
Surgical management and prognosis of phyllodes tumors of the breast
Introduction
Phyllodes tumors (PTs), rare fibroepithelial tumors of the breast, comprise 0.3–1% of all breast tumors and 2–3% of all fibroepithelial breast lesions (1,2). PTs are classified as benign (Grade 1), borderline (Grade 2), or malignant (Grade 3) by the World Health Organization (WHO), according to histological features such as mitotic activity, the degree of stromal cellular atypia, infiltrative tumor margins and stromal overgrowth (3).
Regardless of histological grade, PTs exhibit the potential of local recurrence (4-6). Recurrence occurs relatively common in borderline and malignant PTs while local recurrence (LR) and distant metastasis are rare in benign PTs (2,7). Therefore, based on retrospective data, the National Comprehensive Cancer Network (NCCN) recommends wide local excision (WLE) with a 1 cm margin or more for borderline/malignant PTs but excisional biopsy for benign PTs (8).
However, a lot of published studies and current guidelines have recommended removal of tumors with 1 cm clear margins or even mastectomy (8-10), while several studies questioned the necessity for such large margins in benign phyllodes tumors (BPT), suggesting that surgical margins less than 1 cm are sufficient, with no difference in local or distant recurrence (11-14). BPT are often indistinguishable from fibroadenomas (FAs) in both clinical and histologic manifestations “by core needle biopsy” (15), so they are often not diagnosed preoperatively and may be treated without adequate margins at the initial intervention (16-18). Because of the risks of a second surgery and potentially poorer cosmetic outcomes, surgeons and patients are faced with the dilemmas: after the vacuum-assisted biopsy system (VABS) or local excision (LE) for complete removal of BTP, could we consider a ‘‘wait-and-watch’’ policy as NCCN recommended, instead of re-excision to obtain wide safe margins (19-21).
The aim of our study is to examine the surgical management and prognosis of 238 patients with PTs, and to identify the clinicopathologic risk factors for LR (type of surgical modalities and tumor size), so as to provide optimal treatment for PT patients to avoid LR. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-21-877/rc).
Methods
Patient enrollment
All cases given a diagnosis of PTs and resected from January 1, 2006 and April 30, 2020 at the First Medical Center of Chinese PLA General Hospital, were retrospectively evaluated. The PTs were classified as benign, borderline, or malignant according to the WHO (World Health Organization) guidelines. Surgical treatment was categorized as VABS, LE, WLE, or mastectomy. The study excluded patients with breast cancer, including carcinoma in situ and invasive breast cancer, or a previous breast cancer history and unavailable follow-up data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Chinese PLA General Hospital (No. S2019-152-01) and individual consent for this retrospective analysis was waived.
Clinical data evaluation
The clinicopathologic data and clinical outcomes that were evaluated for each case from outpatient and medical records included age, history of fibroadenoma, type of surgery, tumor size, histological characteristics, tumor recurrence, distant metastasis, and patient survival. Tumor size was grouped according to the American Joint Committee on Cancer TNM (Tumor, Node and Metastasis) staging system categories (T-stage, T1, T2 and T3). Surgical procedures were defined as follows (12,22): (I) VABS: the targeted lesion was excised by the 7G probe (EnCor, SenoRx, Aliso Viejo, CA) until no residual lesions could be detected by ultrasound. (II) LE: tumor excision with the margin of normal breast tissue no more than 1 cm, including excision, lumpectomy and simple excision. (III) WLE: tumor excision with the margin of normal breast tissue more than 1.0 cm, including wide excision, partial mastectomy and breast-conserving surgery (BCS). (IV) Mastectomy: complete removal of breast tissue, including simple mastectomy, total mastectomy, radical mastectomy, or modified radical mastectomy. All the pathological results of this study were independently reviewed by two experienced pathologists.
Follow-up and relapse events
The patients were scheduled for a follow-up assessment at 1 month, at 3 months, at 6 months and every 12 months after the procedure by outpatient and medical records or telephone communication with the patients or their families. Follow-up contents included local recurrence and distant metastasis, survival, and surgical satisfaction, etc. Recurrence cases include the cases of local recurrence and distant metastasis. The number of cases in the hospital during the study period determined the sample size. Patients were censored at last follow-up if still no recurrence or lost to follow-up before November 30, 2020. Censored data are indicated in the figures by vertical ticks.
Statistical analyses
Data were analyzed on IBM SPSS Statistics 23.0 (SPSS Inc., Chicago, IL, USA). Student’s t-test was used for continuous variables to assess statistical significance. χ2 test and Fisher’s exact test were used for categorical variables, as appropriate. χ2 test and Fisher’s exact test were used to determine the correlations among the variables. Kaplan-Meier survival curves and log-rank statistics were used for evaluating disease free survival time (DFS). Multivariate regression analysis was performed using the Cox proportional hazards model. P values <0.05 were considered statistically significant.
