
Impact of financial support on the prognosis of HER2-positive breast cancer from 2002 to 2020: a prospective cohort from western China
Introduction
Breast cancer is one of the most common cancers worldwide with a relatively better prognosis compared with other common cancers (e.g., lung cancer). Using standard treatment based on mastectomy in combination with chemotherapy, the 5-year survival rate of early breast cancer is usually >90%, except for the triple-negative subtype (1,2). In China, advanced-stage breast cancer is more common compared with Europe and the USA (3,4). Better prognosis prompts patients’ stronger treatment willingness, which consequently inspires high medical expense (5). However, expenditure on examinations and treatments, especially targeted therapy, which is necessary for many malignant tumors, can have a significant financial impact on low-income individuals or families. Approximately 87.9% of individuals or families will suffer from catastrophic health expenditures (CHE), which is defined as out-of-pocket expenditure ≥40% of a household’s non-food expenditure before insurance compensation. Even after insurance compensation, 66.3% of patients and their families still suffer CHE (4). The high cost of diagnosis and treatment is the major obstacle for patients who have a lower socioeconomic status to complete standard or optimal treatment regimen (usually regimens containing target drug), leading to relatively worse outcomes (6-8).
Financial support is significantly associated with the cost of cancer treatment, which further impact on patients’ therapy decision and prognosis (9). Previous research reported that higher cost of National Health Service (NHS) is related to the decrease of hospitalizations of breast cancer and lower annual expense due to bring about the patients proportion of early diagnosis and care (10). Although high distress and financial status worsening have been reported in breast cancer patients, the short-term financial assistance had been reported to improve the patients’ quality of life and made them feel more in control of financial decision-making (11). The surgical cost was reported to influence patients’ decision on surgery and the catastrophic burden of breast cancer was likely attenuated under higher household income and an increasing percentage of care covered by insurance (12). Prior to the target therapy era, human epidermal growth factor receptor2 (HER2)-positive breast cancer was associated with poor prognosis, including a high risk of relapse and metastasis, as well as high mortality. The development of anti-HER2 targeted drugs, such as trastuzumab, has significantly improved the outcomes of this breast cancer subtype. After it was approved by the China Food and Drug Administration for the treatment of HER2-positive breast cancer in 2002, trastuzumab became accessible in China and is now the most widely used anti-HER2 monoclonal antibody. However, the price of trastuzumab is up to $1,740/440 mg and it can cost $20,540–$24,164 for a patient to complete a standard adjuvant course of anti-HER2 target therapy. To support patients with affordable access to trastuzumab, the Cancer Foundation of China launched the Herceptin Breast Cancer Assistance Program (BCAP) in 2011, in which patients can receive an extra 8 bottles of Herceptin at no additional cost for every 6 bottles they purchase. The BCAP continued until November 2017, and Herceptin was then covered by Chinese health insurance. Administered and financed by different institutions, there are 2 types of health insurance; the urban and rural schemes. The urban scheme includes the urban resident-based basic health insurance scheme (URBMI), which covers the unemployed, children, and the elderly. The urban employee-based basic health insurance scheme (UEBMI) covers employees. The new rural cooperative medical scheme (NRCMS) covers rural household residents (13). About 60–75% of medical expenses are covered by the urban scheme, whereas only 50% are covered by the rural scheme. The disparity in health insurance coverage, as well what financial assistance (if any) was available at different time points, can have an impact on the economic burden of HER2-positive breast cancer patients in accessing trastuzumab, depending on when they were diagnosed.
As the first monoclonal antibody to significantly improve breast cancer-related survival outcomes, there is no research on the impact of financial support on the long-term prognosis of HER2-positive breast cancer in resource-limited regions of developing countries. Although trastuzumab had not been covered by Chinese insurance before November 2017, the reimbursement rates of urban and rural health insurance for diagnostic- and treatment-related expenses had an impact on whether patients could afford to access trastuzumab treatment. Therefore, exploring the association between the financial support and HER2-positive breast cancer helps to clarify the underneath mechanism and further improve the outcomes of these patients. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-229/rc).
Methods
Study design
This was a real-world prospective cohort study, in which patients received trastuzumab in the adjuvant phase, or both the neoadjuvant and adjuvant phases. Patients were allocated to the trastuzumab treatment group or the no trastuzumab treatment group.
