
Tumor-infiltrating lymphocytes status, programmed death-ligand 1 expression, and clinicopathological features of 41 cases of pure apocrine carcinoma of the breast: a retrospective study based on clinical pathological analysis and different immune statuses
Introduction
Apocrine carcinoma (AC) is a rare and special subtype of breast cancer, accounting for about 0.3–4% of all breast cancers (1-5). The World Health Organization (WHO) Classification of Breast Tumors (2012 edition) classifies any invasive carcinomas with apocrine cell features as invasive carcinomas with apocrine differentiation, including invasive carcinomas with focal apocrine differentiation and extensive apocrine differentiation (pure AC) (6). Pure AC is strictly defined as >90% of cancer cells with apocrine morphological and immunohistochemistry (IHC) characteristics: distinct cell borders, abundant eosinophilic granular cytoplasm, large nuclei with prominent nucleoli, negative expression of estrogen receptor (ER) and progesterone receptor (PR), and high expression of androgen receptor (AR) by IHC (7,8). The 2019 edition of the WHO Classification of Breast Tumors (5) also follows this diagnostic criterion, according to which pure AC accounts for only about 1% of all breast cancers.
Pure AC can be divided into human epidermal growth factor receptor-2 (HER2)-positive and negative types [triple-negative apocrine carcinoma (TNAC)] according to HER2 expression. In clinical trials of AR-positive triple negative breast cancer (TNBC), the combination use of AR inhibitors and programmed death ligand 1 (PD-L1) inhibitors has shown encouraging results(9). The target proteins studied in TNBC in recent years are PD-L1 and AR, but the correlation between AR and PD-L1 was rarely studied. The prognosis of TNBC is significantly correlated with the density of tumor-infiltrating lymphocytes (TILs) and the expression pattern of TILs (P<0.05), and TILs in TNBC have significant prognostic significance according to the recommendations of the International TILs Working Group (10-12). It has been reported that TNBC with low TILs and high PD-L1 status may be a therapeutic target for immune checkpoint inhibitors (13). Only a few cases of pure AC have been reported up to now, so the knowledge and research on this tumor are limited. Moreover, there is still no standardized treatment for this tumor. In our study, 41 cases of pure AC were collected to investigate the correlation between TILs status, PD-L1 expression, and clinicopathological features, in order to improve the understanding of this tumor and provide new treatment insights and offer the possibility of immunotherapy for AC patients. We present the following article in accordance with the REMARK reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-248/rc).
Methods
Clinical data
We developed and implemented a retrospective clinical research study that enrolled 41 patients with pure AC between January 2014 to November 2020. A total of 2,703 cases of invasive breast cancer that were surgically resected in Hubei Cancer Hospital during the same period were collected. Cases diagnosed as AC of the breast were enrolled and reviewed by two senior pathologists according to the 2019 edition of WHO breast tumor classification criteria (7), and 41 cases were finally diagnosed as pure AC. Due to the low prevalence, only 41 cases of pure AC were collected over a 6-year period. The sample size of this study was small, and this sample will continue to be collected for a large sample size supplementary study in the follow-up. The inclusion criteria were as follows: (I) cases in which the postoperative pathology was definitive for AC of the breast; and (II) patients who underwent treatment with radical surgery or mass excision. The exclusion criteria were as follows: (I) patients who underwent only puncture diagnosis; (II) cases of non-primary breast cancer; and (III) cases involving 0% or minimal tumor content in paraffin specimens after neoadjuvant treatment with radiation.
The clinicopathological data of the patients included gender, age, menopause, maximum tumor diameter, histological grade, pathological stage, lymph node metastasis, IHC expression of ER, PR, AR, HER2, and Ki67 in previous pathological tests, and the fluorescence in situ hybridization (FISH) results for HER2 2+, treatment options, and prognosis. The prognostic information of all cases was followed up by telephone. The follow-up information included patient treatment measures, local recurrence, distant metastasis, and death. The follow-up period began when the patient was first diagnosed and ended in January 2021, with a follow-up period of 1–104 months and a median follow-up period of 29 months. OS is described as the time frame between diagnosis and the date of last contact or death from any cause. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethical Committees of Hubei Cancer Hospital (No. LLHBCH2022YN-014) and individual consent for this retrospective analysis was waived.