Result
Clinicopathologic features
According to the inclusion and exclusion criteria, a total of 238 eligible patients were screened out. Table 1 presents the clinicopathologic characteristics of 238 patients included in this study. 171 (71.8%) were classified as benign, 38 (16.0%) as borderline, and 29 (12.2%) as malignant. The mean follow-up was 50.2 months and ranged from 3 to 107 months. The age of the patients ranged from 11 to 72 years, with a mean of 40 years. The mean greatest dimension of the tumors was 3.7 cm, with a median of 2.8 cm. The greatest dimension of the tumors was significantly correlated with the histological grade (P<0.01): BPT were significantly smaller than borderline and malignant tumors. 113 patients (47.5%) had right-sided lesions, 121 (50.8%) had left-sided lesions, and 4 (1.7%) had bilateral PTs. The majority of tumors occurred in the upper outer quadrant (53.9%) of the breast. Also, 33 patients had a previous history of fibroadenoma. Patients with a history of fibroadenoma are more likely to develop borderline or malignant PTs (P<0.01). 55 patients (23.1%) experienced LR and 4 patients (1.7%) occurred distant metastasis simultaneously. The local recurrence rate was lower for BPT (14.6%) compared with borderline tumors (34.2%) and malignant tumors (44.8%), which showed a statistically significant difference (P<0.01).
Table 1
| Variables | Total | Benign | Borderline | Malignant | P value |
|---|---|---|---|---|---|
| Cases (%) | 238 (100.0) | 171 (71.8) | 38 (16.0) | 29 (12.2) | |
| Age (years) | 0.020 | ||||
| Mean | 40.3±12.5 | 39.0±11.7 | 45.2±13.4 | 41.1±14.0 | |
| Range | 11–73 | 11–65 | 13–73 | 17–65 | |
| Follow-up (months) | 0.103 | ||||
| Mean | 50.20±49.0 | 48.6±48.0 | 55.0±26.2 | 57.9±54.5 | |
| Range | 3.0–107.0 | 3.0–107.0 | 16.0–103.0 | 8.0–105.0 | |
| Maximum tumor diameter [M(QR), cm] | 2.8 (1.9) | 2.5 (1.5) | 4.0 (3.2) | 5.0 (3.5) | <0.01 |
| Tumor size (cm) | |||||
| T1 | 52 (21.8) | 49 (28.7) | 3 (7.9) | 0 (0.0) | |
| T2 | 149 (62.6) | 109 (63.7) | 22 (57.9) | 18 (62.1) | |
| T3 | 37 (15.6) | 13 (7.6) | 13 (34.2) | 11 (37.9) | |
| Location, n (%) | 0.244 | ||||
| Left | 121 (50.8) | 82 (48.0) | 25 (65.8) | 14 (48.3) | |
| Right | 113 (47.5) | 86 (50.3) | 13 (34.2) | 14 (48.3) | |
| Bilateral | 4 (1.7) | 3 (1.7) | 0 (0.0) | 1 (3.4) | |
| History of fibroadenoma, n (%) | <0.01 | ||||
| No | 205 (86.1) | 156 (91.2) | 27 (71.0) | 22 (75.9) | |
| Yes | 33 (13.9) | 15 (8.8) | 11 (29.0) | 7 (24.1) | |
| Type of surgery, n (%) | <0.01 | ||||
| VABS | 87 (36.6) | 87 (50.9) | 0 (0.0) | 0 (0.0) | |
| LE | 60 (25.2) | 41 (24.0) | 12 (31.6) | 7 (24.1) | |
| WLE | 65 (27.3) | 35 (20.5) | 18 (47.4) | 12 (41.4) | |
| Mastectomy | 26 (10.9) | 8 (4.6) | 8 (21.0) | 10 (24.5) | |
| AND, n (%) | <0.01 | ||||
| No | 236 (99.2) | 171 (100.0) | 38 (100.0) | 27 (93.1) | |
| Yes | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (6.9) | |
| Bilateral, n (%) | 0.04 | ||||
| No | 234 (98.3) | 168 (98.2) | 38 (100.0) | 28 (96.6) | |
| Yes | 4 (1.7) | 3 (1.8) | 0 (0.0) | 1 (3.4) | |
| Borders, n (%) | 0.004 | ||||
