
The efficacy and safety of microwave ablation versus conventional open surgery for the treatment of papillary thyroid microcarcinoma: a systematic review and meta-analysis
Introduction
Thyroid nodules are common diseases in the endocrine system. Most thyroid nodules are benign, but 5–15% are malignant (1). The most common type of thyroid malignancy is papillary thyroid carcinoma (PTC), which accounts for 70% of thyroid malignancies. Papillary thyroid microcarcinoma (PTMC) refers to a PTC ≤1 cm in diameter (2). In recent years, the incidence and detection rate of thyroid cancer has been rising, and most thyroid cancers are low-risk PTMC (3). Studies have shown that the mortality rate of PTMC patients is about 0–2.2% (4,5). The stable attributable mortality rate of thyroid cancer indirectly reveals the low risk and low mortality of PTMC.
The current standard of care for PTMC is conventional open surgery (OS). However, the risk of OS is high. In OS, normal thyroid tissue may be innocently removed, parathyroid glands are involved, and cervical lymph nodes are dissected (6). Further, various types of complications can occur after OS, which can seriously affect the quality of life and the health of patients.
In recent years, minimally invasive technology has developed rapidly. Ultrasound-guided microwave ablation (MWA) technology has achieved good safety and therapeutic effects in the treatment of benign thyroid nodules, and compared to OS, MWA technology results in fewer postoperative complications, has no effect on thyroid function, causes less trauma, and enables the faster recovery, and improved appearance of some PTMC patients (7-9). However, Tong’s study mentioned that MWA only inactivates cancer nodules and does not remove the surrounding lymph nodes, and has a high risk of residual cancer foci and lymph node metastasis, even after microwave ablation, there are still a few tumors with recurrence and lymph node metastasis, which can not be used as a routine treatment for primary operable primary thyroid cancer (10). Therefore, MWA is a non-radical treatment for tumors, and its use as a routine treatment remains controversial (11).
To date, no systematic review has been conducted on the efficacy and safety of MWA and OS in the treatment of PTMC patients. By summarizing the published literature on the efficacy and safety of MWA versus OS in the treatment of PTMC, we strictly select and evaluate relevant research, extract data and merge them with a meta-analysis, compare the efficacy and safety indexes during surgery, postoperatively, and in a follow-up period to systematically evaluate the advantages and disadvantages of these 2 treatment schemes, then draw comprehensive conclusions and provide an evidence base for clinical decision making. We present the following article in accordance with the MOOSE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-243/rc).
Methods
Literature search strategy
We systematically searched 7 electronic databases, PubMed, Excerpta Medica Database (EMBASE), Cochrane Library, Web of Science, WanFang database, China National Knowledge Infrastructure (CNKI), and Chinese BioMedical Database (CBM), from their establishment to March 2022, using the following keywords: (I) microwave ablation (MWA); (II) open surgery (OS); and (III) papillary thyroid microcarcinoma (PTMC). In the strategy, all these words were combined using the Boolean operators “AND” or “OR”. There were no restrictions on the publication language in the literature search. The search strategy, including the literature search and reference list screening, was developed by 2 of the authors, but it should be noted that this search strategy may not have captured all the relevant studies.
Study selection
To be eligible for inclusion in our meta-analysis, the studies had to meet the following criteria: (I) include patients in the stable stage of PTMC; (II) include a MWA test group; (III) include an OS control group; (IV) examine the main outcome indicators of intra-operative outcomes (i.e., operation time, blood loss, and incision size), post-operative outcomes (i.e., length of hospital stay, and hospitalization expenses), and follow-up outcomes (i.e., complication rate, recurrence rate, and lymph node metastasis) and (V) the study design can be prospective or retrospective, and the publication language can be English or Chinese. Studies were excluded from our meta-analysis for the following reasons: (I) the research examined other diseases; (II) the research compared other interventions; (III) the study lacked available data; and (IV) the article was a review, abstract, or duplicate publication.
Data extraction and quality assessment
The data extraction was performed independently by 2 investigators (N Liu and Q Hu), and any disagreements were resolved by consensus or consultation with a third reviewer. Prespecified data elements were extracted from each study using a structured data abstraction form. The data extracted included the study authors, study design, patient characteristics (i.e., age and gender), year of research duration, and follow-up period. As all the included studies were non-randomized clinical trials, the risk-of-bias assessment was performed using the Cochrane Risk of Bias 2.0.
