
Hypercalcemic crisis caused by primary hyperparathyroidism in a 11-year-old boy: a rare case report and review of the literature
Introduction
Primary hyperparathyroidism (PHPT) is a common endocrine disorder among adults (1). However, PHPT in pediatric patients is rare, with an estimated incidence of 1 case per 300,000 pediatric patients (2). To date, only approximately 200 cases of PHPT among pediatric patients and adolescents have been reported worldwide (3).
One of the unique characteristics of PHPT in pediatric patients is that affected patients tend to have symptoms of PHPT before the diagnosis of hypercalcemia (2), which is in contrast to the dominance of asymptomatic PHPT in adults. This PHPT feature in adults is considered to attribute to the increased frequency of serum calcium level checks in a health screening (4). Meanwhile, delayed diagnosis of PHPT in pediatric patients until the patient becomes symptomatic, could be partly because blood examination is not routinely performed in health screening for school-age children. Furthermore, in general, the symptoms caused by hypercalcemia may not be specific, such as abdominal pain, nausea, or fatigue. Due to the rarity of PHPT in pediatric patients and these non-specific symptoms, making an early diagnosis of PHPT may be difficult in pediatric practice.
Hypercalcemic crisis is defined as an albumin-corrected serum calcium level of >14 mg/dL associated with multiorgan dysfunction and severe symptoms, including coma, mental alteration, anorexia, and nausea (5). Hypercalcemic crisis is a serious complication of PHPT. Delayed diagnosis of PHPT sometimes causes this endocrinology emergency that may even result in fatal consequences if not adequately treated (6). However, because PHPT is likely to be symptomatic in pediatric patients, even if the symptoms are vague, it is usually diagnosed before hypercalcemic crisis is developed. Indeed, there are only six reports of PHPT-induced hypercalcemic crisis in pediatric patients (7-11). Thus, an appropriate treatment approach for this condition has not been demonstrated. In pediatric patients with PHPT, there is the possibility of hereditary diseases that affect the choice of surgical procedure (total parathyroidectomy and auto-transplantation or focused parathyroidectomy). Hence, it would be reasonable that genetic testing is performed prior to surgery. However, in case of a hypercalcemic crisis, we may not have enough time to wait for the results of genetic testing. Therefore, emergency focused parathyroidectomy without the results of genetic testing may be allowed for pediatric patients with a PHPT-induced hypercalcemic crisis.
Here, we report a case of a hypercalcemic crisis caused by PHPT in an 11-year-old boy, review the literature, and discuss the treatment strategy for PHPT-induced hypercalcemic crisis in pediatric patients. We present the following case in accordance with the CARE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-92/rc).
Case presentation
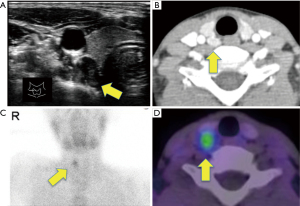
An 11-year-old boy with no history of serious disease or family history of endocrine disorder had abdominal pain and nausea for 3 months for which he visited a clinic. Physical examination and blood tests were performed. As serum calcium was not tested, hypercalcemia was not noticed. Subsequently, symptomatic treatment was initiated. However, his symptoms worsened. Then, the boy was referred to a nearby hospital for further examination. A blood chemistry test showed a markedly raised albumin-corrected serum calcium level (14.3 mg/dL) (normal range, 8.7–10.2 mg/dL) and an increased intact-parathyroid hormone (PTH) level (405 pg/mL) (normal range, 10.0–65.0 pg/mL). Neck ultrasonography revealed a well-defined mass of 15×9 mm in size on the dorsal side of the right lobe of the thyroid gland, which was considered an enlarged right superior parathyroid gland (Figure 1A). Contrast enhanced computed tomography also demonstrated an enlarged upper right parathyroid gland (Figure 1B), which was thought to be an enlarged right superior parathyroid gland. Renal stones were not detected. Tc-99m sestamibi scintigraphy showed an accumulation consistent with this mass, with no other accumulation suggestive of the parathyroid was detected (Figure 1C,1D). His lumber bone mineral density was 0.682 g/cm2, which was relatively low for his age (normal range for a 11-year-old Japanese boy: 0.694–0.706 g/cm2). Based on these findings, he was diagnosed with hypercalcemia due to PHPT. Treatment for hypercalcemia was initiated immediately with intravenous transfusion of normal saline (2,400 mL/day) along with furosemide (60 mg/day) to facilitate calcium excretion. The patient also received calcitonin (40 units/day). However, hypercalcemia worsened (16.5 mg/dL) and he developed hypercalcemic crisis with severe nausea and general fatigue 2 days after admission. He could not take almost any foods due to these symptoms and became lethargic.