| Circumscribed/pushing | 226 (95.0) | 167 (97.7) | 32 (84.2) | 27 (93.1) | |
| Infiltrative | 12 (5.0) | 4 (2.3) | 6 (15.8) | 2 (6.9) | |
| Mitotic activity, n (%) | <0.01 | ||||
| 0–4/10 HPF | 88 (62.9) | 85 (86.8) | 3 (10.3) | 0 (0.0) | |
| 5–9/10 HPF | 34 (24.3) | 12 (12.2) | 21 (74.4) | 1 (7.7) | |
| ≥10/10 HPF | 18 (12.8) | 1 (1.0) | 5 (17.2) | 12 (72.3) | |
| Intra-tumoral necrosis, n (%) | 0.001 | ||||
| No | 231 (97.1) | 170 (99.4) | 36 (94.7) | 25 (86.2) | |
| Yes | 7 (2.9) | 1 (0.6) | 2 (5.3) | 4 (13.8) | |
| Recurrence, n (%) | 0.002 | ||||
| No | 183 (76.9) | 146 (85.4) | 25 (65.8) | 12 (41.4) | |
| Yes | 55 (23.1) | 25 (14.6) | 13 (34.2) | 17 (58.6) | |
| Local recurrence, n (%) | <0.01 | ||||
| No | 187 (78.6) | 146 (85.4) | 25 (65.8) | 16 (55.2) | |
| Yes | 51 (21.4) | 25 (14.6) | 13 (34.2) | 13 (44.8) | |
| Distant metastasis, n (%) | <0.01 | ||||
| No | 234 (98.3) | 171 (100.0) | 38 (100.0) | 25 (86.2) | |
| Yes | 4 (1.7) | 0 (0.0) | 0 (0.0) | 4 (13.8) | |
| Death, n (%) | <0.01 | ||||
| No | 234 (98.3) | 171 (100.0) | 38 (100.0) | 25 (86.2) | |
| Yes | 4 (1.7) | 0 (0.0) | 0 (0.0) | 4 (13.8) |
VABS, vacuum-assisted biopsy system; LE, local excision; WLE, wide local excision; AND, axillary node dissection.
Pathologic features
Infiltration tumor borders were found in 12 patients (5.0 %) and pushing borders were found in 226 patients (95.0%). The tumor borders, mitotic activity and intra-tumoral necrosis all showed significant association with the histological grade (P<0.05). Malignant PTs were more likely to perform infiltrative borders, greater mitotic activity and intra-tumoral necrosis (Table 1).
Follow-up and recurrence
Age was not a significant risk factor for LR (χ2=1.069, P=0.301) or DFS. The statistically significant risk factors for LR and DFS of PTs included tumor size (χ2=8.459, P=0.015), the histological grade (χ2=39.625, P<0.01) and the history of fibroadenoma (χ2=35.500, P<0.01) in univariate analysis (Table 2 and Figure 1). Operative procedures didn't influence the recurrence. 87 (36.6%) patients were treated with VABS without other surgical treatments, and 15 (17.2%) had a recurrence. Of 60 (25.2%) patients undergoing LE, 20 (33.3%) had a recurrence; 65 (27.3%) were managed with WLE, and 13 (20.0%) had a recurrence. 26 (10.9%) patients had a mastectomy, and 26.9% had a recurrence. The association between surgical procedures and recurrence was not statistically significant (χ2=5.754, P=0.124). Cox regression model was further used for survival analysis. In multivariate analysis, histologic grade (P<0.01) and history of fibroadenoma (P<0.01) were independent prognostic factors for LR (Table 3). A significantly lower DFS for the malignant than for the benign grade (OR 3.990; 95% CI: 2.006–7.937). Patients who had the history of fibroadenoma often occurred poor prognosis than those who had no fibroadenoma (OR 3.530; 95% CI: 1.995–6.247). Likewise, tumor size of T3 and T2 and had a greater influence for DFS than that of T1 (OR 1.975; 95% CI: 0.659–5.921 and OR 1.697; 95% CI: 0.678–4.250, respectively).
