
Determining the benefit of neoadjuvant chemotherapy in reduction of axillary dissection rates in Z0011 trial cohort with high nodal burden
Introduction
The Z0011 trial has demonstrated no survival difference in the omission of axillary lymph node dissection (ALND) in T1–2 breast cancer patients with <3 lymph nodes involvement. In patients with a high nodal burden of ≥3 metastatic nodes, an ALND is advocated (1). To avoid an ALND in this group of patients with a high nodal burden, patients could undergo neoadjuvant chemotherapy (NACT) instead. If nodal pathological complete response (pCR) is obtained with NACT, these patients would avoid an ALND, which is associated with multiple complications (2).
NACT could result in nodal pCR in about 40% of patients (3) with a higher rate of nodal pCR observed in patients with certain subtypes such as those positive for human epidermal growth factor receptor2 (HER2). Nevertheless, the benefit of NACT in achieving nodal pCR and consequent reduction of ALND rates in the Z0011 trial patients with high nodal burden has not been previously studied.
It was reported that the number of abnormal nodes on axillary ultrasound could reliably identify the subgroup of patients with high nodal burden in the Z0011 trial cohort (4,5). In particular, in a prior study (5), for patients fulfilling Z0011 trial criteria with a positive percutaneous node biopsy, ≥3 sonographically abnormal nodes was shown to be highly predictive of high nodal burden status, with an odds ratio of 20.72 and area under the receiver operating characteristic curve of 0.747. 92.9% of patients in that study (5) with ≥3 sonographically abnormal nodes demonstrated high nodal burden. Applying this sonographic predictive factor to our cohort of T1–2 patients with nodal metastasis who received NACT, we aim to identify the subgroup of patients with high nodal burden who would have otherwise needed an ALND based on Z0011 trial and determine whether NACT, in this group, could result in nodal pCR with avoidance of ALND. We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-7/rc).
Methods
Newly diagnosed breast cancer patients treated at KK Women’s and Children’s Hospital, Republic of Singapore from 1st September 2005 to 31st Oct 2017 were retrospectively identified from a prospectively maintained database. We identified the Z0011 trial high nodal burden subgroup as T1–2 breast cancer patients with no palpable axillary node, ≥3 abnormal nodes on initial axillary ultrasound and histologically proven nodal metastasis. Among this group of patients, we only included patients who had undergone NACT and an ALND after NACT in order to determine the true nodal status. Patients with occult breast cancer, N3 or distant metastasis, or incomplete data were excluded.
At our institution, in addition to their mammogram and breast ultrasound, most patients would have a dedicated axillary ultrasound with the number of abnormal nodes reported. An abnormal node is defined as having any of these sonographic features: eccentric or uniform cortical thickness ≥3 mm, displacement, or complete effacement of the fatty hilum. Based on ultrasound, only the most suspicious axillary node would be biopsied.
The ultrasounds were performed by trained breast sonographers and the images interpreted by dedicated breast radiologists at a specialized breast centre. The radiologists all had >5 years of experience in breast imaging. The axillary ultrasound would take on average, about 5–10 minutes to complete.
During the study period, patients with nodal metastasis may have been offered an upfront ALND with breast surgery or NACT, depending on the treating clinician’s preference. If receiving NACT, the chemotherapy regime was typically anthracycline and taxane-based, with HER2 positive patients also treated with targeted therapy. After completion of NACT, this group of patients would usually undergo ALND and breast surgery. The choice of breast surgery was decided after discussion with each patient.
Demographics, radiological and pathological features of this group of patients were collected. Isolated tumor cells in ALND after NACT were considered as positive nodal involvement. The nodal pCR based on ALND, was used to determine the benefit of NACT in the reduction of ALND rates in this group of high nodal burden patients.
Statistical analysis
A Fisher exact test was used to compare demographic, radiologic and pathologic variables between patients with ypN0 and ypN+, with P<0.05 defined as statistically significant. Multivariable logistic regression incorporating a backward elimination selection algorithm was used to identify independent predictors of nodal pCR with P defined at <0.05. SAS V9.4 statistical software (Cary, NC, USA) was used for the analysis.
Ethical statement
All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by SingHealth Centralised Institutional Review Board (CIRB Ref: 2020/2147) and waiver of patients’ informed consent was obtained.
Results
Of the 128 breast cancer patients with nodal involvement who received NACT, 72 patients had cT1–2 tumor with no palpable axillary node but histologically proven metastatic lymph node (Figure 1). Of these 72 patients, 1, 47 and 24 patients had 0, 1–2 and ≥3 abnormal nodes on initial ultrasound respectively. The patients with 1–2 abnormal nodes on initial ultrasound had nodal pCR of 38.3%. For the 1 patient with no abnormal lymph node sonographically, she had a positive sentinel lymph node biopsy after her NACT. ALND revealed only that sentinel lymph node to be positive.
