
Lung cavitation in patients with anaplastic thyroid cancer treated with lenvatinib
Introduction
Anaplastic thyroid cancer (ATC) is a rare malignancy, accounting for 1–2% of all thyroid cancers (1). The median survival time from diagnosis is 3.8 months, and the one-year survival rate is 18% (2). Significant indicators of poor ATC prognosis include acute symptoms, tumor size >5 cm, distant metastasis, and a white blood cell (WBC) >10,000/µL (3). Other research has found that age >70 and extrathyroidal infiltration (T4b) also predict a poor prognosis (4). Distant metastasis occurs in 53% of ATC patients, most commonly in the lungs (88%), and bone (15%) (5). In 2015, the Japanese Ministry of Health, Labor, and Welfare approved the clinical use of lenvatinib for ATC. lenvatinib is an orally administered inhibitor of vascular endothelial growth factor receptor (VEGFR)-1, -2, and -3, fibroblast growth factor receptor (FGFR)-1 to -4, platelet-derived growth factor receptor (PDGFR)-α, and rearranged during transfection (RET) and KIT proto-oncogenes (6). Research has found the median progression-free survival (PFS) and the overall survival (OS) in ATC patients to be 7.4 and 10.6 months, respectively, with a response rate of 24% (7). A previous study in our hospital reported adverse effects (AE) of lenvatinib administration for ATC that included hypertension (91%), general fatigue and loss of appetite (65%), proteinuria (61%), and tumorous cutaneous fistulas (26%) (8). In addition, there have been several cases of pneumothorax caused by lung cavitation and collapse after administration of lenvatinib to ATC patients with lung metastasis (9-11). It has been reported that the incidence of lung cavitation was 12% in thyroid cancers with lung metastasis treated with antiangiogenic agents (12). However, as far as ATC patients with lung metastasis are concerned, lung cavitation seems to be frequently observed. In the present study, we aimed to investigate the incidence rate of lung cavitation and impact on prognosis in ATC patients with lung metastasis treated with lenvatinib. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-71/rc).
Methods
Patient selection
All patients with ATC with lung metastasis treated by lenvatinib between November 2015 and May 2021 were identified for review from the electronic medical records of Kanagawa Cancer Center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective study was approved by Review Board of Kanagawa Cancer Center in Japan (No. 2021-88) and individual consent for this retrospective analysis was waived.
Endpoints
The primary objective of this study was to determine the incidence of cavitation of lung metastasis in ATC patients treated with lenvatinib. The secondary objective was to evaluate prognostic factors [age, gender, performance status (PS), acute enlargement, WBC count, primary tumor size, distant metastasis other than lung, initial dose of lenvatinib, lung cavitation and primary tumor resection] in ATC patients with lung metastasis treated with lenvatinib.
Statistical analysis
Medians and interquartile ranges were used as continuous variables and evaluated by the t-test. Categorical data were evaluated by Fisher’s exact test. For time-to-event outcomes, the Cox proportional hazard regression was used. Survival curves were determined using the Kaplan-Meier method. We defined a two-tailed P value of <0.05 as statistically significant. Data analysis was performed using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
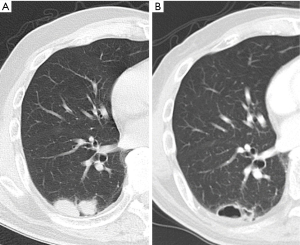
Twenty-six patients were treated with lenvatinib for ATC with lung metastasis. ATC was diagnosed by fine needle aspiration in 4 cases, core needle biopsy in 14 cases, surgical specimens in 7 cases, and clinical course in 1 case. Lung metastasis was also diagnosed by computed tomography findings in all 26 cases. Figure 1 shows an example of lung cavitation of ATC patient during while on treatment of lenvatinib.