Table 2
| Variables | Total | Recurrence, n (%) | 5-year DFS estimates | χ² | P value |
|---|---|---|---|---|---|
| Age (years) | 1.069 | 0.301 | |||
| ≤40 | 111 | 22 (19.8) | 0.790 | ||
| >40 | 127 | 33 (26.0) | 0.720 | ||
| Size | 8.459 | 0.015 | |||
| T1 | 52 | 6 (11.5) | 0.883 | ||
| T2 | 149 | 35 (23.5) | 0.744 | ||
| T3 | 37 | 14 (37.8) | 0.612 | ||
| History of fibroadenoma | 35.500 | <0.01 | |||
| No | 199 | 33 (16.6) | 0.432 | ||
| Yes | 39 | 22 (56.4) | 0.816 | ||
| Surgery | 5.754 | 0.124 | |||
| VABS | 87 | 15 (17.2) | 0.848 | ||
| LE | 60 | 20 (33.3) | 0.635 | ||
| WLE | 65 | 13 (20.0) | 0.359 | ||
| Mastectomy | 26 | 7 (26.9) | 0.848 | ||
| Histologic grade | 39.625 | <0.01 | |||
| Benign | 171 | 25 (14.6) | 0.848 | ||
| Borderline | 38 | 13 (34.2) | 0.635 | ||
| Malignant | 29 | 17 (58.6) | 0.359 | ||
| Borders | 0.011 | 0.916 | |||
| Circumscribed/pushing | 226 | 52 (23.0) | 0.729 | ||
| Infiltrative | 12 | 3 (25.0) | 0.753 | ||
| Intra-tumoral necrosis | 2.436 | 0.119 | |||
| No | 231 | 52 (23.0) | 0.571 | ||
| Yes | 7 | 3 (25.0) | 0.757 |
DFS, disease-free survival; VABS, vacuum-assisted biopsy system; LE, local excision; WLE, wide local excision; T1, T2 and T3, T-stage from the American Joint Committee on Cancer TNM (Tumor, Node and Metastasis) staging system categories.
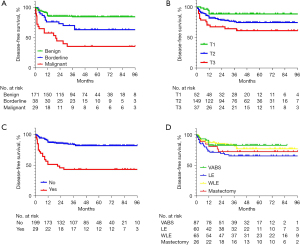
Table 3
| Variables | β value | Standard error | Wald value | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Size | 1.135 | 0.459 | |||
| T2 vs. T1 | 0.489 | 0.464 | 1.114 | 1.697 (0.678, 4.250) | 0.259 |
| T3 vs. T1 | 0.501 | 0.544 | 0.850 | 1.975 (0.659, 5.921) | 0.224 |
| History of fibroadenoma | |||||
| Yes vs. no | 1.261 | 0.291 | 18.759 | 3.530 (1.995, 6.247) | <0.01 |
| Histologic grade | 16.337 | <0.01 | |||
| Borderline vs. benign | 0.438 | 0.379 | 1.332 | 1.549 (0.737, 3.259) | 0.248 |
| Malignant vs. benign | 1.384 | 0.351 | 15.556 | 3.990 (2.006, 7.937) | <0.01 |
T1, T2 and T3, T-stage from the American Joint Committee on Cancer TNM (Tumor, Node and Metastasis) staging system categories.
The risk stratification for LR
The correlation between surgical procedures and LR according to subgrouping by tumor size and histologic grade is presented in Table 4. The greatest tumor diameter less than 3 cm accounted for 55.0% (131/238) and the recurrence rate was 15.3% (20/131). 45.0% (107/238) of PTs were larger than 3cm, and the recurrence rate was 23.4% (25/107). There was no significant difference in the recurrence rate between the two groups (P=0.113). For PTs ≤3 cm, the recurrence rates were 6.8%, 23.3%, 29.6%, 0% in the VABS group, LE group, the WLE group and the mastectomy group, respectively. For PTs >3 cm, the recurrence rates were 71.4%, 43.3%, 13.2%, 28.0% in each group, respectively. The differences between the two subgroups were statistically significant (P<0.01). For BPT, the LR rates in the VABS group were 17.2%, 17.0%, 5.7% and 12.5% in the LE group and the WLE group and the mastectomy group, respectively. Interestingly, the difference between groups was not statistically significant (P=0.397). That is, different surgical treatments had no influence on LR for BPT patients. However, for borderline/malignant PTs, the recurrence rates were 68.4%, 36.7% and 33.3% in the LE group, the WLE group and the mastectomy group, respectively, and the difference was statistically significant (P=0.049).
Table 4
| Variables | Surgery type | χ² | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VABS | % | LE | % | WLE | % | Mastectomy | % | |||
| Tumor size (cm) | 62.240 | <0.01 | ||||||||
| ≤3 | 73 | 6.8 (5/73) | 30 | 23.3 (7/30) | 27 | 29.6 (8/27) | 1 | 0 (0) | 10.461 | 0.010 |
| >3 | 14 | 71.4 (10/14) | 30 | 43.3 (13/30) | 38 | 13.2 (5/38) | 25 | 28.0 (7/25) | 17.928 | <0.01 |
| Histologic grade | 66.562 | <0.01 | ||||||||
| Benign | 87 | 17.2 (15/87) | 41 | 17 (7/41) | 35 | 5.7 (2/35) | 8 | 12.5 (1/8) | 2.29 | 0.397 |
| Borderline/malignant | 0 | 0 | 19 | 68.4 (13/19) | 30 | 36.7 (11/30) | 18 | 33.3 (6/18) | 6.047 | 0.049 |
VABS, vacuum-assisted biopsy system; LE, local excision; WLE, wide local excision.