Statistical analysis
In our study, the meta-analysis was performed with Review Manager 5.4 (The Cochrane Collaboration, 2020) to estimate the different effects between the MWA and OS groups of patients with PTMC. In brief, we used the odds ratio (OR) for the pooling effects of the binary variables and the mean difference (MD) for the continuous variables. The statistical heterogeneity between the studies was assessed using the chi-square test or Cochran’s Q test, and the I2 statistic, which measured inconsistencies across study results and describes the proportion of total variation in study estimates due to heterogeneity rather than sampling errors. If there was no statistical heterogeneity among the included studies, a fixed-effects model was used for the meta-analysis, but if there was heterogeneity among the included studies, a random-effects model was used. The Egger’s test and a funnel plot were used to examine potential publication bias.
Results
Search process
The literature search retrieved 772 unique studies, and after the removal of duplicate files, 676 articles were screened to determine their eligibility. From these, 578 articles were further excluded based on the screening of the abstracts and titles. The full text of 98 articles were reviewed to determine the type of article, the outcome data of interest, and the study design. Ultimately, 13 articles were included in our meta-analysis and subjected to data extraction (12-24). The literature search process, the inclusion and exclusion criteria, and the final sample size are illustrated in Figure 1.
Characteristics of included studies
Table 1 presents a comprehensive description of each trial included in the meta-analysis. All of the 13 included studies were non-randomized retrospective studies. A total of 2,169 patients (1,088 MWA patients and 1,081 OS patients) were included in the meta-analysis. The sample sizes of the studies ranged from 65 to 644. The mean diameter of the nodules was >4 mm. As MWA is a relatively new treatment for PTMC, all the research studies had been published after 2017.
Table 1
| Study | Study design | No. of patients | Gender (M/F) | Age (years)* | Mean diameter of nodule (mm)* | Follow-up | Duration | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MWA | OS | MWA | OS | MWA | OS | MWA | OS | |||||||
| Ma 2017 | Retrospective study | 30 | 35 | 3/27 | 7/28 | 46.10±8.20 | 49.97±10.30 | – | – | 6 months | January 2014 to November 2015 | |||
| Chen 2018 | Retrospective study | 49 | 40 | 9/40 | 5/35 | 44.88±11.04 | 45.78±13.74 | – | – | 24 months | January 2014 to February 2017 | |||
| Li 2018 | Retrospective study | 46 | 46 | 16/32 | 13/33 | 43.63±9.27 | 49.59±9.0 | 4.49±1.55 | 4.29±1.37 | 42 months | February 2014 to August 2017 | |||
| Xu 2018 | Retrospective study | 41 | 46 | 12/29 | 16/30 | 45.8±10.2 | 46.2±11.5 | 8.87±1.01 | 8.13±1.22 | – | January 2012 to October 2017 | |||
| Gong 2019 | Retrospective study | 79 | 73 | 26/53 | 20/53 | 49.32±7.48 | 48.86±7.42 | 6.31±1.71 | 6.28±1.69 | 1 month | May 2014 to February 2018 | |||
| Huo 2019 | Retrospective study | 50 | 50 | 14/36 | 12/38 | 43.66±9.54 | 45.14±9.82 | 7.14±2.26 | 6.95±2.42 | 6 months | January 2016 to December 2017 | |||
| Li 2019 | Retrospective study | 168 | 143 | 36/132 | 29/114 | 47.36±10.75 | 49.18±11.41 | – | – | 12 months | January 2013 to September 2018 | |||
| Wang 2019 | Retrospective study | 35 | 35 | 19/16 | 20/15 | 46.18±8.35 | 45.16±7.58 | – | – | – | December 2017 to December 2018 | |||
| Wang 2020 | Retrospective study | 36 | 36 | 18/18 | 19/17 | 45.6±8.5 | 46.1±8.9 | – | – | 6 months | April 2018 to April 2019 | |||
| Yao 2020 | Retrospective study | 120 | 120 | 19/101 | 22/98 | 45.86±8.30 | 46.02±8.11 | 5.89±1.12 | 5.96±1.05 | 12 months | January 2017 to December 2018 | |||
| Zhou 2020 | Retrospective study | 51 | 50 | 11/40 | 14/36 | 44.72±9.94 | 42.16±10.28 | 4.46±1.39 | 4.30±1.25 | 18 months | May 2014 to January 2017 | |||
| Wang 2021 | Retrospective study | 63 | 83 | 12/51 | 24/59 | 43.56±14.17 | 43.32±10.88 | 4.5±1.1 | 4.5±1.1 | 24 months | 2016 to 2018 | |||
| Zu 2021 | Retrospective study | 320 | 324 | 83/237 | 77/247 | 44.99±10.62 | 43.91±11.47 | 4.99±1.93 | 5.11±1.88 | 7 years | July 2013 to June 2020 | |||
*, the data were presented as mean ± standard deviation. MWA, microwave ablation; OS, open surgery.