Three cases of ATC transformation of recurrent lesions after resection of differentiated thyroid cancer were observed. Of the 23 cases excluding these cases, 4 patients underwent primary resection first, 1 patient underwent resection after weekly paclitaxel (PTX), 1 patient underwent primary resection after lenvatinib treatment, and 1 patient underwent weekly PTX as pretreatment of lenvatinib administration. In the other 16 patients, lenvatinib was started without prior treatment. In three cases, lenvatinib administration were discontinued due to AE: one case of gastrointestinal bleeding, one case of esophageal fistula, and one case of urethral fistula. Twelve patients (46.2%) had lung cavitation during the treatment. Two patients (16.7%) of those with lung cavitation suffered pneumothorax. Three patients (11.5%) had an anaplastic transformation and were excluded from the evaluations of primary tumor size and primary tumor resection. The median age was 73% and 46.2% of the patients were male. Nine patients (34.6%) were current or past smokers. Seventeen patients (65.4%) were initially treated with lenvatinib (24 mg). There were no significant differences in gender, smoking history, initial dose of lenvatinib, or best overall response between the cavitation (+) group and the cavitation (−) group (Table 1). Two cases of the cavitation (+) group presented with pneumothorax. The median OS was 128 days (79–228 days). The cavitation (+) group had a significantly longer OS than the cavitation (−) group [186 days (117–355 days) vs. 89 days (59–179 days), P=0.033] (Table 1). Kaplan-Meier survival curves indicated a median OS duration of 186 days in the cavitation (+) group and 89 days in the cavitation (−) group. A significant difference in OS was observed between the two groups (P=0.0293) (Figure 2). Univariate analysis demonstrated no significant differences in prognosis resulting from age, gender, PS, acute enlargement, WBC >10,000/µL, primary tumor size, distant metastasis other than lung, initial dose of lenvatinib, or primary tumor resection. Lung cavitation was the only significant prognostic factor (hazard ratio: 0.38, 95% CI: 0.16–0.93) (Table 2). Bulla formation in the lungs due to coronavirus disease 2019 (COVID-19) pneumonia has been reported, as well as a case of complications with thyroid cancer (13). In this study, screening tests for COVID-19 were performed at the time of admission or in case of fever, resulting in no cases of complicated COVID-19 infection after COVID-19 era.
Table 1
| Characteristics | Total, n=26 | Cavitation of lung metastasis | P | |
|---|---|---|---|---|
| (+), n=12 | (−), n=14 | |||
| Overall survival (days), median [IQR] | 128 [79–228] | 186 [117–355] | 89 [59–179] | 0.033 |
| Age (years), median [IQR] | 73 [65–79] | 73 [66–75] | 74 [60–82] | 0.974 |
| Gender (male), n (%) | 12 (46.2) | 7 (58.3) | 5 (35.7) | 0.431 |
| Smoker, n (%) | 9 (34.6) | 5 (41.7) | 4 (28.6) | 0.683 |
| Initial dose of lenvatinib (mg), n (%) | ||||
| 24 | 17 (65.4) | 9 (75.0) | 8 (57.1) | 0.094 |
| 20 | 1 (3.8) | 1 (8.3) | 0 (0.0) | |
| 14 | 7 (26.9) | 1 (8.3) | 6 (42.9) | |
| 10 | 1 (3.8) | 1 (8.3) | 0 (0.0) | |
| Best overall response, n (%) | ||||
| PR | 6 (23.1) | 2 (16.7) | 4 (28.6) | 0.428 |
| SD | 17 (65.4) | 8 (66.7) | 9 (64.3) | |
| PD | 2 (7.7) | 2 (16.7) | 0 (0.0) | |
| NE | 1 (3.8) | 0 (0.0) | 1 (7.1) | |
IQR, interquartile range; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable.
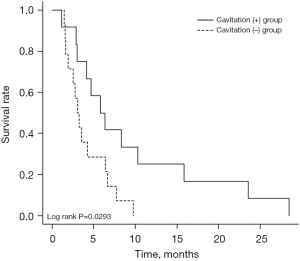
Table 2
| Prognostic factors | HR | 95% CI | P |
|---|---|---|---|
| Age >70 (years, n=16) | 1.1 | 0.48–2.47 | 0.839 |
| Gender (male, n=12) | 0.92 | 0.41–2.03 | 0.828 |
| PS =0 (n=16) | 0.84 | 0.37–1.93 | 0.685 |
| Acute enlargement of primary tumor (n=15) | 1.95 | 0.80–4.74 | 0.142 |
| White blood cell >10,000 (/μL, n=14) | 1.04 | 0.48–2.31 | 0.907 |
| Primary tumor size >5 (cm, n=10) | 1.01 | 0.41–2.47 | 0.982 |
| Distant metastasis other than lung (n=13) | 1.2 | 0.54–2.67 | 0.659 |
| Initial dose of lenvatinib =24 (mg, n=17) | 0.46 | 0.19–1.10 | 0.081 |
| Cavitation of lung metastasis (n=14) | 0.38 | 0.16–0.93 | 0.035 |
| Primary tumor resection (n=6) | 0.49 | 0.19–1.29 | 0.150 |
PS, performance status; HR, hazard ratio; CI, confidence interval.