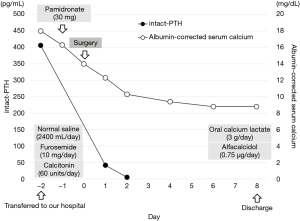
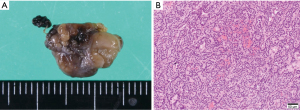
The child was then transferred to our hospital and received intensive care. On the transfer day, his albumin-corrected serum calcium level further increased to 18.0 mg/dL (Figure 2). Meanwhile, his serum phosphorus level was low (1.6 mg/dL) (normal range, 3.9–5.8 mg/dL). The other data was as follows, magnesium 1.4 mg/dL (normal range, 1.8–2.3 mg/dL), alkaline phosphatase 325 U/L (normal range, 154–431 U/L), 25-hydroxyvitamin D 25.5 ng/mL (normal range: more than 20.0 ng/mL), 1,25-dihydroxyvitamin D3 113 pg/mL (normal range, 20–70 pg/mL), calcitonin 3.38 pg/mL (normal range: less than 9.52 pg/mL), PTH-related peptide <1.0 pmol/L (normal range: less than 1.1 pmol/L), prolactin 2.0 ng/mL (normal range, 3.6–16.3 ng/mL), adrenocorticotropic hormone (ACTH) 43.4 pg/mL (normal range, 7.2–63.3 pg/mL), and insulin 9.4 µU/mL (normal range, 5.0–25.0 µU/mL). The calcium level in his urine was 15.5 mg/dL, and calcium/creatinine clearance ratio in urine was 1.67. Renal tubular reabsorption of phosphate was low as 61.0% (normal range, 80.0–94.0%), whereas fractional excretion of calcium was high 0.08 (normal range, 0.02–0.04). As it was necessary to lower the serum calcium level, a single dose of pamidronate (30 mg) was administered in addition to intravenous transfusion of normal saline (70–150 mL/hour adjusted according to the urine output), furosemide (10 mg/day), and calcitonin (60 units/day). Two days later, the albumin-corrected serum calcium level decreased to 14.0 mg/dL (Figure 2). However, his symptoms did not completely improve and he still could not take any foods. Therefore, we performed an emergency parathyroidectomy 2 days after the transfer to our hospital. The enlarged right superior parathyroid gland was resected. We also aimed to resect the right inferior normal parathyroid gland in consideration of the possibility of hereditary disease. However, we could not detect the right inferior parathyroid gland. Considering the possible presence of right inferior parathyroid glands in the right paratracheal adipose tissue and right upper pole of the thymus (12), we resected these tissues as well. Fifteen minutes after the resection of the right superior parathyroid gland, the intact-PTH level decreased to 41.7 pg/dL. Albumin-corrected serum calcium level decreased to 12.3 mg/dL the next day (Figure 2). The size and weight of the right superior parathyroid gland was 15×10 mm and 320 mg, respectively. Histopathological examination revealed that the enlarged gland was as an adenoma (Figure 3), and neither the excised adipose tissue nor the thymus contained any parathyroid tissue. The postoperative course was uneventful, and the symptoms caused by PHPT immediately improved. The albumin-corrected serum calcium level gradually decreased to 8.8 mg/dL on postoperative day 6 (Figure 2). To prevent hypocalcemia, oral calcium lactate (3 g/day) and alfacalcidol (0.75 µg/day) were administered, and their doses were gradually decreased according to the serum calcium level. Six months after surgery, he continued to receive alfacalcidol (0.25 µg/day), and his albumin-corrected serum calcium and intact-PTH levels were maintained within normal limits. As his lumbar bone mineral density improved to 0.774 g/cm2 6 months after surgery, alfacalcidol will be stopped in the near future. Since PHPT occurred at a young age, the possibility of genetic disease was considered. Therefore, we performed genetic testing for multiple endocrine neoplasia type 1 (MEN1) and hyperparathyroidism-jaw tumor syndrome (HPT-JT) after the operation. However, we did not detect any pathogenic mutations in the MEN1 gene, a causative gene for MEN1, by Sanger sequencing and multiplex ligation-dependent probe amplification. In addition, we did not detect any pathogenic mutations in the CDC73 gene, which is responsible for HPT-JT, by next-generation sequencing. To date, although the serum calcium levels of his parents has not been checked, no genetic predisposition has been identified in this family.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian/parent for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Herein, we report a case of PHPT-induced hypercalcemic crisis that occurred in an 11-year-old boy who had been treated with emergency parathyroidectomy following failed medical treatment. Previous reports showed that PHPT rarely developed hypercalcemic crisis in pediatric patients (2,13-15). Together with the rarity of PHPT in pediatric patients itself, hypercalcemic crisis caused by PHPT in pediatric patients has been considered an even rarer entity. PHPT-induced hypercalcemic crisis in pediatric patients has been described in a relatively large number of patient cohort studies (2,13-15), but few cases have been reported with detailed clinicopathological characteristics or clinical courses. After extensive literature research, we found six English reports of hypercalcemic crisis caused by PHPT in patients aged ≤18 years with detailed clinicopathological characteristics and clinical courses (7-11) (Table 1).