Interpretation of TILs
According to the 2014 International Working Group on TILs interpretation criteria (10,14), TILs include lymphocytes and plasma cells. In the hematoxylin-eosin (H&E) slices, microscope magnification of ×200–400 (ocular ×10, with an objective of ×20–×40), the ratio of TILs in the stromal areas (%) of the invasive cancer area was evaluated as a whole, not the number of stromal cells, not focusing only on hot-spot areas. TILs that were outside the tumor border, in areas of ductal carcinom in situ (DCIS), in tumor zones with crush artifacts, necrosis, regressive hyalinization as well as in the previous core biopsy site, and around the normal lobules were excluded. We set 50% as the threshold for assessing the status of TILs, and the pure AC cases were divided into TILs ≥50% and TILs <50% groups (15).
IHC staining and interpretation
Formalin-fixed paraffin-embedded (FFPE) samples from 14 of the 41 samples of patients who underwent surgical resection within 2 years (from January 2019 to November 2020) were selected for PD-L1 IHC staining, which was conducted on a Ventana automatic immunohistochemical staining instrument (Agilent Technologies, Basel, Switzerland) using the EnVision method. The reagents used in this study were purchased from ABCAM (Cambridge, UK). The primary antibody PD-L1 (clone number SP142) was applied at a working concentration of 1:100, and the secondary antibody was used in the Optiview amplification kit (ROCHE, Basel, Switzerland). The interpretation of PD-L1 was based on previously reported literature (16), using an immune cell (IC) scoring algorithm for the overall assessment of IC staining based on the entire tumor area, and calculating the percentage of the area within the tumor area occupied by IC of any intensity PD-L1 staining, rather than a random field of view for evaluation, with a PD-L1 positive threshold of IC ≥1%. IC cell types include lymphocytes, macrophages, dendritic cells, granulocytes, plasma cells, etc. Tumor areas evaluated for PD-L1 include invasive carcinoma and surrounding tumor-associated interstitium, excluding DICS, lobular carcinoma in situ (LCIS), and necrosis. For 10 cases where the HER2 (ROCHE, Basel, Switzerland, clone number 4B5) IHC staining had been performed in previous pathological examinations, HER2 (DAKO, Basel, Switzerland, clone number EP3) was verified on the Dako automatic immunohistochemical staining instrument, and the IHC staining conditions were consistent with those of previous HER2 IHC tests. The IHC results were evaluated by two trained pathologists using a double-blind reading method.
Statistical analysis
We used either Pearson chi-square test or Spearman rank correlation to detect the association between different HER2 expression and clinicopathological variables. We used either Fisher’s exact test to detect the association between PD-L1 positive and negative expression and clinicopathological variables. Survival curves were plotted using the Kaplan-Meier method [95% confidence interval (CI)] and the differences were compared using the log-rank test. The Univariate Cox regression model was used, taking covariates (all clinicopathological features) into account (15). All variables with P<0.05 were included in the multivariate Cox model. All tests were two-sided tests, and statistical significance was defined as P<0.05. SPSS (26.0, Chicago, USA) was used to analyze data.
Results
Clinical information
A total of 2,446 out of 2,703 invasive breast carcinomas contained information on axillary lymph node dissection, of which 40.1% (980/2,446) had lymph node metastasis. There were 41 cases of pure AC in our cohort; all of the patients were females, aged 32–75 years (mean: 59 years), and 53.7% (22/41) of the patients were over 59 years old. Furthermore, 85.4% (35/41) of patients were menopausal and 14.6% (6/41) were of childbearing age. Among all of the postoperative cases, 2.4% (1/41) of patients received radiotherapy, 70.7% (29/41) of patients received chemotherapy, 22.0% (9/41) of patients received both radiotherapy and chemotherapy, and 4.9% (2/41) of patients had not undergone postoperative treatment. HER2-positive patients (22.0%, 9/41) were treated with Herceptin-targeted therapy. Axillary lymph node metastasis occurred in 48.8% (20/41) of patients. According to the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system, there were 5 cases (5/41, 12.2%) in stage I, 24 cases (24/41, 58.5%) in stage II, 9 cases (9/41, 21.9%) in stage III, and 3 cases (3/41, 7.3%) in stage IV. The follow-up time ranged from 1 to 104 months. At the end of the follow-up period, there were four cases lacking clinical information about tumor metastasis/recurrence and two cases lacking information on patient survival. Tumor metastasis/recurrence occurred in 5.4% (2/37) patients, and 10.3% (4/39) patients died.