Discussion
In the present study, we stratified patients according to histologic and surgical factors, and analyzed the clinicopathologic risk factors for the LR in 238 PTs. Previous literature reported that the rate of relapse for overall, benign, borderline, and malignant PT are 8–36%, 10–17%, 14–25% and 23–30%, respectively (3,12). The corresponding rates in our study were 23.1%, 14.6%, 34.2%, and 58.6%, and we observed a slightly higher rate of recurrence for borderline and malignant PT. The possible reason is that the number of patients with borderline/malignant PTs in this study was relatively small, and bias could not be avoided. Previous studies also demonstrated that the risk of LR was significantly increased from benign to borderline to malignant PTs (6,9,23,24), which are similar to our results. Patients who had the history of fibroadenoma often occurred LR than those who had no fibroadenoma (OR 3.798; 95% CI: 2.013–7.164). Hence, PTs patients with the history of fibroadenoma are advised for periodic reexamination and should be alert to the risk of secondary or even multiple recurrence of PTs. Considering that PTs and fibroadenoma both are breast fibroepithelial tumors, similarities in clinical presentation, imaging and tissue sampling, relevant studies suggest that there may be a partial correlation between them (25-27).
As we know, factors associated with local recurrence include surgical margins, tissue border, nuclear atypia and stromal overgrowth, number of tumors, histology grade and pleomorphism (28-30). In this study, local recurrence occurred in 55 cases (23.1%), most of which occurred during the first 3 years after initial treatment. The results of our study suggest that histologic grade (P<0.01), type of surgery (P=0.006) and history of fibroadenoma (P<0.01) were significant prognostic indicators for LR on multivariate analysis. To our surprise, the size of tumor was not associated with DFS (P=0.459) (Table 3). Jang, et al. reported that tumor size and surgical margin status was a major prognostic factor for local recurrence, whereas a systematic review and meta-analysis reported in 54 studies with 9,234 individual cases that mitoses, tumor border and necrosis, surgical margin status, stromal cellularity, stromal atypia, stromal overgrowth, and type of surgery may be risk factors for LR (23,24).
Surgery is the preferred treatment for PTs (31). However, the type and the extent of surgery remains controversial because the surgical margin may be associated with the LR of PTs. A lot of published studies and current guidelines have recommended tumors removal with 1 cm clear margins or even mastectomy (8-10). However, some studies reported no increase of LR even with positive surgical margin (32,33). A meta-analysis of 13 studies suggested a clear association between LR rates and width of margins. Regardless of the tumor grade, surgical margins ≥10 mm showed a lower risk of LR than margins <10 mm (2). Interestingly, another review found no difference between 1 and 10 mm margins in recurrence rate and suggested that 1 mm margins were acceptable for BPT (34). In addition, most of studies discussed the effect of open surgery and margin status on recurrence rates, while few studies have been conducted on the treatment of BPT with VABS (35). Therefore, apart from the 151 patients who underwent conventional open surgery for patients, this study also included a total of 87 patients treated with VABS and then explored the influence of the different management strategies on different PT grades and tumor size.
VABS is a minimally invasive procedure that can remove lesions under ultrasonography guidance, without re-aim or re-insertion. As we know, VABS is not only a diagnostic biopsy method, but also an alternative treatment for small benign breast diseases (36-38). However, given the rates of misdiagnose and residual tumor, PTs diagnosed after VABS should be surgically excised (39). Previous studies demonstrated that no difference existed in the recurrence-free survival of patients treated with VABS and open surgery (20,21,40). Our study also found no association between surgery methods and local recurrence rate for BPT (P=0.397), but a higher recurrence rate in VABS and LE subgroups than in WLE and mastectomy subgroups for borderline/malignant tumors (P<0.01). In the subgroup with the maximum diameter of the lesion less than or equal to 3 cm, VABS group had the lowest recurrence rate (6.8%) than LE (23.3%) and WLE (29.6%) (P=0.010). On the contrary, in the subgroup with the maximum diameter greater than 3 cm, VABS group had the highest recurrence rate (71.4%) than LE (43.3%), WLE (13.2%) and mastectomy groups (P<0.01). Therefore, we suggest that VABS or LE can be treated for BPT with small mass (<3 cm), whereas WLE or even mastectomy should be conducted for borderline/malignant PTs with large mass (>3 cm), just as recommended as current guidelines.