Table 1
| Case | Authors | Age/sex | FH of PHPT | Serum Ca (mg/dL) | i-PTH (pg/mL) | Symptoms | Culprit parathyroid gland | Initial treatment | Days to the surgery | Operation procedure | Pathological diagnosis | i-PTH after surgery (pg/mL) | Postoperative course | Genetic mutation test | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Choudhry et al. (7) | 13/f | None | 18.5 | 1,198 | Severe abdominal pain | Lt inferior | Normal saline; furosemide; calcitonin pamidronate | 2 days | Lt inferior parathyroidectomy (unilateral neck exploration) | Adenoma | 51.0 | Symptom free, Ca and alfacalcidol supplementation | MEN1 | No mutation |
| 2 | Walczyk et al. (11) | 15/m | None | 14.5 | 880 | Gait disturbance | Rt inferior | Normal saline; furosemide; pamidronate | N.D | Rt inferior parathyroidectomy (unilateral neck exploration) | Adenoma | N.D | Symptom free, Ca and alfacalcidol supplementation | N.D | N.D |
| 3 | Sala et al. (10) | 16/f | None | 14.9 | 184 | Severe nausea; altered mental status | Rt inferior | Normal saline; furosemide; pamidronate | N.D | Rt inferior parathyroidectomy (unilateral neck exploration) | Adenoma | N.D | Symptom free, Ca and alfacalcidol supplementation | N.D | N.D |
| 4 | Pal et al. (9) | 12/m | None | 14.1 | 203 | GTCS; severe abdominal pain; severe nausea | Lt inferior | Normal saline; furosemide | N.D | Lt inferior parathyroidectomy (bilateral neck exploration) | Adenoma | 28.0 | Symptom free, no supplementation | MEN1 | No mutation |
| 5 | Pal et al. (9) | 16/m | None | 14.5 | 2,491 | GTCS; paresthesia; decreased alertness | Lt inferior | Normal saline; furosemide; zoledronate | 5 days | Lt inferior parathyroidectomy (bilateral neck exploration) | Adenoma | 43.0 | Symptom free, no supplementation | MEN1 | No mutation |
| 6 | Mamedova et al. (8) | 16/f | Yes | 16.7 | 2,207 | Pathologic fracture; severe nausea; progressive weight loss | Rt inferior | Normal saline; denosumab | 22 days | Rt inferior parathyroidectomy (unilateral neck exploration) | Adenoma | 6.7 | Symptom free, Ca and alfacalcidol supplementation | CDC73 | Exon 2 176C>T |
| 7 | Our case | 11/m | None | 18.0 | 406 | Severe nausea; severe general fatigue | Rt superior | Normal saline; furosemide; calcitonin pamidronate | 2 days | Rt superior parathyroidectomy (unilateral neck exploration) | Adenoma | 41.7 | Symptom free, Ca and alfacalcidol supplementation | MEN1, CDC73 | No mutation |
FH, family history; PHPT, primary hyperparathyroidism; Ca, calcium; i-PTH, intact-parathyroid hormone; N.D, not described; GTCS, generalized tonic-clonic convulsion.