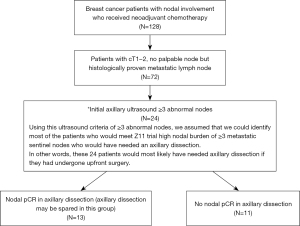
A total of 24 patients were included in the analysis. The mean age was 55.2 years (range, 31–70 years). 87.5% and 12.5% underwent mastectomy and lumpectomy respectively (Table 1). 91.7% and 29.2% had invasive ductal carcinoma and grade III cancer. 54.2% and 41.7% had positivity for estrogen receptor (ER) and HER2 respectively. Progesterone receptor (PR) positivity was 33.3%. The mean tumor size based on ultrasound was 31.5 mm (range, 6–50 mm). 13 patients (54.2%) achieved nodal pCR. 10 patients (41.7%) achieved tumor pCR.
Table 1
| Characteristics | N (%) (n=24) |
|---|---|
| Age (years) | |
| <50 | 7 (29.2) |
| ≥50 | 17 (70.8) |
| Surgery | |
| Mastectomy | 21 (87.5) |
| Wide local excision | 3 (12.5) |
| Completed chemotherapy | |
| Yes | 17 (70.8) |
| No | 7 (29.2) |
| Sonographic tumor size (mm) | |
| 0–20 | 6 (25.0) |
| 21–50 | 18 (75.0) |
| Histology | |
| Invasive ductal carcinoma | 22 (91.7) |
| Invasive lobular carcinoma/others | 2 (8.3) |
| Tumor grade | |
| I, II | 13 (54.2) |
| III | 7 (29.2) |
| Missing | 4 (16.6) |
| ER | |
| Positive | 13 (54.2) |
| Negative | 11 (45.8) |
| PR | |
| Positive | 8 (33.3) |
| Negative | 16 (66.7) |
| HER2 | |
| Positive | 10 (41.7) |
| Negative | 14 (58.3) |
| ypT (mm) | |
| 0–20 | 17 (70.8) |
| 21–50 | 6 (25.0) |
| >50 | 1 (4.2) |
| Nodal pCR | |
| Yes | 13 (54.2) |
| No | 11 (45.8) |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; pCR, pathological complete response.
Seven (29.2%) patients did not complete the entire course of chemotherapy mainly due to the side effects from chemotherapy.
Based on tumor subtype, there were 10 patients (41.7%) with ER/PR positivity and HER2 negativity and 4 patients (16.7%) who was triple negative. There were 10 patients (41.7%) with HER2 positivity. Among the ER/PR+ HER2− tumors, 30% achieved nodal pCR. In the triple negative (TN) and HER2 positive group, the nodal pCR rate was 75% and 70% respectively.
Univariate analysis revealed age as a continuous variable (P=0.024) and ypT (P=0.006) to be statistically significant factors for nodal pCR (Table 2). Multivariable analysis incorporating backward elimination only showed ypT (P=0.006) to be statistically significant for nodal pCR.
Table 2
| Variable | Patients with ypN0 (n=13), n (%) | Patients with ypN+ (n=11), n (%) | P value |
|---|---|---|---|
| Age (years) | 0.0778 | ||
| <50 | 6 (46.2) | 1 (9.1) | |
| ≥50 | 7 (53.8) | 10 (90.9) | |
| Surgery | 0.2228 | ||
| Mastectomy | 10 (76.9) | 11 (100) | |
| Wide local excision | 3 (23.1) | 0 (0) | |
| Completed chemotherapy | 0.1819 | ||
| Yes | 11 (84.6) | 6 (54.5) | |
| No | 2 (15.4) | 5 (45.5) | |
| Radiological | |||
| Sonographic tumor size (mm) | 0.4780 | ||
| 0–20 | 4 (30.8) | 2 (18.2) | |
| 21–50 | 9 (69.2) | 9 (81.8) | |
| Pathologic features on core biopsy | |||
| Histology | 1.0000 | ||
| Invasive ductal carcinoma | 12 (92.3) | 10 (90.9) | |
| Invasive lobular carcinoma/others | 1 (7.7) | 1 (9.1) | |
| Tumor grade | 0.3498 | ||
| I, II | 5 (38.5) | 8 (72.7) | |
| III | 5 (38.5) | 2 (18.2) | |
| Missing | 3 (23.0) | 1 (9.1) | |
| ER | 0.1228 | ||
| Positive | 5 (38.5) | 8 (72.7) | |
| Negative | 8 (61.5) | 3 (27.3) | |
| PR | 0.0825 | ||
| Positive | 2 (15.4) | 6 (54.5) | |
| Negative | 11 (84.6) | 5 (45.5) | |
| HER2 | 0.2397 | ||
| Positive | 7 (53.8) | 3 (27.3) | |
| Negative | 6 (46.2) | 8 (72.7) | |
| ypT (mm) | 0.0311 | ||
| 0–20 | 12 (92.3) | 5 (45.5) | |
| 21–50 | 1 (7.7) | 5 (45.5) | |
| >50 | 0 (0) | 1 (9.1) | |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Discussion
Using the ultrasound criteria of ≥3 abnormal nodes to identify the Z0011 trial patients with high nodal burden, 54.2% of these patients could achieve nodal pCR post NACT, hence avoiding an ALND. The nodal pCR rate varied with molecular subtype and was highest for the TN subtype. To the best of our knowledge, this is the first study attempting to determine the reduction rate of ALND by NACT in the Z0011 trial postulated high nodal burden patients who otherwise would have required an upfront ALND.