Results of quality assessment
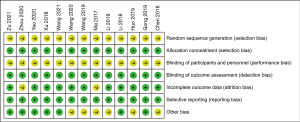
The quality of the included studies and the risk of bias were assessed in accordance with the Cochrane Risk of Bias 2.0. Of the 13 articles, all were non-randomized clinical trials, 2 articles did not include all the outcome variables of interest, and 3 articles had a short or no follow-up period. The summary bias assessment of each included article were shown in Figure 2.
Results of meta-analysis
The results of the meta-analysis are summarized in Table 2.
Table 2
| Outcome of interest | Studies (n) | Patients (n) | MD/OR | 95% CI | P | I2 (%) |
|---|---|---|---|---|---|---|
| Intra-operative outcomes | ||||||
| Operation time | 10 | 1,641 | –44.85 (MD) | –58.08 to –31.62 | <0.00001 | 100 |
| Blood loss | 9 | 1,552 | –23.37 (MD) | –29.57 to –17.17 | <0.00001 | 99 |
| Incision size | 5 | 1,083 | –47.04 (MD) | –81.93 to –12.14 | 0.008 | 100 |
| Post-operative outcomes | ||||||
| Length of hospital stay | 9 | 1,401 | –4.19 (MD) | –5.46 to –2.92 | <0.00001 | 99 |
| Hospitalization expenses | 5 | 1,166 | –85.65 (MD) | –133.86 to –37.45 | 0.0005 | 100 |
| Follow-up outcomes | ||||||
| Complication rate | 12 | 2,023 | 0.23 (OR) | 0.16 to 0.33 | <0.00001 | 0 |
| Recurrence rate | 8 | 1,670 | 0.8 (OR) | 0.37 to 1.77 | 0.59 | 0 |
| Lymph node metastasis | 8 | 1,659 | 0.71 (OR) | 0.26 to 1.95 | 0.51 | 0 |
MD, mean difference; OR, odds ratio; CI, confidence interval.
Intra-operative outcomes
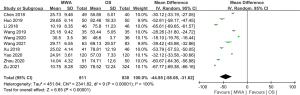
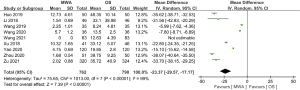
Of the 13 studies, 10 (comprising 1,641 patients) examined operation time. The MWA group had a significantly shorter operation time than the OS group (MD =–44.85, 95% CI: –58.08 to –31.62; P<0.00001, random-effects model; see Figure 3). Of the 13 studies, 9 analyzed blood loss. The pooled results showed that the MWA group had significantly less blood loss than the OS group (MD =–23.37, 95% CI: –29.57 to –17.17; P<0.00001, random-effects model; see Figure 4). Of the 13 studies, 5 (comprising 1,083 patients) reported on incision size. Overall, the pooled estimate showed that the incision size of the MWA group was significantly less than that of the OS group (MD =–47.04, 95% CI: –81.93 to –12.14; P=0.008, random-effects model; see Figure 5).

Post-operative outcomes
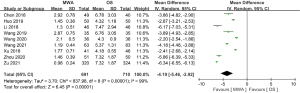
Of the 13 studies, 9 (comprising 1,401 patients) evaluated the length of hospital stay. The pooled result showed MWA had a significant beneficial effect in decreasing patients’ length of hospital stay (MD =–4.19, 95% CI: –5.46 to –2.92; P<0.00001, random-effects model; see Figure 6). Of the 13 studies, 5 assessed hospitalization expenses. The pooled data revealed that the hospitalization expenses of the MWA group were significantly less than those of the OS group (MD =–85.65, 95% CI: –133.86 to –37.45; P=0.0005, random-effects model; see Figure 7).