Because it is necessary to lower serum calcium levels, the initial treatment consisting of normal saline infusion and/or furosemide was immediately started for all six patients shown in Table 1. However, bisphosphonate (etidronate, pamidronate, or zoledronate) was used to decrease serum calcium levels in four out of six cases (7,9-11), while in one case, denosumab, a human monoclonal antibody of the receptor activator of nuclear factor kappa-B ligand, was administered (8). In addition, calcitonin was used in one case (7). In our case, both a bisphosphonate (pamidronate) and calcitonin were used to suppress bone resorption. As the bones are involved in the elevation of the serum calcium level in our case, a bisphosphonate and calcitonin would have some effect on decreasing the serum calcium level. With regard to other anti-bone resorption agents, cinacalcet, which is currently used to treat secondary hyperparathyroidism and parathyroid carcinoma, can be an option in the future. Although serum calcium levels were decreased by these medical treatments to some extent, parathyroidectomy was performed as radical treatment soon after hospitalization (1–5 days after admission) in five out of six patients (7,9-11). Another patient (case 6) underwent surgery 22 days after admission. This case had family history of PHPT, and hence genetic testing was performed before surgery (8). Emergency surgeries after initial treatment might be imperative because a hypercalcemic crisis can result in fatal consequences in cases where prompt and proper treatment cannot be provided, and surgery is the only curative treatment for PHPT (6). In our case, the boy was diagnosed with a hypercalcemic crisis with a highly elevated albumin-corrected serum calcium level (18.0 mg/dL) and associated severe nausea. However, emergency surgery prevented a possible fatal consequence.
Although emergency surgery for hypercalcemic crisis is necessary to prevent fatal outcomes, surgical procedures should be chosen carefully because the possibility of hereditary diseases such as MEN1, MEN2A, HPT-JT, and familial isolated PHPT is higher in pediatric patients with PHPT than in adults (16). When genetic testing confirms pathogenic mutations in MEN1, RET, or CDC73, which are genes responsible for MEN1, MEN2A, or HPT-JT, respectively, total parathyroidectomy and auto-transplantation with bilateral neck exploration should be performed. However, as it takes a couple of weeks to obtain the results of genetic testing, we need to decide on a treatment strategy without information on gene mutations in a patient with hypercalcemic crisis that requires emergency surgery. Therefore, we consider a focused parathyroidectomy as an appropriate treatment to promptly lower the serum calcium level with minimal invasion in pediatric patients with a hypercalcemic crisis caused by PHPT. Indeed, all six previously reported cases of hypercalcemic crisis in pediatric patients (shown in Table 1) underwent removal of one enlarged parathyroid gland, which resulted in a favorable decrease in serum calcium levels. Our patient also underwent resection of the enlarged parathyroid gland, and serum calcium level decreased to the normal range. In two cases (cases 4 and 5), bilateral neck exploration was performed, but the glands other than the responsible one were preserved because they were of normal size. The serum intact-PTH levels decreased to normal levels soon after the surgery (9). Furthermore, in all the cases shown in Table 1, the resected gland was histopathologically diagnosed with adenoma.
Among hereditary diseases associated with PHPT, MEN1 is the most prevalent; the reported frequency of MEN1 in pediatric patients diagnosed with PHPT is approximately 13% (17). Clinical presentation of MEN1-associated PHPT has been reported to be mild or asymptomatic, with mild elevation of serum calcium and intact PTH levels (18). Shariq et al. (19) reported the clinical features of 60 patients with MEN1 aged under 18 years old, and none of the patients presented with hypercalcemic crisis. Thus, hypercalcemic crisis seems less likely to occur in pediatric patients with MEN1. HPT-JT is another hereditary disease that can present with PHPT in pediatric patients. Among seven cases of hypercalcemic crisis in pediatric patients shown in Table 1, one case (case 6) was diagnosed as HPT-JT, as evidenced by a pathogenic mutation in CDC73 (8). A single parathyroid tumor is usually responsible for the development of PHPT in patients with HPT-JT. Furthermore, the resected parathyroids in a patient with HPT-JT are likely to be pathologically adenomas or carcinomas, but not hyperplasia (20). Hence, focused parathyroidectomy may be a feasible option for HPT-JT. Altogether, we consider that focused parathyroidectomy is a reasonable strategy for pediatric patients with PHPT-induced hypercalcemic crisis. Meanwhile, hypercalcemic crisis due to PHPT could be a consequence of the delayed diagnosis of PHPT. The symptoms of PHPT are heterogeneous, as shown in Table 1, and are expected to be vague and non-specific. Therefore, early diagnosis of PHPT may be difficult. However, when treating pediatric patients with non-specific symptoms, serum calcium levels should be considered because it can help us recognize the possibility of PHPT before the onset of hypercalcemic crisis. The important differential diagnosis for hypercalcemia in pediatric patients is familial hypocalciuric hypercalcemia (FHH), which shows hypercalcemia with low fractional excretion of calcium (≤0.01). In our case, because fractional excretion of calcium was high as 0.08, FHH was ruled out.
PHPT-induced hypercalcemic crisis is a rare condition in pediatric patients. Although a genetic test should be generally considered for a child with PHPT, emergency focused parathyroidectomy prior to genetic testing is an appropriate strategy when the patient presents with a hypercalcemic crisis caused by PHPT. A serum calcium level check is important to differentiate PHPT before the onset of hypercalcemic crisis when pediatric patients present with non-specific symptoms.