Data and participants
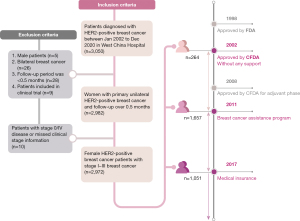
We identified 3,050 patients who were pathologically diagnosed with HER2-positive breast cancer between January 2002 and December 2020 at Sichuan University, West China Hospital, based on the Breast Cancer Information Management System (BCIMS) (13,14). The BCIMS is a patient registry database that collects information, including demographic and clinicopathological characteristics, treatment regimens, laboratory examinations, and follow-up visits. The rate of loss to follow up in the BCIMS is low, and the loss to follow-up rate is lower than 0.4% since 2017. Although trastuzumab was initially approved as adjuvant therapy for HER2-positive breast cancer in 2008, it has been accessible in China since 2002. Among the 3,050 patients, we further included female patients with primary unilateral clinical stage I–III disease. There are 7 patients have received adjuvant treatment of trastuzumab before 2008 in our database. Therefore, we included patients diagnosed with HER2-positive breast cancer between 2002 and 2008. Seventy-eight patients were excluded due to male breast cancer, bilateral disease, stage 0 or stage IV disease, the follow-up period being too short (<0.5 months), and those participating in clinical trials (Figure 1). HER2-positive cancer was defined as pathological immunohistochemical staining, showing HER2 “3+” or fluorescence in situ hybridization (FISH) suggesting HER2 gene amplification (15). The clinical stage of tumor was defined according to the National Comprehensive Cancer Network clinical practice guideline (16). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and data used in this study was collected from BCIMS, which was approved by the Biological and Medical Ethics Committee (BMEC) of West China Hospital (2012 approval No. 130). Informed consent was taken from all the patients.
Follow up
Patients were regularly followed up via in-person appointment or telephone until death or December 31, 2020, whichever came first.
Variables
To analyze the impact of financial support on trastuzumab use, patients were divided into the following 3 groups, based on when they were diagnosed with breast cancer and what financial support was provided: (I) before 2011 (no financial support); (II) 2011–2017 (BCAP support); and (III) 2018 onwards (health insurance support). To further analyze the association between financial support and survival outcomes, patients were reclassified according to health insurance type and reimbursement ratio. In the insurance-type classification, patients were categorized into the urban scheme group, in which patients were insured by the URBMI or UEBMI, and the rural scheme groups, in which patients were insured by the NRCMS. In the reimbursement ratio categorization, health insurance was additionally categorized according to the reimbursement rate (≤50% and >50%). The cut-off value of the reimbursement rate was decided according to maximally selected rank statistics (17). In the Cox regression analysis, demographic characteristics (including age, region, level of education, body mass index, and menopause), clinicopathological characteristics (including T/N stage, histologic grade, and Ki-67 status), and treatment (including surgery, chemotherapy, radiotherapy, and endocrine therapy) were included as confounders due to the dependent or independent association with survival outcome reported in previous research (18,19).
Outcome evaluation
The main endpoints included overall survival (OS) and invasive disease-free survival (iDFS). Breast cancer-specific survival (BCSS) was used as the secondary endpoint. OS and BCSS were defined as the time interval from the date of diagnosis to death due to any reason and due to breast cancer, respectively. iDFS was defined as the time interval from the date of diagnosis to the occurrence of any of the following events: ipsilateral invasive breast cancer relapse and progression, local or regional invasive breast cancer recurrence and progression, pathologically confirmed contralateral invasive breast cancer, distant metastasis, non-breast secondary primary invasive cancer, or death due to any reason (20).
Statistical analysis
Differences in demographic, clinicopathological, and treatment characteristics between the trastuzumab treatment group and the no trastuzumab treatment group were performed using χ2-test and Fisher’s exact test (Table 1). To determine the association between financial support and trastuzumab use, logistic regression analysis was performed with adjustments for demographic characteristics in model A, and additionally for clinicopathological factors in model B. Treatments were further adjusted to analyze correlations between treatment types and trastuzumab use in model C. We performed competing risk model analyses to calculate the cumulative rates and the 95% confidence interval (CI) of overall mortality, invasive-disease event rate, and breast cancer-specific mortality. To estimate the hazard ratios (HRs) of financial support, we used multivariate Cox proportional hazard regression analysis with adjustments for demographic factors (model A), clinicopathological factors (model B), and treatment types (model C) by comparing different insurance schemes, reimbursement rates, and diagnostic time periods, respectively. Schoenfeld residuals were calculated using the Cox proportional hazards model. To detect the potential causal relationship between financial support and survival outcomes with respect to trastuzumab treatment, mediation analysis was performed. Sensitivity analysis was performed by clustering patients according to reimbursement rate (≤50% and >50%) and insurance type (urban and rural scheme), respectively, to further reveal the relationship between financial support and trastuzumab treatment. Patients whose medical costs were out of pocket or covered by commercial insurance were defined as uninsured. Mediation analysis was based on the counterfactual approach (21), and used quasi-Bayesian approximation (R package mediation, version 4.5.0). A detailed analysis is provided in the Supplementary Information (22). R package version 4.1.0 was used for the statistical analysis, and statistical significance was defined as P<0.05 under the two-sided test.