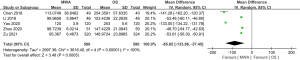
Follow-up outcomes
Of the 13 studies, 12 (comprising 2,023 patients) examined complications. The complication rate of the MWA group was significantly decreased compared to that of the OS group (OR =0.23, 95% CI: 0.16 to 0.33; P<0.00001, fixed-effects model; see Figure 8). Of the 13 studies, 8 (comprising 1670 patients), examined the recurrence rate, but no significant difference was found between the MWA group and the OS group (OR =0.80, 95% CI: 0.37 to 1.77; P=0.59, fixed-effects model; see Figure 9). Similarly, a meta-analysis of the differences in lymph node metastasis between the 2 groups was conducted, but no significant difference was found (OR =0.71, 95% CI: 0.26 to 1.95; P=0.51, fixed-effects model; see Figure 10).
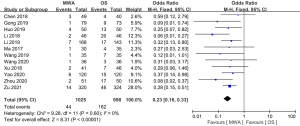
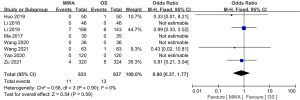
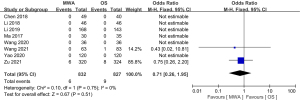
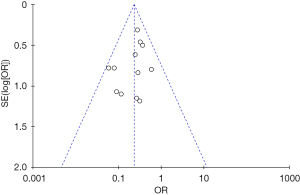
Results of publication bias
If >10 studies were included in the meta-analysis, the data were evaluated for publication bias. Thus, we examined the funnel plot for the complication rate. As Figure 11 showed, the plot was approximately symmetrical, and Egger’s test also revealed no significant publication bias (P=0.718).
Discussion
Traditional thyroidectomy is a mature technique used in the treatment of PTMC, and the effect is accurate, but due to the need for an incision of 6–8 cm, the injury is relatively large and bleeding is relatively high (25,26). Additionally, hematomas can compress the trachea and esophagus, and even cause suffocation, the neck flap, muscles, nerves, and blood vessels can be greatly damaged during the operation, normal blood and lymphatic reflux can be destroyed, and parathyroid-gland damage can cause complications, such as hypocalcemia convulsions (27,28). With the improvement of modern minimally invasive technology, MWA technology has been applied in the clinical treatment of various benign and malignant tumors, such as those in the liver, lungs, and kidneys (29-31). This minimally invasive treatment, which has a number of advantages, including less adverse reactions, a shorter operation time, less intra-operative blood loss, and a shorter postoperative hospital stay, is increasingly being used in the treatment of thyroid surgery, and its clinical treatment has been widely recognized (32,33).
In this study, a meta-analysis was conducted of 13 studies to compare the efficacy of MWA and OS in the treatment of PTMC. Compared to the OS group, the MWA group had a shorter operation time (MD =–44.85, 95% CI: 5.73 to 20.68; P<0.00001), less intra-operative blood loss (MD =–23.37, 95% CI: –29.57 to –17.17; P<0.00001), a smaller surgical incision (MD =–47.04, 95% CI: –81.93 to –12.14; P=0.008), a shorter postoperative hospital stay (MD =–4.19, 95% CI: –5.46 to –2.92; P<0.00001), lower hospitalization expenses (MD =–85.65, 95% CI: –133.86 to –37.45; P=0.0005), and fewer complications (OR =0.23, 95% CI: 0.16 to 0.33; P<0.00001), and the differences were statistically significant. However, there was no significant difference in the incidence of recurrence (OR =0.80, 95% CI: 0.37 to 1.77; P=0.59) or lymph node metastasis (OR =0.71, 95% CI: 0.26 to 1.95; P=0.51) between the 2 treatment methods during the follow-up period. The above results showed that MWA has a good effect on PTMC.