Pathological features
The maximum diameter of tumors was 0.47–10.00 cm (mean maximum diameter: 2.41 cm). Under low magnification, the carcinoma tissue exhibited solid sheets, nest, micropapillary, and cord-like growth patterns. In some cases, medium to high-grade apocrine type ductal carcinoma in situ was observed. Under high magnification, the tumor cells exhibited a large size and distinct cell borders. The cells had abundant cytoplasm, large mostly central nuclei, and prominent eosinophilic nucleoli (Figure 1A,1B), most of which were eosinophilic and granular, some of which were in the form of tiny vacuoles. The Nottingham histological grades were all grade II–III: 73.2% were grade II (30/41) and 26.8% were grade III (11/41). Different degrees of inflammatory cell infiltration were seen in the stroma; the evaluation of TILs status showed that 80.5 % of TILs were <50% (33/41) and 19.5% of TILs were ≥50% (8/41) (Figure 1C,1D).
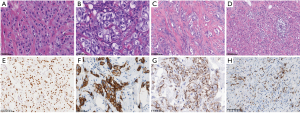
The IHC of previous pathological examinations showed negative ER and PR, as well as diffuse, strongly positive expression of AR (Figure 1E). HER2 was expressed to varying degrees (Figure 1F), of which 2.44% scored 0 (1/41), 17.07% scored 1+ (7/41), 41.46% scored 2+ (17/41), and 39.02% scored 3+ (16/41). Seventeen of the 2+ cases were tested by FISH, and the results showed that one case was HER2-positive, while the remaining 16 cases were HER2-negative. Of the 41 cases, 41.5% were HER2-positive (17/41) and 58.5% were negative (24/41). IHC staining for HER2 in 24.4% (10/41) of the cases showed cytoplasmic granular and microglobulin staining (Figure 1G), of which three were HER2 positive and seven were negative, with staining results of 1+ (two cases), 2+ (five cases), and 3+ (three cases). The FISH results for 2+ were all negative. Of the 14 cases tested for PD-L1, 50% (7/14) were positive for PD-L1, showing a dark brown dot or linear staining of the membrane/cytoplasm (Figure 1H).
Clinicopathological characteristics of HER2-positive and negative pure AC
Among the 41 cases of pure AC, a comparative analysis of the HER2-positive and negative groups revealed that the expression of TILs and the proliferation of Ki67 in the HER2 positive group were significantly higher than those in the HER2 negative group (P=0.032, P=0.003) (Table 1). Meanwhile, the pathological parameters such as age, menopause, histological grade, and lymph node metastasis were not statistically different between these two groups. PD-L1 staining was 50% (7/14) positive in 14 cases of AC. The PD-L1 positive rates in the HER2-negative and positive groups were 66.7% (4/6) and 37.5% (3/8), respectively, and this difference was not statistically significant (P=0.592). Two of the 14 cases presented TILs ≥50%, which were positive for PD-L1 and Ki67 >20%. In the remaining 12 samples with TILs <50%, 41.7% (5/12) were PD-L1 positive and 58.3% (7/12) were PD-L1 negative. All of the 14 PD-L1-negative samples had TILs <50% (Table 2), and there was no statistically significant difference between PD-L1 expression and the pathological parameters such as age, menopause, histological grade, and lymph node metastasis.