Interestingly, it should be noted that the 87 patients included in this study who received VABS were not diagnosed as BPT preoperatively, resulting in unclear surgical margin status (positive or negative). Previous studies also demonstrated that surgical margins have no correlation with recurrence rate in BPT (41-43). These results are similar to our own: the local recurrence rates of each surgery group are 17.2%, 17.0%, 5.7% and 12.5%, respectively (P=0.397). Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions) also recommended VABS treatment for the lesion which lacks high-risk cytological features, although more prolonged observation is necessary (44,45). Thus, VABS is an effective and safe biopsy and treatment procedure for BPT. These results suggested a “wait-and-watch” policy for patients with unexpected benign subtypes, instead of unnecessary re-excision, leading to an unsatisfied breast volume deficit and undue breast deformity. For broadline/malignant PTs, the vast majority of authors performed a wide excision with margins at least 10 mm to reduce the recurrence rate of LR (6,12,46-48). The role of radiation therapy (RT) as an adjuvant method for local control remains controversial (12,49-51). Zeng et al. showed that adjuvant RT for borderline/malignant PT decreased the LR rate in patients undergoing BCS (50). However, Boutrus et al. showed that RT was not suitable for BPT but may improve local recurrence free survival for borderline/malignant PT (52). We did not assess RT as a risk factor due to the limited data and more data are needed for further exploration of this issue.
Our study does have several potential limitations. First, this was a single-center, retrospective study, resulting in inevitable inherent bias. For example, the patients who received VABS and LE were more likely to have smaller tumors. Even when we considered these factors in our multivariate analysis, the study still included other factors that could not be adjusted for, such as breast density, family history, and socioeconomic factors and physicians’ subjective factors. Second, surgical margin status (positive or negative) and margin length (1 mm, 10 mm or >10 mm) was unclear. Hence, recurrent and residual PTs are difficult to differentiate for patients of VABS and LE group. Third, a further study with a larger sample size and longer follow-up is necessary.
Conclusions
Histologic grade, type of surgery and history of fibroadenoma were independent prognostic factors for LR while tumor size had no significance for LR in multivariate analysis. Given that our data suggest no association between surgery methods and local recurrence rate for BPT, we suggested a “wait-and-watch” policy for patients with unexpected benign subtypes, instead of unnecessary re-excision. In addition, VABS or LE can be treated for BPT with small mass, whereas WLE or even mastectomy should be conducted for borderline/malignant PTs with large mass.
Acknowledgments
Funding: This work was supported by the Project of Big Data of PLA General Hospital (grant No. 2018MBD-017).
Footnote
Reporting Checklist: The authors have completed the STOBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-21-877/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-877/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-877/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-21-877/coif). XL serves as an Editor-in-Chief of Gland Surgery from May 2022 to April 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Chinese PLA General Hospital (No. S2019-152-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Y, Kleer CG. Phyllodes Tumor of the Breast: Histopathologic Features, Differential Diagnosis, and Molecular/Genetic Updates. Arch Pathol Lab Med 2016;140:665-71. [Crossref] [PubMed]
- Toussaint A, Piaget-Rossel R, Stormacq C, et al. Width of margins in phyllodes tumors of the breast: the controversy drags on?-a systematic review and meta-analysis. Breast Cancer Res Treat 2021;185:21-37. [Crossref] [PubMed]
- Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO classification of Tumours of the Breast, 4th edn. Lyon: IARC Press; 2012.
- Mangi AA, Smith BL, Gadd MA, et al. Surgical management of phyllodes tumors. Arch Surg 1999;134:487-92; discussion 492-3. [Crossref] [PubMed]
- Spitaleri G, Toesca A, Botteri E, et al. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol 2013;88:427-36. [Crossref] [PubMed]