MWA uses electrodes to generate microwaves. The polar molecules in the tissue move at high speed in the microwave field and generate heat by friction. The temperature of the tissue increases under the action of thermal energy, and is followed by dehydration, protein denaturation, and irreversible necrosis (34,35). Finally, under the immune phagocytosis of the body, the necrotic tissue slowly shrinks and disappears, which achieves the purpose of eliminating local tumors. Ablation only causes damage to a part of the normal thyroid tissue and does not affect thyroid function. Thus, the long-term use of thyroid hormone replacement drugs can be avoided, and it has little effect on patients’ quality of life (36). The ablation is generally performed under local anesthesia, and due to precise positioning by ultrasound, the location, size, texture, and blood supply of the tumor nodules can be comprehensively explored (37). Additionally, by reducing the risk of tissue damage around the thyroid gland, such as the esophagus, trachea, and parathyroid glands, and protecting the surrounding tissue by the intraoperative fluid barrier, the treatment effect is enhanced and the risk of complications is reduced. Compared to OS, MWA has obvious advantages, such as its use of general anesthesia and a larger incision, especially for patients at high surgical risk, with scar constitution, a short lifespan, or those who need surgery as soon as possible (38).
In the included studies, the most common complications in the MWA group were transient hoarseness or choking after drinking water, which mainly occurred because the target nodule ablation was close to the dorsal capsule, the “liquid barrier” was insufficient, or during the puncture process (39). If the gland and surrounding tissue are squeezed or the ablation time is too long, the “liquid isolation zone” shrinks, and thermal damage leads to nerve damage. Additionally, the lever prying method requires the operator to have extensive practical experience. If the timing is not good for lever prying, it is easy to damage the nerve or cause residual tumor (40). In the OS group, complications may have been reduced due to advances in surgical techniques, including the use of intraoperative neuromonitoring. Additionally, no patients in either group experienced life-threatening complications, such as postoperative bleeding, laryngeal edema, or dyspnea (41).
As awareness of thyroid cancer increases, it is hoped that in the future, the biological behavior of indolent PTC will be screened as ablation indications, and ablations will be able to be performed with certainty (42). It has been reported that central lymph node metastases does not affect the prognosis of PTMC patients. However, the use of MWA to treat patients with potential central lymph node metastases should be considered with caution, and patients suitability should be strictly controlled (43). To avoid the excessive pursuit of minimally invasive surgery, the preoperative screening of non-invasive PTMC is key; however, the questions of how to identify non-invasive PTMC patients and accurately evaluate preoperative lymph nodes require further study (44).
This study had a number of limitations. First, MWA treatment of thyroid cancer is currently uncommon, and various studies on MWA were only in the explorative stage. Second, the included studies were all studies that had been conducted in the past 5 years; thus, the long-term effects of MWA are not yet known, especially the long-term recurrence rate and postoperative cancer metastasis rate. Finally, the included studies were all non-randomized clinical trials, and the lack of randomized, double-blind, large-sample randomized controlled trial data may have reduced the quality of the present study.
Conclusions
In conclusion, compared to OS, the use of MWA in the treatment of PTMC has the advantages of producing less trauma, a faster recovery, and fewer complications. The efficacy of MWA is similar to that of OS, and its clinical application value is high. However, the current follow-up time of studies is short, and the MWA technique may still have uncertain hidden dangers, such as tumor recurrence and lymph node metastasis. Long-term follow-up studies with large samples need to be conducted to further confirm our results, and the suitability of MWA for patients needs to be further evaluated.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-243/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-243/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feng K, Liu Y, Xu LJ, et al. Long noncoding RNA PVT1 enhances the viability and invasion of papillary thyroid carcinoma cells by functioning as ceRNA of microRNA-30a through mediating expression of insulin like growth factor 1 receptor. Biomed Pharmacother 2018;104:686-98. [Crossref] [PubMed]