Discussion
Datar et al. reported that 10 out of 83 patients (12%) developed lung cavitation during antiangiogenic agents treatment in thyroid cancers (12). They also described that lung cavitation had no significant effect on survival (12). In the study, antiangiogenic agents contained lenvatinib and sorafenib, and thyroid cancers included any type of histology (papillary thyroid cancer, follicular thyroid cancer, poorly differentiated thyroid cancer and ATC) (12). To our knowledge, the present study is the first report in which the incidence and the prognosis of lung cavitation patients of only ATC with lung metastasis treated with lenvatinib.
In our study, the incidence of lung cavitation was 46.2%, and it is speculated that the difference in Datar’s results is due to the difference of patient’s background, tumoral micro environment and its sensitivity to angiogenesis inhibitors. As for tumoral micro environment, it has been reported that peritumoral micro-vessel density is significantly lower in ATC than in differentiated thyroid cancer, which may result in the difference in the cavitation due to tumor necrosis (14).
Surprisingly, patients who developed lung cavitation had a significantly better prognosis than those who did not, which remains unclear. Otherwise, the presence of lung cavitation was not associated with the best overall response (Table 1). Two cases out of six partial response (PR) cases (33%) were discontinued due to fatal AE (esophageal fistula, and urethral fistula), on the other hand, 17 stable disease (SD) cases had no fatal AE and were not discontinued. There might be some cases in which lenvatinib was too effective, resulting in a shortened prognosis.
There have been several reports of lung cavitation in patients with other cancers treated with angiogenesis inhibitors. In non-small cell lung cancer, lung cavitation was observed in 19–24% of patients treated with angiogenesis inhibitors (15,16). Like in our study, Huang et al. found that prognosis was better when lung cavitation occurred in non-small cell lung cancer treated with angiogenesis inhibitor. They speculate that this may be because all of their lung cavitation patients (100%) achieved PR, compared to only 36.5% of their non-cavitation patients (17). Otherwise, Nishino et al. reported that it has not been any significant difference in OS or PFS between patients with and without lung cavitation (16). They have classified patterns of lung cavitation into three categories: (I) development of a cavity within the dominant lung tumor, (II) development of cavitation in non-dominant nodules, and (III) development of cavitation in non-dominant nodules with adjacent interstitial abnormalities (16). Peng et al. described that bevacizumab treatment for lung metastasis of colon cancer resulted in lung cavitation in 20% of patients. In case that lung cavitation occurred, the OS was significantly better, but not the PFS (18). Thus, although cavitation of lung tumors (primary and metastatic) treated with antiangiogenic agents may be an indicator of a favorable prognosis, further studies are needed.
As for the mechanism, it was speculated that lung tumoral cavitation was resulted from central tumor necrosis induced by the inhibition of tumor-associated angiogenesis, as shown in preclinical models (19).
Disruption of lung cavitation can lead to pneumothorax. That was observed in two cases in our study. Previously, three cases of pneumothorax during treatment with lenvatinib for ATC with lung metastasis have been reported. Yamazaki et al. reported a case of pneumothorax on day 34 of treatment with lenvatinib for ATC with lung metastasis. Three thoracic drainages and one adhesion therapy were performed. Recurrence of the pneumothorax and persistent pneumoperitoneum meant that the lenvatinib dose had to be reduced from 24 to 14 mg (9). The second case of bilateral pneumothorax occurred on day 50 of lenvatinib treatment for ATC with lung metastasis. Bilateral thoracic drainage was ineffective, resulting in bilateral partial pneumonectomy (11). In the third previously reported case, lenvatinib treatment was given after total thyroidectomy for ATC with lung metastasis. Bilateral pneumothorax developed two months after the treatment began. The patient underwent thoracic drainage and pleural adhesions. Lenvatinib was discontinued and the patient was transferred to a hospice after external irradiation for local recurrence (10).
When lung cavitation occurs, internal tumor necrosis can be observed on imaging and this necrosis is thought to cause bronchopleural fistulas to form, leading to pneumothorax (20).
In a study of pneumothorax that occurred during chemotherapy, 12% of patients died during treatment of the pneumothorax. The causes were interstitial pneumonia following pleurodesis and cancer progression during drug therapy interruption (21).
Our study has a limitation worth noting. This was a single-center retrospective study that included a small number of patients. Further studies with larger cohorts should investigate the correlation between lung cavitation and prognosis. Despite this limitation, we believe that our results of this study are important given that this has been the first study to investigate the association between the lung cavitation and OS in ATC patients with lung metastasis treated by lenvatinib.