Acknowledgments
We would like to thank Editage (https://www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-92/rc
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-92/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-92/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian/parent for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet 2018;391:168-78. [Crossref] [PubMed]
- Mallet EWorking Group on Calcium Metabolism. Primary hyperparathyroidism in neonates and childhood. The French experience (1984-2004). Horm Res 2008;69:180-8. [PubMed]
- Alagaratnam S, Kurzawinski TR. Aetiology, Diagnosis and Surgical Treatment of Primary Hyperparathyroidism in Children: New Trends. Horm Res Paediatr 2015; Epub ahead of print. [Crossref] [PubMed]
- Liu Y, Guo S, Wu J, et al. Changes in clinical patterns of Chinese patients with primary hyperparathyroidism in the past 12 years: a single-center experience. Endocr Connect 2021;10:1428-34. [Crossref] [PubMed]
- Ahmad S, Kuraganti G, Steenkamp D. Hypercalcemic crisis: a clinical review. Am J Med 2015;128:239-45. [Crossref] [PubMed]
- Cannon J, Lew JI, Solórzano CC. Parathyroidectomy for hypercalcemic crisis: 40 years' experience and long-term outcomes. Surgery 2010;148:807-12; discussion 812-3. [Crossref] [PubMed]
- Choudhry KS, Malik MZ, Buggs-Saxton C. Hypercalcemic crisis due to primary hyperparathyroidism in systemic lupus erythematosus (SLE). Lupus 2013;22:847-50. [Crossref] [PubMed]
- Mamedova E, Kolodkina A, Vasilyev EV, et al. Successful Use of Denosumab for Life-Threatening Hypercalcemia in a Pediatric Patient with Primary Hyperparathyroidism. Horm Res Paediatr 2020;93:272-8. [Crossref] [PubMed]
- Pal R, Dutta A, Agrawal K, et al. Primary Hyperparathyroidism Presenting as Posterior Reversible Encephalopathy Syndrome: A Report of Two Cases J Clin Res Pediatr Endocrinol 2020;12:432-8.
- Sala TD, Mureşan S, Roman R, et al. Hypercalcaemic Crisis Due to Primary Hyperparathyroidism: Report of Two Cases. J Crit Care Med (Targu Mures) 2019;5:34-9. [Crossref] [PubMed]
- Walczyk A, Szalecki M, Kowalska A. Primary hyperparathyroidism: a rare endocrinopathy in children. Two case reports. Endokrynol Pol 2011;62:346-50. [PubMed]
- Reitz RJ 3rd, Dreimiller A, Khil A, et al. Ectopic and supernumerary parathyroid glands in patients with refractory renal hyperparathyroidism. Surgery 2021;169:513-8. [Crossref] [PubMed]
- Cronin CS, Reeve TS, Robinson B, et al. Primary hyperparathyroidism in childhood and adolescence. J Paediatr Child Health 1996;32:397-9. [Crossref] [PubMed]
- Sharanappa V, Mishra A, Bhatia V, et al. Pediatric Primary Hyperparathyroidism: Experience in a Tertiary Care Referral Center in a Developing Country Over Three Decades. World J Surg 2021;45:488-95. [Crossref] [PubMed]
- Wang W, Kong J, Nie M, et al. Primary hyperparathyroidism in Chinese children and adolescents: A single-centre experience at Peking Union Medical College Hospital. Clin Endocrinol (Oxf) 2017;87:865-73. [Crossref] [PubMed]
- Fraser WD. Hyperparathyroidism. Lancet 2009;374:145-58. [Crossref] [PubMed]
- Kollars J, Zarroug AE, van Heerden J, et al. Primary hyperparathyroidism in pediatric patients. Pediatrics 2005;115:974-80. [Crossref] [PubMed]
- Lamas C, Navarro E, Casterás A, et al. MEN1-associated primary hyperparathyroidism in the Spanish Registry: clinical characterictics and surgical outcomes. Endocr Connect 2019;8:1416-24. [Crossref] [PubMed]
- Shariq OA, Lines KE, English KA, et al. Multiple endocrine neoplasia type 1 in children and adolescents: Clinical features and treatment outcomes. Surgery 2022;171:77-87. Erratum in: Surgery 2022;171:1708-11. [Crossref] [PubMed]
- DeLellis RA, Mangray S. Heritable forms of primary hyperparathyroidism: a current perspective. Histopathology 2018;72:117-32. [Crossref] [PubMed]