- Yan J, Qiu T, Lu J, et al. Microwave ablation induces a lower systemic stress response in patients than open surgery for treatment of benign thyroid nodules. Int J Hyperthermia 2018;34:606-10. [Crossref] [PubMed]
- Alharbi SF, Abba AA, Hafiz AA, et al. A case of papillary hyperplasia of thyroid misdiagnosed as papillary thyroid carcinoma: Case report and review of literature. Clin Case Rep 2021;9:e04867. [Crossref] [PubMed]
- Lloret Linares C, Troisvallets D, Sellier P, et al. Micrometastasis of papillary thyroid carcinoma in a human immunodeficiency virus-infected patient: a case report and discussion. Med Oncol 2010;27:756-9. [Crossref] [PubMed]
- Kim NH, Beak SK, Baik SH, et al. A patient with micropapillary thyroid carcinoma and macronodular lung metastasis: stable disease for eight years without treatment. Thyroid 2009;19:309-11. [Crossref] [PubMed]
- Singh A, Butuc R, Lopez R. Metastatic papillary thyroid carcinoma with absence of tumor focus in thyroid gland. Am J Case Rep 2013;14:73-5. [Crossref] [PubMed]
- Moreland AJ, Ziemlewicz TJ, Best SL, et al. High-powered microwave ablation of t1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol 2014;28:1046-52. [Crossref] [PubMed]
- Maetani I, Ukita T, Inoue H, et al. Transpapillary microwave coagulation therapy for recanalizing self-expandable metallic stents occluded by tumor ingrowth: initial experience. Endoscopy 2001;33:719-23. [Crossref] [PubMed]
- Chernock RD, El-Mofty SK, Becker N, et al. Napsin A expression in anaplastic, poorly differentiated, and micropapillary pattern thyroid carcinomas. Am J Surg Pathol 2013;37:1215-22. [Crossref] [PubMed]
- Tong Y, Sun P, Yong J, et al. Radiogenomic Analysis of Papillary Thyroid Carcinoma for Prediction of Cervical Lymph Node Metastasis: A Preliminary Study. Front Oncol 2021;11:682998. [Crossref] [PubMed]
- Han ZY, Dou JP, Cheng ZG, et al. Efficacy and safety of percutaneous ultrasound-guided microwave ablation for cervical metastatic lymph nodes from papillary thyroid carcinoma. Int J Hyperthermia 2020;37:971-5. [Crossref] [PubMed]
- Ma F, Huang P, Qi R, et al. Study on Ultrasound-guided Percutaneous Microwave Ablation and Surgical Resection for Papillary Thyroid Microcarcinoma. Chin J Ultrasound Med 2017;33:399-402.
- Xu B, Zhou NM, Cao WT, et al. Comparative study on operative trauma between microwave ablation and surgical treatment for papillary thyroid microcarcinoma. World J Clin Cases 2018;6:936-43. [Crossref] [PubMed]
- Chen H, Zhao C, Huang P. Microwave ablation and surgical resection of papillary thyroid microcarcinoma: comparative analysis of clinical efficacy, safety and economy. Chin J Ultrasound Med 2018;15:275-9.
- Li J, Liu Y, Liu J, et al. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia 2018;34:653-9. [Crossref] [PubMed]
- Li J, Liu Y, Liu J, et al. A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia 2019;36:640-6. [Crossref] [PubMed]
- Wang Y. Analysis of ultrasound-guided microwave ablation in the treatment of thyroid micro papillary carcinoma and surgical resection. Guide Chin Med 2019;17:116-7.
- Gong H, Liu W, Yao Z. Ultrasound-guided microwave ablation, radiofrequency ablation and surgical resection in the treatment of thyroid micropapilla carcinoma. Clin rehab cancer Chin 2019;26:781-4.
- Huo Q, Zhou N, Cao W, et al. Ultrasound-guided MWA in the treatment of TMC and its effect on thyroid hormones. Southwest Med National Defense 2019;29:146-8.
- Wang Z. Clinical efficacy and safety of ultrasound-guided percutaneous microwave ablation and surgical resection in the treatment of thyroid micro papillary carcinoma. J Med Theor & Prac 2020;33:1788-90.
- Yao L, Wang J, Wu J. Effect of ultrasound-guided microwave ablation versus surgery in treatment of thyroid papillary microcarcinoma. J Clin Med Prac 2020;24:40-4.
- Zhou H, Li G, Chen Y. Ultrasound-guided percutaneous microwave ablation for papillary thyroid microcarcinoma. Modern Med J 2020;48:373-8.
- Zu Y, Liu Y, Zhao J, et al. A cohort study of microwave ablation and surgery for low-risk papillary thyroid microcarcinoma. Int J Hyperthermia 2021;38:1548-57. [Crossref] [PubMed]
- Wang X, Niu X, Mu S, et al. Analysis and evaluation of the efficacy of ultrasound-guided microwave ablation for papillary thyroid microcarcinoma. Int J Hyperthermia 2021;38:1476-85. [Crossref] [PubMed]
- Lee JK, Choi JU, Oh SE, et al. Clinical Characteristics of Thyroid Micropapillary Carcinoma. Acoust Res Lett 2009;52:679.