- Wei J, Tan YT, Cai YC, et al. Predictive factors for the local recurrence and distant metastasis of phyllodes tumors of the breast: a retrospective analysis of 192 cases at a single center. Chin J Cancer 2014;33:492-500. [Crossref] [PubMed]
- Barrio AV, Clark BD, Goldberg JI, et al. Clinicopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol 2007;14:2961-70. [Crossref] [PubMed]
- Bevers TB, Helvie M, Bonaccio E, et al. Breast Cancer Screening and Diagnosis, Version 4.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:1362-89. [Crossref] [PubMed]
- Yom CK, Han W, Kim SW, et al. Reappraisal of conventional risk stratification for local recurrence based on clinical outcomes in 285 resected phyllodes tumors of the breast. Ann Surg Oncol 2015;22:2912-8. [Crossref] [PubMed]
- Bhargav PR, Mishra A, Agarwal G, et al. Phyllodes tumour of the breast: clinicopathological analysis of recurrent vs. non-recurrent cases. Asian J Surg 2009;32:224-8. [Crossref] [PubMed]
- Kim GE, Kim JH, Lee KH, et al. Stromal matrix metalloproteinase-14 expression correlates with the grade and biological behavior of mammary phyllodes tumors. Appl Immunohistochem Mol Morphol 2012;20:298-303. [Crossref] [PubMed]
- Kim S, Kim JY, Kim DH, et al. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat 2013;141:353-63. [Crossref] [PubMed]
- Sawalhi S, Al-Shatti M. Phyllodes tumor of the breast: a retrospective study of the impact of histopathological factors in local recurrence and distant metastasis. Ann Saudi Med 2013;33:162-8. [Crossref] [PubMed]
- Tremblay-LeMay R, Hogue JC, Provencher L, et al. How Wide Should Margins Be for Phyllodes Tumors of the Breast? Breast J 2017;23:315-22. [Crossref] [PubMed]
- Van Osdol AD, Landercasper J, Andersen JJ, et al. Determining whether excision of all fibroepithelial lesions of the breast is needed to exclude phyllodes tumor: upgrade rate of fibroepithelial lesions of the breast to phyllodes tumor. JAMA Surg 2014;149:1081-5. [Crossref] [PubMed]
- Kalambo M, Adrada BE, Adeyefa MM, et al. Phyllodes Tumor of the Breast: Ultrasound-Pathology Correlation. AJR Am J Roentgenol 2018;210:W173-9. [Crossref] [PubMed]
- Chao TC, Lo YF, Chen SC, et al. Sonographic features of phyllodes tumors of the breast. Ultrasound Obstet Gynecol 2002;20:64-71. [Crossref] [PubMed]
- Komenaka IK, El-Tamer M, Pile-Spellman E, et al. Core needle biopsy as a diagnostic tool to differentiate phyllodes tumor from fibroadenoma. Arch Surg 2003;138:987-90. [Crossref] [PubMed]
- Park HL, Pyo YC, Kim KY, et al. Recurrence Rates and Characteristics of Phyllodes Tumors Diagnosed by Ultrasound-guided Vacuum-assisted Breast Biopsy (VABB). Anticancer Res 2018;38:5481-7. [Crossref] [PubMed]
- Ouyang Q, Li S, Tan C, et al. Benign Phyllodes Tumor of the Breast Diagnosed After Ultrasound-Guided Vacuum-Assisted Biopsy: Surgical Excision or Wait-and-Watch? Ann Surg Oncol 2016;23:1129-34. [Crossref] [PubMed]
- Chao X, Jin X, Tan C, et al. Re-excision or "wait and watch"-a prediction model in breast phyllodes tumors after surgery. Ann Transl Med 2020;8:371. [Crossref] [PubMed]
- Chen WH, Cheng SP, Tzen CY, et al. Surgical treatment of phyllodes tumors of the breast: retrospective review of 172 cases. J Surg Oncol 2005;91:185-94. [Crossref] [PubMed]
- Lu Y, Chen Y, Zhu L, et al. Local Recurrence of Benign, Borderline, and Malignant Phyllodes Tumors of the Breast: A Systematic Review and Meta-analysis. Ann Surg Oncol 2019;26:1263-75. [Crossref] [PubMed]
- Jang JH, Choi MY, Lee SK, et al. Clinicopathologic risk factors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol 2012;19:2612-7. [Crossref] [PubMed]
- Tan J, Ong CK, Lim WK, et al. Genomic landscapes of breast fibroepithelial tumors. Nat Genet 2015;47:1341-5. [Crossref] [PubMed]
- Vidal M, Peg V, Galván P, et al. Gene expression-based classifications of fibroadenomas and phyllodes tumours of the breast. Mol Oncol 2015;9:1081-90. [Crossref] [PubMed]
- Ng CCY, Md Nasir ND, Loke BN, et al. Genetic differences between benign phyllodes tumors and fibroadenomas revealed through targeted next generation sequencing. Mod Pathol 2021;34:1320-32. [Crossref] [PubMed]
- Zhou ZR, Wang CC, Yang ZZ, et al. Phyllodes tumors of the breast: diagnosis, treatment and prognostic factors related to recurrence. J Thorac Dis 2016;8:3361-8. [Crossref] [PubMed]