Table 1
| Characteristics | HER2-negative (N=24) | HER2-positive (N=17) | P value |
|---|---|---|---|
| Age, years | 59.4±10.2 | 57.4±8.8 | 0.476 |
| Menopause, n (%) | 0.991 | ||
| Yes | 21 (87.5) | 14 (82.4) | |
| No | 3 (12.5) | 3 (17.6) | |
| Tumor size, cm | 2.3±2.0 | 2.5±1.0 | 0.350 |
| Histologic grade, n (%) | 0.303 | ||
| 2 | 19 (79.2) | 11 (64.7) | |
| 3 | 5 (20.8) | 6 (35.3) | |
| Lymph node status, n (%) | 0.412 | ||
| Negative | 11 (45.8) | 10 (58.8) | |
| Positive | 13 (54.2) | 7 (41.2) | |
| TILs, n (%) | 0.032 | ||
| <50% | 22 (91.7) | 11 (64.7) | |
| ≥50% | 2 (8.3) | 6 (35.3) | |
| Ki67, n (%) | 0.003 | ||
| ≤20% | 14 (58.3) | 2 (11.8) | |
| >20% | 10 (41.7) | 15 (88.2) | |
AC, apocrine carcinoma; HER2, human epidermal growth factor receptor-2; TILs, tumor-infiltrating lymphocytes.
Table 2
| Characteristics | PD-L1-negative (N=7) | PD-L1-positive (N=7) | P value |
|---|---|---|---|
| HER2, n (%) | 0.592 | ||
| Negative | 2 (28.6) | 4 (57.1) | |
| Positive | 5 (71.4) | 3 (42.9) | |
| TILs, n (%) | 0.462 | ||
| <50% | 7 (100.0) | 5 (71.4) | |
| ≥50% | 0 (0.0) | 2 (28.6) | |
| Ki67, n (%) | 1.000 | ||
| ≤20% | 2 (28.6) | 2 (11.8) | |
| >20% | 5 (71.4) | 5 (88.2) | |
HER2, human epidermal growth factor receptor-2; TILs, tumor-infiltrating lymphocytes; AC, apocrine carcinoma; PD-L1, programmed death-ligand 1.
Prognostic analysis
The follow-up period ended in January 2021. Univariate analysis was performed on the clinicopathological features of the 41 cases of pure AC. The Kaplan-Meier survival curves showed that the overall survival time of HER2-positive patients was longer than that in HER2-negative patients [hazard ratio (HR) 0.03, 95% CI: 0.00–infinite, P=0.211] (Figure 2A). Among the 17 HER2-positive patients, 76.5% (13/17) were treated with chemotherapy and 23.5% (4/17) received radiotherapy. Of these patients, 52.9% (9/17, seven of radiotherapy and two of chemoradiotherapy) received Herceptin-targeted therapy.

Among the 24 HER2-negative patients, 4.2% (1/24) received radiotherapy, 66.7% (16/24) received chemotherapy, 20.8% (5/24) received chemoradiotherapy, and 8.3% (2/24) did not receive chemoradiotherapy. Moreover, none of these patients were treated with targeted therapy. The overall survival rate was higher in patients with Ki67 >20% than in patients with Ki67 ≤20% (HR 0.28, 95% CI: 0.03–2.74, P=0.245) (Figure 2B). Of the 25 patients with Ki67 >20%, 68.0% (17/25) received chemotherapy, 24.0% (6/25) received chemoradiotherapy, and 8.0% (2/25) did not receive chemoradiotherapy. Among these patients, 32.0% (8/25, six radiotherapy and two chemoradiotherapy) received Herceptin-targeted therapy. Of the 16 patients with Ki67 ≤20%, 6.3% (1/16) received radiotherapy, 75.0% (12/16) received chemotherapy, and 18.75% (3/16) received chemoradiotherapy, of which 6.3% (1/16, 1 radiotherapy) received Herceptin-targeted therapy.
TILs ≥50%, TILs <50%, Ki67 >20%, and Ki67 ≤20% were defined as TILshigh, TILslow, Ki67high, and Ki67low, respectively. TILs and Ki67 co-expression analysis with the TILshigh/Ki67high, TILshigh/Ki67low, TILslow/Ki67low, and TILslow/Ki67high subgroups revealed no significant differences in overall survival between the groups (HR 1.25, 95% CI: 0.58–2.70, P=0.452), but only the TILshigh/Ki67high group did not have any death events (Figure 2C). In 14 cases of pure AC, the PD-L1 expression was positive in all TILshigh/Ki67high groups, and the positive expression of PD-L1 was 50% in the TILslow/Ki67low group (Figure 2D).