Table 1
| Characteristics | Total (n=2,972) | No trastuzumab treatment (n=1,430) | Trastuzumab treatment (n=1,542) | P value |
|---|---|---|---|---|
| Age, n (%) | <0.001*** | |||
| <55 years | 2,154 (72.5) | 993 (69.4) | 1,161 (75.3) | |
| ≥55 years | 818 (27.5) | 437 (30.6) | 381 (24.7) | |
| Insurance type, n (%) | <0.001*** | |||
| Rural | 711 (23.9) | 442 (30.9) | 269 (17.4) | |
| Urban | 2,183 (73.5) | 936 (65.5) | 1,247 (80.9) | |
| Other† | 78 (2.6) | 52 (3.6) | 26 (1.7) | |
| Reimbursement rate, n (%) | <0.001*** | |||
| ≤50% | 752 (25.3) | 504 (35.2) | 248 (16.1) | |
| >50% | 2,149 (72.3) | 861 (60.2) | 1,288 (83.5) | |
| NA | 71 (2.4) | 65 (4.5) | 6 (0.4) | |
| Year at diagnosis, n (%) | <0.001*** | |||
| Before 2011 | 264 (8.9) | 213 (14.9) | 51 (3.3) | |
| 2011–2017 | 1,657 (55.8) | 983 (68.7) | 674 (43.7) | |
| From 2018 | 1,051 (35.4) | 234 (16.4) | 817 (53.0) | |
| Region, n (%) | <0.001*** | |||
| Rural | 968 (30.0) | 563 (39.4) | 405 (26.3) | |
| Urban | 2,004 (70.0) | 867 (60.6) | 1,137 (73.7) | |
| Level of education‡, n (%) | <0.001*** | |||
| ≤6 years | 483 (16.3) | 294 (20.6) | 189 (12.3) | |
| 7–12 years | 1,698 (57.1) | 874 (61.1) | 824 (53.4) | |
| >12 years | 744 (25.0) | 219 (15.3) | 525 (34.0) | |
| NA | 47 (1.6) | 43 (3.0) | 4 (0.2) | |
| Body mass index, n (%) | <0.001*** | |||
| <25 | 2,249 (75.7) | 1,020 (71.3) | 1,229 (79.7) | |
| ≥25 | 684 (23.0) | 374 (26.2) | 310 (20.1) | |
| NA | 39 (1.3) | 36 (2.5) | 3 (0.2) | |
| Menopause, n (%) | 0.755 | |||
| Pre | 1,627 (54.7) | 780 (54.5) | 847 (54.9) | |
| Post | 1,311 (44.1) | 637 (44.5) | 674 (43.7) | |
| NA‡ | 34 (1.1) | 13 (0.9) | 21 (1.4) | |
| T stage, n (%) | 0.014* | |||
| 0–1 | 873 (29.4) | 415 (29.0) | 458 (29.7) | |
| 2 | 1,607 (54.1) | 803 (56.2) | 804 (52.1) | |
| 3–4 | 483 (16.3) | 205 (14.3) | 278 (18.0) | |
| NA | 9 (0.3) | 7 (0.5) | 2 (0.2) | |
| N stage, n (%) | 0.919 | |||
| 0 | 1,239 (41.7) | 605 (42.3) | 634 (41.1) | |
| 1 | 885 (29.8) | 421 (29.4) | 464 (30.1) | |
| 2 | 402 (13.5) | 190 (13.3) | 212 (13.7) | |
| 3 | 444 (14.9) | 212 (14.8) | 232 (15.0) | |
| NA | 2 (0.1) | 2 (0.1) | 0 (0.0) | |
| Anatomic stage, n (%) | 0.225 | |||
| I | 558 (18.8) | 258 (18.0) | 300 (19.5) | |
| II | 1,392 (46.8) | 693 (48.5) | 699 (45.3) | |
| III | 1,022 (34.4) | 479 (33.5) | 543 (35.2) | |
| Histologic grade, n (%) | 0.015* | |||
| I–II | 730 (24.6) | 333 (23.3) | 397 (25.7) | |
| III | 1,655 (55.7) | 839 (58.7) | 816 (52.9) | |
| NA | 587 (19.8) | 258 (18.0) | 329 (21.3) | |
| Hormone receptor, n (%) | 0.299 | |||
| Negative | 1,183 (39.8) | 555 (38.8) | 628 (40.7) | |
| Positive | 1,776 (59.8) | 869 (60.8) | 907 (58.8) | |
| NA | 13 (0.4) | 6 (0.4) | 7 (0.5) | |
| Ki-67, n (%) | <0.001*** | |||
| <14% | 245 (8.2) | 144 (10.1) | 101 (6.5) | |
| ≥14% | 2,613 (87.9) | 1,227 (85.8) | 1,386 (89.9) | |
| NA | 114 (3.8) | 59 (4.1) | 55 (3.6) | |
| Surgery, n (%) | 0.813 | |||
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Yes | 2,972 (100.0) | 1,430 (100.0) | 1,542 (100.0) | |
| Chemotherapy, n (%) | <0.001*** | |||
| No | 84 (2.8) | 77 (5.4) | 7 (0.4) | |
| Yes | 2,888 (97.2) | 1,353 (94.6) | 1,535 (99.6) | |
| Radiotherapy, n (%) | 0.584 | |||
| No | 1,967 (66.2) | 954 (66.7) | 1,013 (65.7) | |
| Yes | 1,005 (33.8) | 476 (33.3) | 529 (34.3) | |
| Endocrine therapy, n (%) | 0.798 | |||
| No | 1,472 (49.5) | 705 (49.3) | 767 (49.7) | |
| Yes | 1,500 (50.5) | 725 (50.7) | 775 (50.2) | |
*P<0.05, ***P<0.001. †, patients who paid out of pocket, patients with commercial insurance, and patients with an unknown insurance type. ‡, levels of education indicate primary school level (<6 years), secondary school level (6–12 years), and college or above level (>12 years of education), respectively. Patients with missing information were excluded from the corresponding statistics analysis. NA, not available.