- Zhou ZR, Wang CC, Sun XJ, et al. Prognostic factors in breast phyllodes tumors: a nomogram based on a retrospective cohort study of 404 patients. Cancer Med 2018;7:1030-42. [Crossref] [PubMed]
- Di Liso E, Bottosso M, Lo Mele M, et al. Prognostic factors in phyllodes tumours of the breast: retrospective study on 166 consecutive cases. ESMO Open 2020;5:e000843. [Crossref] [PubMed]
- Salvadori B, Cusumano F, Del Bo R, et al. Surgical treatment of phyllodes tumors of the breast. Cancer 1989;63:2532-6. [Crossref] [PubMed]
- Chng TW, Lee JY, Lee CS, et al. Validation of the Singapore nomogram for outcome prediction in breast phyllodes tumours: an Australian cohort. J Clin Pathol 2016;69:1124-6. [Crossref] [PubMed]
- Moo TA, Alabdulkareem H, Tam A, et al. Association Between Recurrence and Re-Excision for Close and Positive Margins Versus Observation in Patients with Benign Phyllodes Tumors. Ann Surg Oncol 2017;24:3088-92. [Crossref] [PubMed]
- Shaaban M, Barthelmes L. Benign phyllodes tumours of the breast: (Over) treatment of margins - A literature review. Eur J Surg Oncol 2017;43:1186-90. [Crossref] [PubMed]
- Park HL, Kwon SH, Chang SY, et al. Long-term follow-up result of benign phyllodes tumor of the breast diagnosed and excised by ultrasound-guided vacuum-assisted breast biopsy. J Breast Cancer 2012;15:224-9. [Crossref] [PubMed]
- Grady I, Gorsuch H, Wilburn-Bailey S. Long-term outcome of benign fibroadenomas treated by ultrasound-guided percutaneous excision. Breast J 2008;14:275-8. [Crossref] [PubMed]
- Kibil W, Hodorowicz-Zaniewska D, Popiela TJ, et al. Vacuum-assisted core biopsy in diagnosis and treatment of intraductal papillomas. Clin Breast Cancer 2013;13:129-32. [Crossref] [PubMed]
- Wang ZL, Liu G, He Y, et al. Ultrasound-guided 7-gauge vacuum-assisted core biopsy: Could it be sufficient for the diagnosis and treatment of intraductal papilloma? Breast J 2019;25:807-12. [Crossref] [PubMed]
- Youk JH, Kim H, Kim EK, et al. Phyllodes tumor diagnosed after ultrasound-guided vacuum-assisted excision: should it be followed by surgical excision? Ultrasound Med Biol 2015;41:741-7. [Crossref] [PubMed]
- Zhang SL, Lian ZQ, Yu HY, et al. Effect of ultrasound-guided vacuum-assisted excision verus open surgery for benign phyllodes tumors of breast on postoperative local recurrence. Zhonghua Wai Ke Za Zhi 2020;58:110-3. [PubMed]
- Cowan ML, Argani P, Cimino-Mathews A. Benign and low-grade fibroepithelial neoplasms of the breast have low recurrence rate after positive surgical margins. Mod Pathol 2016;29:259-65. [Crossref] [PubMed]
- Teo JY, Cheong CS, Wong CY. Low local recurrence rates in young Asian patients with phyllodes tumours: less is more. ANZ J Surg 2012;82:325-8. [Crossref] [PubMed]
- Graña López L, Vázquez Caruncho M, Villares Armas Á. Percutaneous removal of benign phyllodes tumor of the breast: An alternative to surgery. Breast J 2018;24:1035-7. [Crossref] [PubMed]
- Rageth CJ, O'Flynn EAM, Pinker K, et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat 2019;174:279-96. [Crossref] [PubMed]
- Lucioni M, Rossi C, Lomoro P, et al. Positive predictive value for malignancy of uncertain malignant potential (B3) breast lesions diagnosed on vacuum-assisted biopsy (VAB): is surgical excision still recommended? Eur Radiol 2021;31:920-7. [Crossref] [PubMed]
- Strode M, Khoury T, Mangieri C, et al. Update on the diagnosis and management of malignant phyllodes tumors of the breast. Breast 2017;33:91-6. [Crossref] [PubMed]
- Guillot E, Couturaud B, Reyal F, et al. Management of phyllodes breast tumors. Breast J 2011;17:129-37. [Crossref] [PubMed]
- Wang F, Jia Y, Tong Z. Comparison of the clinical and prognostic features of primary breast sarcomas and malignant phyllodes tumor. Jpn J Clin Oncol 2015;45:146-52. [Crossref] [PubMed]
- Kim YJ, Kim K. Radiation therapy for malignant phyllodes tumor of the breast: An analysis of SEER data. Breast 2017;32:26-32. [Crossref] [PubMed]
- Zeng S, Zhang X, Yang D, et al. Effects of adjuvant radiotherapy on borderline and malignant phyllodes tumors: A systematic review and meta-analysis. Mol Clin Oncol 2015;3:663-71. [Crossref] [PubMed]
- Mitus JW, Blecharz P, Jakubowicz J, et al. Phyllodes tumors of the breast. The treatment results for 340 patients from a single cancer centre. Breast 2019;43:85-90. [Crossref] [PubMed]
- Boutrus RR, Khair S, Abdelazim Y, et al. Phyllodes tumors of the breast: Adjuvant radiation therapy revisited. Breast 2021;58:1-5. [Crossref] [PubMed]