Discussion
Invasive breast carcinoma may exhibit focal to extensive apocrine differentiation. Vranic et al. (7) first proposed the concept of pure AC of the breast, which only accounts for about 1% of all breast cancers and has been reported in cases and small samples in China and abroad (17). It has been suggested that there is no significant difference in the biological behavior of pure AC and common invasive cancer (17), but due to the present limits to our understanding of morphology and academic discussion, these opinions have no practical clinical significance. In recent years, with the increasing numbers of cases and the development of research, the understanding of this special subtype of breast cancer has gradually deepened.
The present study included 41 cases of pure AC, which accounted for 1.5% (41/2,703) of all breast cancers admitted during the same period, and the total incidence rate was similar to that reported in the literature. Our study revealed that this special subtype of breast cancer is more likely to occur in postmenopausal elderly women compared to invasive ductal carcinoma. The mean age of onset in this patient cohort was 59 years, 53.7% (22/41) patients were over 59 years old, and 85.4% (35/41) patients were menopausal, which is consistent with previously reported, and there were no significant differences in the clinical manifestations, imaging features, and preferred sites between the patients (18-20). Tanaka et al. (21) reported that pure AC patients had lower rates of axillary lymph node metastasis than non-pure AC patients; however, the rate of axillary lymph node metastasis in our cohort (48.8%, 20/41) was higher than that of non-AC patients (40.1%, 980/2,446) during the same period. The reason for this inconsistency may be that there were fewer enrolled cases, making the data of Tanaka et al. scholars’ group and our group differ in statistical results (21). Another reason may be racial differences between the patients. A domestic study of HER2-negative pure AC showed that 50% (9/18) of patients had axillary lymph node metastasis, with 1–10 positive lymph nodes (22). Also, most patients had earlier TNM staging: 38.9% (7/18) were stage I, 50% (9/18) were stage II, and 11.1% (2/18) were stage III (22). In this study, 12.2% (5/41), 58.5% (24/41), and 21.9% (9/41) were stages I, II, and III, respectively, and the staging distribution was later than that of the aforementioned domestic research.
The most characteristic morphology of pure AC is cytological characteristics. The carcinoma cells retain the characteristics of benign apocrine metaplasia cells, with distinct cell borders, large volume, and abundant cytoplasm, most of which are eosinophilic and granular, and some of which may be in the form of tiny vacuoles. The nuclei of AC cells are approximately three times larger than those of normal apocrine cells (three-fold rule). Also, there are one or more prominent eosinophilic nucleoli, and mitoses can be seen. The histological structure of pure AC is similar to that of non-apocrine breast carcinoma, which can exhibit various growth patterns (such as solid sheet, nested, micropapillary, and cord-like), and is often combined with middle-high grade apocrine ductal carcinoma in situ, necrosis, and calcification can also be seen. According to the strict definition of pure AC, its immunophenotype is constantly negative for ER and PR, and strongly positive for AR. Pure Ac can be divided into HER2-positive and HER2-negative types (TNAC) based on HER2 expression (3,22,23). We found that the HER2-positive type (41.5%) was slightly less prevalent than the HER2-negative type (58.5%). We also observed that in 24.4% (10/41) of the HER2-positive cases, HER2 appeared cytoplasmic granular and micro-spherical staining when using ROCHE company’s HER2 primary antibody for IHC staining. According to the HER2 interpretation criteria, only invasive tumor cells with membrane staining should be interpreted, and cytoplasmic staining should be ruled out. FISH verification of these special HER2 staining cases showed that 30.0% (3/10) were HER2 positive, 70.0% (7/10) were HER2 negative, and all of the cases with IHC staining of 2+ were FISH negative. This expression pattern has never been observed in previous routine HER2 IHC assays in non-apocrine breast cancers and is currently found only in AC. To clarify whether this is related to the performance of different clone numbers of antibodies, IHC validation staining of a HER2 primary antibody from DAKO was performed on these 10 cases, and the interpretation results were consistent with those of ROCHE, but no cytoplasmic granular staining was observed in any of them. We speculated that the HER2 cytoplasmic granular staining may be caused by the HER2 primary antibody from ROCHE, but the specific reason requires further investigation.
