignancy risk in adolescents. Correlation with cyto-histological findings. Endocrinol Diabetes Nutr (Engl Ed) 2021;68:728-34. [PubMed]
- Zhang J, Fu WX, Li WP, et al. Diagnostic value of thyroid micronodules with high b-value diffusion weighted imaging: Comparative study with high-resolution ultrasound. Eur J Radiol 2021;143:109912. [Crossref] [PubMed]
- Radzina M, Ratniece M, Putrins DS, et al. Performance of Contrast-Enhanced Ultrasound in Thyroid Nodules: Review of Current State and Future Perspectives. Cancers (Basel) 2021;13:5469. [Crossref] [PubMed]
(English Language Editor: J. Teoh)