Results
Patient baseline characteristics and the impact of financial support on trastuzumab use
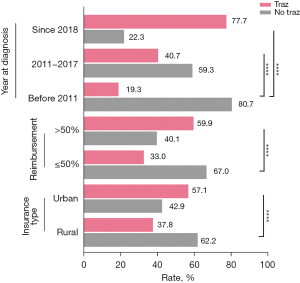
A total of 2,972 female patients with stages I–III unilateral HER2-positive breast cancer were included in the study. The median age at diagnosis was 49 years [interquartile range (IQR): 43–55 years]. A total of 1,542 patients underwent adjuvant or neoadjuvant trastuzumab therapy, followed by adjuvant trastuzumab therapy combined with chemotherapy regimen, and 1,430 patients were treated with adjuvant chemotherapy only. An increasing trend in the proportion of patients treated with trastuzumab from the earliest time period (before 2011) to the most recent time period (from 2018) was observed. When compared with the no trastuzumab treatment group, significantly more patients in the trastuzumab treatment group had urban insurance and were reimbursed at a rate >50% (P<0.001) (Table 1). Furthermore, the proportion of patients treated with trastuzumab surpassed that of their untreated counterparts from 2018. The ratio of trastuzumab treatment was reasonably higher for patients reimbursed at a higher rate (≥50%) or insured by the urban scheme than those reimbursed at a lower rate (<50%) or insured by the rural scheme, respectively (Figure 2). The proportion of patients who paid out of pocket or were covered by commercial insurance was higher in the no trastuzumab treatment group compared with the treatment group. We also observed that patients in the trastuzumab treatment group were more likely to have a higher level of education and a lower body mass index (P<0.001), and their tumors were more aggressive in terms of T stage and Ki-67 intensity (P<0.001) compared with patients in the no trastuzumab treatment group (Table 1). There was no significant difference in terms of menopause status, lymph node stage, and anatomic stage between the groups with or without trastuzumab treatment. There was no difference in therapy regimen, except for chemotherapy, between the groups with or without trastuzumab (Table 1). Additionally, we observed that there was no significant change in the ratio of clinical characteristics, including T/N stage, anatomic stage, hormone receptor status, and Ki-67 expression intensity from the earliest time period to the most recent time period (Figure S1).
Association between financial support and trastuzumab use
In a further exploration of the association between financial support and trastuzumab use, we found that poor financial support or underinsured patients had a reduced likelihood of trastuzumab use during the adjuvant treatment phase. Logistic regression analysis found that patients diagnosed with breast cancer during 2011–2017 [odds ratio (OR): 3.19, 95% CI: 2.20–4.71, P<0.001] and from 2018 (OR: 22.09, 95% CI: 14.76–33.73, P<0.001) showed stronger propensity for trastuzumab treatment. Similarly, patients with urban insurance (OR: 1.71, 95% CI: 1.40–2.10, P<0.001) and those with a higher reimbursement rate (>50%, OR: 2.63, 95% CI: 2.17–3.20, P<0.001) were more likely to have trastuzumab treatment during the neoadjuvant and adjuvant phases compared with those covered by the rural scheme or those with a lower reimbursement rate (Table 2).