The distribution of tumor TILs in our cohort of 41 ACs ranged from 1–80%, with a median value of 20%, which was consistent with the previous domestic research report (22). Our results indicated that 80.5% (33/41) of patients had TILs <50% and 19.5% (8/41) had TILs ≥50%. The 41 AC cases were divided into HER2-positive and negative groups based on their HER2 status. The HER2-positive group had higher TIL expression and Ki67 proliferation index than the HER2-negative group, suggesting that HER2-positive patients had higher TIL levels and tumor proliferation activity. Meanwhile, by observing the 14 cases of AC with PD-L1 (SP142) IHC staining, we found that PD-L1 expression and TILs status were differentially expressed between the HER2-negative and positive groups, but none of them were statistically significant, which may be related to the limited sample size. Recent studies on T cell dynamics have demonstrated that the response of T cells in TILs to immune checkpoint blockade (ICB) may depend on the recruitment of peripheral T cells, which, together with PD-L1 and the tumor mutational burden (TMB), represent different origins and mechanisms of ICB responses (24,25). The differential expression shown by the data in our cohort may help to reasonably design combined treatment strategies to improve the treatment response of AC patients. The 50% positive expression rate of PD-L1 is higher compared to that in generalized TNAC (26,27), while a domestic study (22) have reported a PD-L1 positivity rate of 11.2% (2/17). There is also a lack of clinical data on the use of PD-L1 inhibitors in pure AC.
The study on the gene expression profile of breast cancer have proposed the “molecular apocrine type” (LAR), which is characterized by the absence of ER and high expression of AR, and most tumors are HER2-positive, suggesting that it is related to the HER2 signaling pathway (28). There is a certain correlation and overlap between this molecular type and histopathological AC, but it is not fully equated, and not all LAR breast cancers meet the diagnostic criteria for AC. Pure AC strongly expresses AR, and the activation of the AR signaling pathway is its distinctive feature. Previous studies have reported that forkhead box A1 (FOXA1) and HER2 activation can up-regulate AR levels (29,30), and AR antagonists have achieved good efficacy in the treatment of prostate cancer, providing new ideas to clinicians regarding AR as a new therapeutic target (31-33). Gucalp et al. (34) discovered encouraging results in a clinical trial in patients with metastatic AR-positive TNBC treated with AR antagonists. There are currently more than 10 clinical trials of AR antagonists targeting breast cancer in progress (35,36).
The survival curves of our 41 patients showed that the cumulative survival of HER2-positive pure AC patients was higher than that of HER2-negative patients, and the cumulative survival of the Ki67 >20% group was higher than that of Ki67 ≤20% group, although this was not statistically significant. Moreover, 52.9% (9/17) of HER2-positive patients received Herceptin-targeted therapy; it is presumed that the cumulative survival benefit may be related to the application of HER2-targeted drugs in patients with this phenotype. The survival curves of TILs and Ki67 co-expression in the TILshigh/Ki67high, TILshigh/Ki67low, TILslow/Ki67low, and TILslow/Ki67high groups showed that the TILshigh/ Ki67high phenotype may have the best prognosis. There were no deaths in the TILshigh/Ki67high group. Among the six cases in the TILshigh/Ki67high group, five were treated with chemotherapy, and one received radiotherapy. Furthermore, five were HER2-positive and two were treated with Herceptin-targeted therapy (one of radiotherapy and one of chemotherapy). The co-expression survival curve showed that the TILslow/Ki67low phenotype likely had the worst prognosis. Of the 14 cases in the TILslow/Ki67low group, one received radiotherapy, 10 received radiotherapy, and three received radiotherapy and chemotherapy. However, a larger sample study is needed to verify these results.
In the 14 cases of AC, PD-L1 expression was positive in the TILshigh/Ki67high group, which may have potential value for immunotherapy. There are currently numerous potential molecular targets in AC. Vranic et al. (8,37) found that about 32% of tumors were HER2-positive, and the HER2-positive type in this study was 41.5% (17/41), which indicates consistency between the studies. Vranic et al. scholars’ study revealed mutations in tumor protein p53 (TP53), B-raf proto-oncogene (BRAF)/Kirsten rat sarcoma virus (KRAS), and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PI3KCA)/phosphatase and tensin homolog (PTEN) genes by next-generation sequencing (NGS) detection, and Lehmann-Che et al. also found that PIK3CA/PTEN/ protein kinase B (AKT) and TP53 alterations were most common in AC (37,38). AC with PIK3CA mutations and high AR expression may be sensitive to combined treatment with PIK3CA and AR inhibitors (39). These potential molecular targets provide the possibility for molecularly-targeted therapies for AC, but functional studies are still needed to verify them.