Conclusions
Lung cavitation occurred in 46.2% of patients treated with lenvatinib for ATC with lung metastasis. Patients who developed lung cavitation had a significantly better prognosis than those who did not.
Acknowledgments
We thank Enago (https://www.enago.jp/) for editing this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-71/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-71/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Review Board of Kanagawa Cancer Center in Japan (No. 2021-88) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kilfoy BA, Devesa SS, Ward MH, et al. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev 2009;18:1092-100. [Crossref] [PubMed]
- Sugitani I, Miyauchi A, Sugino K, et al. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg 2012;36:1247-54. [Crossref] [PubMed]
- Sugitani I, Kasai N, Fujimoto Y, et al. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg 2001;25:617-22. [Crossref] [PubMed]
- Kebebew E, Greenspan FS, Clark OH, et al. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 2005;103:1330-5. [Crossref] [PubMed]
- Venkatesh YS, Ordonez NG, Schultz PN, et al. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer 1990;66:321-30. [Crossref] [PubMed]
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621-30. [Crossref] [PubMed]
- Takahashi S, Kiyota N, Yamazaki T, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol 2019;15:717-26. [Crossref] [PubMed]
- Iwasaki H, Yamazaki H, Takasaki H, et al. Lenvatinib as a novel treatment for anaplastic thyroid cancer: A retrospective study. Oncol Lett 2018;16:7271-7. [Crossref] [PubMed]
- Yamazaki H, Iwasaki H, Yamashita T, et al. A Case of Pneumothorax after Treatment with Lenvatinib for Anaplastic Thyroid Cancer with Lung Metastasis. Case Rep Endocrinol 2018;2018:7875929. [Crossref] [PubMed]
- Kazzaz FI, Cabanillas ME, Bashoura L, et al. Bilateral spontaneous pneumothoraces in anaplastic thyroid cancer. Respir Med Case Rep 2019;26:197-9. [Crossref] [PubMed]
- Lee HN, Chung WS, Jang HJ, et al. Bilateral pneumothorax in a patient with anaplastic thyroid carcinoma and lung metastasis during lenvatinib therapy: a case report. Gland Surg 2020;9:1579-83. [Crossref] [PubMed]
- Datar S, Cabanillas M, Dadu R, et al. Pulmonary cavitation in patients with thyroid cancer receiving antiangiogenic agents. BMC Cancer 2020;20:1181. [Crossref] [PubMed]
- Toda S, Matsui A, Yasukawa M, et al. Pulmonary cavitation in a patient with coronavirus disease 2019 during lenvatinib treatment for thyroid carcinoma: a case report. Ann Palliat Med 2021; Epub ahead of print. [Crossref] [PubMed]
- Gulubova M, Ivanova K, Ananiev J, et al. VEGF expression, microvessel density and dendritic cell decrease in thyroid cancer. Biotechnol Biotechnol Equip 2014;28:508-17. [Crossref] [PubMed]
- Crabb SJ, Patsios D, Sauerbrei E, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol 2009;27:404-10. [Crossref] [PubMed]
- Nishino M, Cryer SK, Okajima Y, et al. Tumoral cavitation in patients with non-small-cell lung cancer treated with antiangiogenic therapy using bevacizumab. Cancer Imaging 2012;12:225-35. [Crossref] [PubMed]
- Huang C, Wang X, Wang J, et al. Incidence and clinical implication of tumor cavitation in patients with advanced non-small cell lung cancer induced by Endostar, an angiogenesis inhibitor. Thorac Cancer 2014;5:438-46. [Crossref] [PubMed]
- Peng Y, Chen Y, Zhang X, et al. Tumoral cavitation in colorectal cancer patients with unresectable lung metastasis treated with bevacizumab and chemotherapy. J Cancer Res Clin Oncol 2018;144:1339-46. [Crossref] [PubMed]
- Huang X, Molema G, King S, et al. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 1997;275:547-50. [Crossref] [PubMed]
- Iida T, Yabana T, Nakagaki S, et al. A Rupture of a Lung Metastatic Lesion of Colon Cancer, Leading to Pneumothorax Caused by Bevacizumab. Intern Med 2016;55:3125-9. [Crossref] [PubMed]
- Yamada N, Abe N, Usui K, et al. Clinical analysis of 12 cases of pneumothorax during intensive chemotherapy for malignant neoplasms. Gan To Kagaku Ryoho 2010;37:1519-23. [PubMed]