Table 2
| Financial support | Model Aa | Model Bb | Model Cc | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||
| By year of diagnosis | ||||||||
| Before 2011 | 1.00 | – | 1.00 | – | 1.00 | – | ||
| 2011–2017 | 2.72 (1.94–3.88) | <0.001 | 2.87 (2.00–4.22) | <0.001 | 3.19 (2.20–4.71) | <0.001*** | ||
| >2018 | 13.70 (9.61–19.88) | <0.001 | 15.07 (10.28–22.53) | <0.001 | 22.09 (14.76–33.73) | <0.001*** | ||
| By insurance type† | ||||||||
| Rural | 1.00 | – | 1.00 | – | 1.00 | – | ||
| Urban | 1.71 (1.41–2.08) | <0.001 | 1.69 (1.38–2.07) | <0.001 | 1.71 (1.40–2.10) | <0.001*** | ||
| By reimbursement rate‡ | ||||||||
| ≤50% | 1.00 | – | 1.00 | – | 1.00 | – | ||
| >50% | 2.55 (2.12–3.07) | <0.001 | 2.59 (2.14–3.15) | <0.001 | 2.63 (2.17–3.20) | <0.001*** | ||
***P<0.001. †, sixty patients paid out of pocket or had commercial insurance; 18 patients had no insurance information available and were excluded in this analysis. ‡, seventy-one patients had missing information for reimbursement rate and were excluded. a, odds ratios (ORs) were adjusted for age at diagnosis (<55 years or ≥55 years), level of education (≤6 years, 7–12 years, >12 years), and menopause status (pre-menopause or post-menopause). b, ORs were adjusted for T stage (0–1, 2, or 3–4), N stage (0, 1, 2, or 3), Ki-67 status (<14% or ≥14%), hormone receptor status (negative and positive), and histologic grade (I–II, III, NA). c, ORs were adjusted for surgery (no or yes), chemotherapy (no/salvage, adjuvant/neoadjuvant), radiotherapy (no or yes), and endocrine therapy (no or yes), if applicable. NA, not available.
Association between financial support and survival outcomes
The decision to undergo trastuzumab treatment is significantly affected by HER2-positive breast cancer patients’ ability to afford treatment. Therefore, we further analyzed the impact of financial support on survival. During the median follow up of 3.9 years (IQR: 1.9–6.4 years), there were 196 deaths, with 153 due to breast cancer. According to the adjusted demographic characteristics in model A, the overall mortality risk of patients diagnosed with breast cancer from 2018 significantly decreased by 57% (95% CI: 13–79%) compared with those diagnosed before 2011. There was no significant difference in overall mortality risk between patients who were diagnosed during 2011–2017 (HR: 0.73, 95% CI: 0.49–1.09) and those diagnosed before 2011. Additionally, the overall mortality risk of patients diagnosed with breast cancer from 2011 to 2017 and from 2018 decreased by 37% (95% CI: 4–59%) and 64% (95% CI: 26–83%) in model C, respectively, compared with those who were diagnosed before 2011. To further confirm the association between financial support and OS, we performed sensitivity analysis by grouping patients according to their health insurance type and reimbursement rate. Among patients with urban insurance, overall mortality decreased from 100% to 61% (95% CI: 43–86%) compared with those who were covered by rural insurance. In addition, we found that overall mortality decreased by 32% (95% CI: 6–51%) in patients with a reimbursement rate ≥50% than those with a reimbursement ratio <50%. Similar trends were also found for iDFS (Table 3) and BCSS (Table S1).
Table 3
| Variable | Cases (n=2,987) | Events, n (%) | Model Aa | Model Bb | Model Cc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Overall survival | ||||||||||
| By year at diagnosis | ||||||||||
| Before 2011 | 264 | 48 (18.2) | 1.00 | 1.00 | 1.00 | |||||
| 2011–2017 | 1,657 | 136 (8.2) | 0.73 (0.49–1.09) | 0.127 | 0.66 (0.43–1.00) | 0.048 | 0.63 (0.41–0.96) | 0.030* | ||
| >2018 | 1,051 | 12 (1.1) | 0.43 (0.21–0.87) | 0.019 | 0.42 (0.20–0.85) | 0.017 | 0.36 (0.17–0.74) | 0.006** | ||
| By insurance type† | ||||||||||
| Rural | 711 | 74 (10.4) | 1.00 | 1.00 | 1.00 | |||||
| Urban | 2,183 | 112 (5.1) | 0.53 (0.38–0.74) | <0.001 | 0.56 (0.40–0.79) | 0.005 | 0.61 (0.43–0.86) | 0.005** | ||
| By reimbursement‡ | ||||||||||
| ≤50% | 752 | 84 (11.2) | 1.00 | 1.00 | 1.00 | |||||
| >50% | 2,149 | 95 (4.4) | 0.63 (0.46–0.87) | <0.001 | 0.64 (0.45–0.89) | 0.007 | 0.68 (0.49–0.94) | 0.02* | ||
| iDFS | ||||||||||
| By year at diagnosis | ||||||||||