In summary, pure AC is a rare and specific type of breast cancer with differential expression of HER2, TILs, and PD-L1, suggesting the possibility of treatment for patients who have different immune statuses and are suitable for exploratory directions such as combined immunotherapy. With the increasing numbers of cases, the application of molecular detection techniques, and the development of drug clinical trials, our understanding and treatment of this tumor will inevitably become clearer and more effective.
Acknowledgments
Funding: This work was supported by the 2020 Jingjian·Tongshu Microsatellite Instability Research Fund Project (Beijing Jingjian Foundation for The Advancement of Pathology, No. JJTS2020-017).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-248/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-248/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-248/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethical Committees of Hubei Cancer Hospital (No. LLHBCH2022YN-014) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan PH, Ellis IO. Myoepithelial and epithelial-myoepithelial, mesenchymal and fibroepithelial breast lesions: updates from the WHO Classification of Tumours of the Breast 2012. J Clin Pathol 2013;66:465-70. [Crossref] [PubMed]
- Dellapasqua S, Maisonneuve P, Viale G, et al. Immunohistochemically defined subtypes and outcome of apocrine breast cancer. Clin Breast Cancer 2013;13:95-102. [Crossref] [PubMed]
- Vranic S, Gatalica Z. An Update on the Molecular and Clinical Characteristics of Apocrine Carcinoma of the Breast. Clin Breast Cancer 2022;22:e576-85. [Crossref] [PubMed]
- Cserni G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica 2020;112:25-41. [Crossref] [PubMed]
- Tan PH, Ellis I, Allison K, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020;77:181-5. [Crossref] [PubMed]
- Lakhani SR. WHO Classification of Tumours of the Breast. WHO classification of tumours of the breast; 2012.
- Vranic S, Tawfik O, Palazzo J, et al. EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol 2010;23:644-53. [Crossref] [PubMed]
- Quinn CM, D'Arcy C, Wells C. Apocrine lesions of the breast. Virchows Arch 2022;480:177-89. [Crossref] [PubMed]
- Zhu Y, Zhu X, Tang C, et al. Progress and challenges of immunotherapy in triple-negative breast cancer. Biochim Biophys Acta Rev Cancer 2021;1876:188593. [Crossref] [PubMed]
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Hida AI, Watanabe T, Sagara Y, et al. Diffuse distribution of tumor-infiltrating lymphocytes is a marker for better prognosis and chemotherapeutic effect in triple-negative breast cancer. Breast Cancer Res Treat 2019;178:283-94. [Crossref] [PubMed]
- Fuchs TL, Pearson A, Pickett J, et al. Why pathologists and oncologists should know about tumour-infiltrating lymphocytes (TILs) in triple-negative breast cancer: an Australian experience of 139 cases. Pathology 2020;52:515-21. [Crossref] [PubMed]
- Tomioka N, Azuma M, Ikarashi M, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer 2018;25:34-42. [Crossref] [PubMed]
- Laenkholm AV, Callagy G, Balancin M, et al. Incorporation of TILs in daily breast cancer care: how much evidence can we bear? Virchows Arch 2022;480:147-62. [Crossref] [PubMed]
- Mangia A, Saponaro C, Vagheggini A, et al. Should Tumor Infiltrating Lymphocytes, Androgen Receptor, and FOXA1 Expression Predict the Clinical Outcome in Triple Negative Breast Cancer Patients? Cancers (Basel) 2019;11:1393. [Crossref] [PubMed]
- Vennapusa B, Baker B, Kowanetz M, et al. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl Immunohistochem Mol Morphol 2019;27:92-100. [Crossref] [PubMed]
- Abati AD, Kimmel M, Rosen PP. Apocrine mammary carcinoma. A clinicopathologic study of 72 cases. Am J Clin Pathol 1990;94:371-7. [Crossref] [PubMed]