- Gao R, Jia X, Liang Y, et al. Papillary Thyroid Micro Carcinoma: The Incidence of High-Risk Features and Its Prognostic Implications. Front Endocrinol (Lausanne) 2019;10:74. [Crossref] [PubMed]
- Hou J, Zhang Y, Fan Y, et al. Risk factors of skip lateral lymph node metastasis in papillary thyroid carcinoma. Eur Arch Otorhinolaryngol 2021;278:493-8. [Crossref] [PubMed]
- Rui ZY, Liu Y, Zheng W, et al. A retrospective study of the risk factors and the prognosis in patients with papillary thyroid carcinoma depending on the number of lymph node metastasis. Clin Exp Med 2021;21:277-86. [Crossref] [PubMed]
- Gillams A. Tumour ablation: current role in the liver, kidney, lung and bone. Cancer Imaging 2008;9 Spec No A:S68-S70.
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38:135-43. [Crossref] [PubMed]
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol 2013;82:e11-6. [Crossref] [PubMed]
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol 2018;144:771-9. [Crossref] [PubMed]
- Mohamad Yusof A, Jamal R, Muhammad R, et al. Integrated Characterization of MicroRNA and mRNA Transcriptome in Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne) 2018;9:158. [Crossref] [PubMed]
- Zhou YL, Gao EL, Zhang W, et al. Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J Surg Oncol 2012;10:67. [Crossref] [PubMed]
- Yue W, Chen L, Wang S, et al. Locoregional control of recurrent papillary thyroid carcinoma by ultrasound-guided percutaneous microwave ablation: A prospective study. Int J Hyperthermia 2015;31:403-8. [Crossref] [PubMed]
- Zhou W, Chen Y, Zhang L, et al. Percutaneous Microwave Ablation of Metastatic Lymph Nodes from Papillary Thyroid Carcinoma: Preliminary Results. World J Surg 2019;43:1029-37. [Crossref] [PubMed]
- Yang Z, Yin L, Zeng Y, et al. Diagnostic and prognostic value of tumor-infiltrating B cells in lymph node metastases of papillary thyroid carcinoma. Virchows Arch 2021;479:947-59. [Crossref] [PubMed]
- Shen Y, Cai XY, Dong JN, et al. Comparative study of ultrasound-guided percutaneous microwave ablation and endoscopic thyroidectomy for thyroid papillary microcarcinoma. J Chin Clin Med Imag 2019;25:18-22.
- Kang YY, Li JJ, Sun JX, et al. Genome-wide scanning for CHD1L gene in papillary thyroid carcinoma complicated with type 2 diabetes mellitus. Clin Transl Oncol 2021;23:2536-47. [Crossref] [PubMed]
- Bohinc BN, Parker JC, Hope WW, et al. Micropapillary thyroid carcinoma and concomitant ectopic thyroid tissue in the adrenal gland: metastasis or metaplasia? Thyroid 2011;21:1033-8. [Crossref] [PubMed]
- Dettmer M, Perren A, Moch H, et al. Comprehensive MicroRNA expression profiling identifies novel markers in follicular variant of papillary thyroid carcinoma. Thyroid 2013;23:1383-9. [Crossref] [PubMed]
- Lino-Silva LS, Domínguez-Malagón HR, Caro-Sánchez CH, et al. Thyroid gland papillary carcinomas with "micropapillary pattern," a recently recognized poor prognostic finding: clinicopathologic and survival analysis of 7 cases. Hum Pathol 2012;43:1596-600. [Crossref] [PubMed]
- Cui T, Jin C, Jiao D, et al. Safety and efficacy of microwave ablation for benign thyroid nodules and papillary thyroid microcarcinomas: A systematic review and meta-analysis. Eur J Radiol 2019;118:58-64. [Crossref] [PubMed]
- Shen K, Xue S, Xie Y, et al. Comparison of thermal ablation and routine surgery for the treatment of papillary thyroid microcarcinoma: a systematic review and Meta-analysis. Int J Hyperthermia 2020;37:913-24. [Crossref] [PubMed]