| Before 2011 | 264 | 70 (26.5) | 1.00 | 1.00 | 1.00 | |||||
| 2011–2017 | 1,657 | 288 (17.4) | 0.91 (0.67–1.23) | 0.575 | 0.54 (0.47–0.61) | <0.001 | 0.52 (0.47–0.59) | <0.001*** | ||
| From 2018 | 1,051 | 47 (4.5) | 0.63 (0.42–0.96) | 0.035 | 0.34 (0.27–0.43) | <0.001 | 0.33 (0.26–0.41) | <0.001*** | ||
| By insurance type † | ||||||||||
| Rural | 711 | 129 (18.1) | 1.00 | 1.00 | 1.00 | |||||
| Urban | 2,183 | 260 (11.9) | 0.72 (0.56–0.91) | 0.007 | 0.75 (0.59–0.95) | 0.019 | 0.73 (0.57–0.92) | 0.009** | ||
| By reimbursement ‡ | ||||||||||
| ≤50% | 752 | 142 (18.9) | 1.00 | 1.00 | 1.00 | |||||
| >50% | 2,149 | 243 (11.3) | 0.87 (0.70–1.09) | 0.233 | 0.87 (0.70–1.09) | <0.001 | 0.48 (0.38–0.62) | <0.001*** | ||
*P<0.05, **P<0.01, ***P<0.001. †, sixty patients paid out of pocket or had commercial insurance; 18 patients had no insurance information available and were excluded in this analysis. ‡, seventy-one patients with missing information on reimbursement rate were excluded. a, HRs were adjusted for age at diagnosis (<55 or ≥55 years), menopause status (pre-menopause or post-menopause), and level of education (≤6, 7–12, >12 years). b, HRs were adjusted for T stage (0–1, 2, or 3–4), N stage (0, 1, 2, or 3), Ki-67 levels (<14% or ≥14%), hormone receptor status (negative or positive), and histologic grade of tumor (I–II, III, NA). c, HRs were adjusted for surgery (no or yes), chemotherapy (no/salvage, adjuvant/neoadjuvant), radiotherapy (no or yes), and endocrine therapy (no or yes), if applicable. CI, confidence interval; HR, hazard ratio; iDFS, invasive disease-free survival; NA, not available.
Although the reimbursement of trastuzumab differed between health insurances, we did not find an association between health insurance type and trastuzumab treatment in patients diagnosed with breast cancer from 2018 (Table S2). There was a trend of patients with urban insurance having better survival outcomes than those with rural insurance in the subgroup of patients diagnosed with breast cancer from 2018 (Table S3). Four patients who paid out of pocket or had commercial insurance, and 8 patients with no insurance information available, were excluded in the analysis.
Causal mediation analysis showed that greater financial support (represented by year of diagnosis), including the BCAP (2011–2017), medical insurance (from 2018), and higher health insurance reimbursement, were associated with trastuzumab use, which in turn significantly improved survival outcomes, especially OS. The financial support (including BCAP and health insurance) improved OS [direct effect (DE): 9.33; indirect effect (IE): 1.84; P<0.001] and iDFS (DE: 9.22; IE: 1.80; P<0.001), as they encouraged trastuzumab treatment by providing financial assistance. Besides, a significant improving effect in OS (DE: 41.98; IE: 9.58; P=0.012) and iDFS (DE: 15.99; IE: 3.61; P=0.040) attributed to urban insurance were observed. We also found that higher reimbursement ratio prompted the OS (DE: 1.28; IE: 1.45; P=0.024) remarkably, while no significant effect of reimbursement ratio that mediated by the trastuzumab was seen in the iDFS (DE: −0.002; IE: 7.91; P=0.980) (Figure 3 and Table 4). Moreover, trends in OS and iDFS were also observed in the subgroup of patients who were diagnosed with breast cancer during 2011–2017 and from 2018, although these were not statistically significant. Mediation analysis for the BCSS is shown in Table S4.
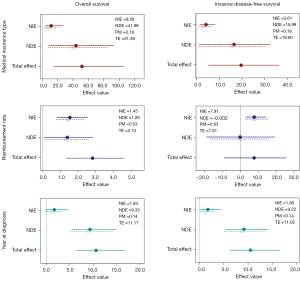
Table 4
| Variable | Year of diagnosis | P value | Insurance type | P value | Reimbursement rate | P value |
|---|---|---|---|---|---|---|
| Overall survival | ||||||
| NIE | 1.84 | 0.042 | 9.58 | <0.001 | 1.45 | <0.001 |
| NDE | 9.33 | <0.001 | 41.98 | 0.012 | 1.28 | 0.024 |
| PM | 0.14 | 0.042 | 0.18 | 0.002 | 0.53 | <0.001 |
| iDFS | ||||||
| NIE | 1.80 | 0.050 | 3.61 | <0.001 | 7.91 | <0.001 |
| NDE | 9.22 | <0.001 | 15.99 | 0.040 | –0.002 | 0.980 |
| PM | 0.14 | 0.050 | 0.18 | 0.012 | 0.63 | 0.350 |
iDFS, invasive disease-free survival; NDE, natural direct effect; NIE, natural indirect effect; PM, proportion of the total effect of financial support on patient prognosis mediated by trastuzumab.