- Zhang N, Zhang H, Chen T, et al. Dose invasive apocrine adenocarcinoma has worse prognosis than invasive ductal carcinoma of breast: evidence from SEER database. Oncotarget 2017;8:24579-92. [Crossref] [PubMed]
- Tsutsumi Y. Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol 2012;42:375-86. [Crossref] [PubMed]
- Alvarenga CA, Paravidino PI, Alvarenga M, et al. Reappraisal of immunohistochemical profiling of special histological types of breast carcinomas: a study of 121 cases of eight different subtypes. J Clin Pathol 2012;65:1066-71. [Crossref] [PubMed]
- Tanaka K, Imoto S, Wada N, et al. Invasive apocrine carcinoma of the breast: clinicopathologic features of 57 patients. Breast J 2008;14:164-8. [Crossref] [PubMed]
- Sun X, Zuo K, Yao Q, et al. Invasive apocrine carcinoma of the breast: clinicopathologic features and comprehensive genomic profiling of 18 pure triple-negative apocrine carcinomas. Mod Pathol 2020;33:2473-82. [Crossref] [PubMed]
- Ismail S, Kherbek H, Skef J, et al. Triple-negative apocrine carcinoma as a rare cause of a breast lump in a Syrian female: a case report and review of the literature. BMC Womens Health 2021;21:396. [Crossref] [PubMed]
- Yost KE, Chang HY, Satpathy AT. Recruiting T cells in cancer immunotherapy. Science 2021;372:130-1. [Crossref] [PubMed]
- Xie Y, Xie F, Zhang L, et al. Targeted Anti-Tumor Immunotherapy Using Tumor Infiltrating Cells. Adv Sci (Weinh) 2021;8:e2101672. [Crossref] [PubMed]
- Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:405-11. [Crossref] [PubMed]
- Hwang SY, Park S, Kwon Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol Ther 2019;199:30-57. [Crossref] [PubMed]
- Zhao S, Ma D, Xiao Y, et al. Molecular Subtyping of Triple-Negative Breast Cancers by Immunohistochemistry: Molecular Basis and Clinical Relevance. Oncologist 2020;25:e1481-91. [Crossref] [PubMed]
- Naderi A, Meyer M, Dowhan DH. Cross-regulation between FOXA1 and ErbB2 signaling in estrogen receptor-negative breast cancer. Neoplasia 2012;14:283-96. [Crossref] [PubMed]
- Cruz RGB, Madden SF, Brennan K, et al. A Transcriptional Link between HER2, JAM-A and FOXA1 in Breast Cancer. Cells 2022;11:735. [Crossref] [PubMed]
- Mills AM, E, Gottlieb C, M, Wendroth S, et al. Pure Apocrine Carcinomas Represent a Clinicopathologically Distinct Androgen Receptor-Positive Subset of Triple-Negative Breast Cancers. Am J Surg Pathol 2016;40:1109-16. [Crossref] [PubMed]
- Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol 2018;36:884-90. [Crossref] [PubMed]
- Newton EE, Mueller LE, Treadwell SM, et al. Molecular Targets of Triple-Negative Breast Cancer: Where Do We Stand? Cancers (Basel) 2022;14:482. [Crossref] [PubMed]
- Gucalp A, Traina TA. Androgen receptor-positive, triple-negative breast cancer. Cancer 2017;123:1686-8. [Crossref] [PubMed]
- Gerratana L, Basile D, Buono G, et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat Rev 2018;68:102-10. [Crossref] [PubMed]
- Hamm C, Fifield BA, Kay A, et al. A prospective phase II clinical trial identifying the optimal regimen for carboplatin plus standard backbone of anthracycline and taxane-based chemotherapy in triple negative breast cancer. Med Oncol 2022;39:49. [Crossref] [PubMed]
- Vranic S, Marchiò C, Castellano I, et al. Immunohistochemical and molecular profiling of histologically defined apocrine carcinomas of the breast. Hum Pathol 2015;46:1350-9. [Crossref] [PubMed]
- Lehmann-Che J, Hamy AS, Porcher R, et al. Molecular apocrine breast cancers are aggressive estrogen receptor negative tumors overexpressing either HER2 or GCDFP15. Breast Cancer Res 2013;15:R37. [Crossref] [PubMed]
- Lehmann BD, Bauer JA, Schafer JM, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 2014;16:406. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