Though AMAROS trial (6) had shown that early breast cancer patients with positive sentinel nodes could be treated safely with radiotherapy instead of ALND, the study included very few patients of high nodal burden of ≥3 metastatic sentinel lymph nodes, hence the current axilla treatment for this group of patients with high nodal burden remained an ALND.
However, ALND is associated with multiple complications. On the other hand, NACT had shown encouraging rates of nodal pCR. Targeted axillary dissection, which involved the removal of the clipped node and sentinel nodes, could be performed after NACT to identify the patients with nodal pCR, hence sparing these patients an ALND. Various ways of localizing the clipped node, which was the initially metastatic node, have been described. These methods included hookwire localization, radioactive seed, the wireless non-radioactive methods such as Savi Scout, Magseed, radiofrequency localizer etc. (7). Each localizing method is associated with its own advantages and pitfalls and had aided in the identification of patients with nodal pCR after NACT, hence sparing them an ALND. To date, however, it is unknown if NACT could reduce the need for ALND in these Z0011 trial high nodal burden patients who would otherwise have needed an ALND, which was the aim of this study.
Although our study population was defined on the assumption that ≥3 sonographically abnormal nodes is highly predictive of the high nodal burden group in the Z0011 trial, we were unable to compare the demographics, radiological and pathological features in our cohort with that of the high nodal burden patients in the Z0011 trial (8) since these features were not reported in the Z0011 trial. However, it has been reported that T1–2 patients who did not fulfil the Z0011 criteria of axillary preservation because of 3 or more metastatic sentinel lymph nodes tended to have higher proportions of T2 and grade III cancers (9) which was similar to our cohort.
NACT could result in nodal pCR, with the pathologic features such as high grade (10), ER negativity and HER2 positivity (11) reported as the most significant factors influencing the nodal pCR rates. In a retrospective study by Pilewskie et al. (12), they found that in cT1–2 patients with nodal disease and ER/PR+ HER2− subtype tumors, they had lower ALND rates (15%) if they underwent upfront surgery with breast conservation instead of NACT (34%). There were 2 reasons for this finding. Firstly, this subtype responds poorly to NACT, hence resulting in little nodal pCR. Secondly, if an upfront surgery was performed instead, this group of patients could adopt Z0011 trial criteria of performing an ALND only if there were ≥3 metastatic nodes, hence allowing axillary preservation in patients with low nodal burden. These two reasons accounted for the lower ALND rates in the upfront surgery group compared to the patients who had undergone NACT.
However, the above study did not distinguish the effect of NACT in the high nodal burden group specifically but determined the effect of NACT in the Z0011 trial cohort collectively. Similarly, in our cohort with ER/PR+ HER2− tumors who underwent NACT, the ALND rate was also reported high at 70%. This high figure is not surprising as our cohort consisted specifically of the supposedly high nodal burden group who would require an upfront ALND. NACT, however, did minimize the risk of ALND in this tumor subtype by 30%.
Conversely, in the same study (12), cT1–2N0 TN or HER2 patients who had NACT had reduced rates of ALND. This was also similar to our cohort with the TN group and the HER2 positive group having the highest nodal pCR rates among the various tumor subtypes.
In our study, ypT was statistically associated with the nodal pCR. This finding was consistent with literature which reported similar findings (13). Breast tumor pCR have been reported to be highly correlated with nodal pCR (14).
Though patients with percutaneous biopsy proven metastatic nodal disease could have a higher nodal disease compared to those detected on sentinel lymph node biopsy alone (15), 47% of the T1–2 patients with positive percutaneous biopsy would still qualify for Z0011 trial of axillary preservation (16). In particular, our cohort for analysis consisted of cT1–2N0 patients with positive percutaneous nodal biopsy. The sonographic criteria of ≥3 sonographically abnormal lymph nodes was then applied to define the high nodal burden group who would not qualify for axillary preservation in Z0011 trial. This group of patients underwent NACT instead.
A strength of the paper included documentation of the number of abnormal nodes in the axillary ultrasound reports which was not practiced worldwide. In addition, an ALND was also performed to determine nodal pCR.