Discussion
To the best of our knowledge, the present study is the first to explore the impact of financial support on trastuzumab use and long-term outcomes in a Chinese registry of HER2-positive breast cancer patients. We observed a significant association between financial support and survival outcome, which was partially mediated by trastuzumab use.
Trastuzumab is the most effective anti-HER2 target drug in breast cancer. Numerous clinical trials and research have confirmed its effectiveness in both early and metastatic breast cancer (23). Although published studies have demonstrated that early trastuzumab use is cost-effective for treatment (24), only a few patients can afford it without health insurance or other financial support (25,26). Various factors, including medical insurance, race, level of education, and clinical stage of the tumor, have been reported to have an impact on trastuzumab use (27-29). Lack of health insurance and a low reimbursement rate are the main factors hindering trastuzumab use (28). Reeder-Hayes et al. reported that there exists trastuzumab use disparity among different races although living in the same country, which is primarily caused by socioeconomic status (e.g., economy, job, source of transportation to treatment, and level of social support) (30). Due to relatively better socioeconomic status, trastuzumab use among white women with HER2-positive breast cancer has been reported to be higher than that in black women (30). Moreover, it has also been reported that this disparity is could lead to inter-ethnic differences in prognosis (30). Besides, geographic factor as one of the representatives of socioeconomic status, was proved to be another cause the disparity of access to trastuzumab in occident (31). Similarly, our research demonstrated that socioeconomic status, which was represented by health insurance and level of education, affected the use of trastuzumab in resource-limited regions of developing countries, such as western and northeastern China. The long-term follow-up data in the present research demonstrated that patients with a lower level of health insurance had poorer survival outcomes. With the assistance of the BCAP, the total cost of trastuzumab adjuvant therapy was reduced by 50% of the original price. When trastuzumab was covered by health insurance in China, the out-of-pocket expenditure among urban residents was reduced by 25–40% of the original price, while among rural residents, it was reduced by 50% of the original cost. We observed that financial support, including the BCAP and health insurance, was associated with increased survival outcomes by providing financial assistance for trastuzumab therapy, which might have been otherwise unaffordable, highlighting the importance of trastuzumab in early HER2-positive breast cancer therapy. Based on these findings, we found room for the improvement in the prognosis of patients with low reimbursement ratio or those without health insurance, in part because they did not receive enough anti-HER2 therapy. Health insurance type is partly reflective of socioeconomic status, and the latter is reported to affect the incidence of breast cancer (3,32). The global breast cancer epidemiology study showed that countries with higher human development index (HDI) had a greater incidence and lower mortality of breast cancer than those with a lower HDI (33). Therefore, studies are needed to assess the cost-effectiveness of improving health insurance policies and increasing financial support for breast cancer patients in resource-limited regions to improve outcomes (33).
Commercial insurance is generally purchased through out-of-pocket payment and the number of patients in this subset who had commercial insurance was low. Therefore, we categorized 6 (0.2%) commercially insured patients into the uninsured subset; this was not likely to impact our overall outcomes. We did not include occupation in our analysis due to a significant number of patients not having an occupation with a fixed income. Based on our previous study, we characterized patients’ socioeconomic status by combining their health insurance type, level of education, as well as region (13). Besides, the patients in the present study were mostly came from the western China, limiting the representativeness of the findings to a wider range of HER2-positive breast cancer patients and the generalization of the findings to patients in other regions.
In conclusion, our findings suggest that greater financial support is significantly associated with improvement in the long-term outcomes of early HER2-positive breast cancer by promoting the use of trastuzumab treatment in the resource-limited regions.
Acknowledgments
Funding: This work was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC21035 to XZ) and the Key Research and Development Project of the Science & Technology Department of Sichuan Province (No. 2021YFS0235 to TL).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-229/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-229/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-229/coif). XZ reports that this work was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC21035 to XZ). TL reports that this work was supported by the Key Research and Development Project of the Science & Technology Department of Sichuan Province (No. 2021YFS0235 to TL). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and data used in this study was collected from BCIMS, which was approved by the Biological and Medical Ethics Committee (BMEC) of West China Hospital (2012 approval No. 130). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pu M, Messer K, Davies SR, et al. Research-based PAM50 signature and long-term breast cancer survival. Breast Cancer Res Treat 2020;179:197-206. [Crossref] [PubMed]
- Polley MC, Leon-Ferre RA, Leung S, et al. A clinical calculator to predict disease outcomes in women with triple-negative breast cancer. Breast Cancer Res Treat 2021;185:557-66. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Sun CY, Shi JF, Fu WQ, et al. Catastrophic Health Expenditure and Its Determinants Among Households With Breast Cancer Patients in China: A Multicentre, Cross-Sectional Survey. Front Public Health 2021;9:704700. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- San Miguel Y, Gomez SL, Murphy JD, et al. Age-related differences in breast cancer mortality according to race/ethnicity, insurance, and socioeconomic status. BMC Cancer 2020;20:228. [Crossref] [PubMed]
- Dreyer MS, Nattinger AB, McGinley EL, et al. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat 2018;167:1-8. [Crossref] [PubMed]
- Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin 2018;68:153-65. [Crossref] [PubMed]
- Wharam JF, Zhang F, Lu CY, et al. Breast Cancer Diagnosis and Treatment After High-Deductible Insurance Enrollment. J Clin Oncol 2018;36:1121-7. [Crossref] [PubMed]
- Mennini FS, Trabucco Aurilio M, Gazzillo S, et al. An Analysis of the Social and Economic Costs of Breast Cancer in Italy. Int J Environ Res Public Health 2021;18:9005. [Crossref] [PubMed]
- Semin JN, Palm D, Smith LM, et al. Understanding breast cancer survivors' financial burden and distress after financial assistance. Support Care Cancer 2020;28:4241-8. [Crossref] [PubMed]
- Greenup RA, Rushing C, Fish L, et al. Financial Costs and Burden Related to Decisions for Breast Cancer Surgery. J Oncol Pract 2019;15:e666-76. [Crossref] [PubMed]
- Xie Y, Valdimarsdóttir UA, Wang C, et al. Public health insurance and cancer-specific mortality risk among patients with breast cancer: A prospective cohort study in China. Int J Cancer 2021;148:28-37. [Crossref] [PubMed]
- Peng Z, Wei J, Lu X, et al. Diagnosis and treatment pattern among rural and urban breast cancer patients in Southwest China from 2005 to 2009. Oncotarget 2016;7:78168-79. [Crossref] [PubMed]
- Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18-43. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Abraham J, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:452-78. [Crossref] [PubMed]
- Laska E, Meisner M, Wanderling J. Exact distribution of a maximally selected Wilcoxon and a new hybrid test of symmetry. Stat Med 2014;33:4292-305. [Crossref] [PubMed]
- McDonald ES, Clark AS, Tchou J, et al. Clinical Diagnosis and Management of Breast Cancer. J Nucl Med 2016;57:9S-16S. [Crossref] [PubMed]
- Pondé NF, Zardavas D, Piccart M. Progress in adjuvant systemic therapy for breast cancer. Nat Rev Clin Oncol 2019;16:27-44. [Crossref] [PubMed]
- Gourgou-Bourgade S, Cameron D, Poortmans P, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials). Ann Oncol 2015;26:2505-6. [Crossref] [PubMed]
- Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137-50. [Crossref] [PubMed]
- Valente MJ, Rijnhart JJM, Smyth HL, et al. Causal Mediation Programs in R, Mplus, SAS, SPSS, and Stata. Struct Equ Modeling 2020;27:975-84. [Crossref] [PubMed]
- Loibl S, Gianni L. HER2-positive breast cancer. Lancet 2017;389:2415-29. [Crossref] [PubMed]
- Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene 2007;26:3637-43. [Crossref] [PubMed]
- Daroudi R, Akbari Sari A, Nahvijou A, et al. The Economic Burden of Breast Cancer in Iran. Iran J Public Health 2015;44:1225-33. [PubMed]
- Gershon N, Berchenko Y, Hall PS, et al. Cost effectiveness and affordability of trastuzumab in sub-Saharan Africa for early stage HER2-positive breast cancer. Cost Eff Resour Alloc 2019;17:5. [Crossref] [PubMed]
- Li J, Shao Z, Xu B, et al. Use of trastuzumab as an adjuvant/neoadjuvant therapy in patients with HER2-positive breast cancer in China: The Nvwa study. Medicine (Baltimore) 2018;97:e10350. [Crossref] [PubMed]
- Lammers P, Criscitiello C, Curigliano G, et al. Barriers to the Use of Trastuzumab for HER2+ Breast Cancer and the Potential Impact of Biosimilars: A Physician Survey in the United States and Emerging Markets. Pharmaceuticals (Basel) 2014;7:943-53. [Crossref] [PubMed]
- Freedman RA, Hughes ME, Ottesen RA, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer 2013;119:839-46. [Crossref] [PubMed]
- Reeder-Hayes K, Peacock Hinton S, Meng K, et al. Disparities in Use of Human Epidermal Growth Hormone Receptor 2-Targeted Therapy for Early-Stage Breast Cancer. J Clin Oncol 2016;34:2003-9. [Crossref] [PubMed]
- Ades F, Senterre C, Zardavas D, et al. Are life-saving anticancer drugs reaching all patients? Patterns and discrepancies of trastuzumab use in the European Union and the USA. PLoS One 2017;12:e0172351. [Crossref] [PubMed]
- Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat 2019;177:537-48. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
(English Language Editor: R. Scott)