Limitations included a small sample size and retrospective nature with possible selection bias of the patients with high risk tumor subtypes for NACT. The high nodal burden group from Z0011 trial was predicated on the number of abnormal axillary nodes on initial axillary ultrasound, which had been reported to be a highly predictive factor. Though this predictive factor does not identify all cases of high nodal burden, in reality, there is no other way in which this ALND reduction rate could be determined. The most certain way to determine the group of patients with high nodal burden would be to perform a sentinel lymph node biopsy and only include those with ≥3 metastatic sentinel lymph nodes. However, then we would not be able to assess the effect of NACT on the removed lymph nodes to assess nodal pCR rate.
In conclusion, in the postulated subgroup of T1–2 breast cancer patients with high nodal burden who will require an ALND, NACT could result in nodal pCR in 54.2% of these patients. Nodal pCR was also associated with ypT. The nodal pCR benefit was noted most significantly with the TN and HER2+ subtype but could still result in nodal pCR in 30% of ER/PR+ HER2 negative patients. As a result, NACT should be offered in this identified high nodal burden group to minimize ALND risk.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-7/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-7/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-7/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by SingHealth Centralised Institutional Review Board (CIRB Ref: 2020/2147) and waiver of patients’ informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg 2016;264:413-20. [Crossref] [PubMed]
- Wernicke AG, Shamis M, Sidhu KK, et al. Complication rates in patients with negative axillary nodes 10 years after local breast radiotherapy after either sentinel lymph node dissection or axillary clearance. Am J Clin Oncol 2013;36:12-9. [Crossref] [PubMed]
- Lim GH, Teo SY, Gudi M, et al. Initial results of a novel technique of clipped node localization in breast cancer patients postneoadjuvant chemotherapy: Skin Mark clipped Axillary nodes Removal Technique (SMART trial). Cancer Med 2020;9:1978-85. [Crossref] [PubMed]
- Lim GH, Teo SY, Allen JC Jr, et al. Determining Whether High Nodal Burden in Early Breast Cancer Patients Can Be Predicted Preoperatively to Avoid Sentinel Lymph Node Biopsy. J Breast Cancer 2019;22:67-76. [Crossref] [PubMed]
- Lim GH, Upadhyaya VS, Acosta HA, et al. Preoperative predictors of high and low axillary nodal burden in Z0011 eligible breast cancer patients with a positive lymph node needle biopsy result. Eur J Surg Oncol 2018;44:945-50. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Laws A, Dillon K, Kelly BN, et al. Node-Positive Patients Treated with Neoadjuvant Chemotherapy Can Be Spared Axillary Lymph Node Dissection with Wireless Non-Radioactive Localizers. Ann Surg Oncol 2020;27:4819-27. [Crossref] [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [Crossref] [PubMed]
- Bonneau C, Hequet D, Estevez JP, et al. Impact of axillary dissection in women with invasive breast cancer who do not fit the Z0011 ACOSOG trial because of three or more metastatic sentinel lymph nodes. Eur J Surg Oncol 2015;41:998-1004. [Crossref] [PubMed]
- Lim LY, Miao H, Lim JS, et al. Outcome after neoadjuvant chemotherapy in Asian breast cancer patients. Cancer Med 2017;6:173-85. [Crossref] [PubMed]
- Montagna G, Mamtani A, Knezevic A, et al. Selecting Node-Positive Patients for Axillary Downstaging with Neoadjuvant Chemotherapy. Ann Surg Oncol 2020;27:4515-22. [Crossref] [PubMed]
- Pilewskie M, Zabor EC, Mamtani A, et al. The Optimal Treatment Plan to Avoid Axillary Lymph Node Dissection in Early-Stage Breast Cancer Patients Differs by Surgical Strategy and Tumor Subtype. Ann Surg Oncol 2017;24:3527-33. [Crossref] [PubMed]
- Yan Z, Wong A, Ng RP, et al. Association of the initial number of sonographically abnormal nodes with nodal pathological response and its implication. Clin Imaging 2021;78:19-21. [Crossref] [PubMed]
- Lim GH, Gudi M, Teo SY, et al. Would Removal of All Ultrasound Abnormal Metastatic Lymph Nodes Without Sentinel Lymph Node Biopsy Be Accurate in Patients with Breast Cancer with Neoadjuvant Chemotherapy? Oncologist 2020;25:e1621-7. [Crossref] [PubMed]
- Lloyd P, Theophilidou E, Newcombe RG, et al. Axillary tumour burden in women with a fine-needle aspiration/core biopsy-proven positive node on ultrasonography compared to women with a positive sentinel node. Br J Surg 2017;104:1811-5. [Crossref] [PubMed]
- Pilewskie M, Mautner SK, Stempel M, et al. Does a Positive Axillary Lymph Node Needle Biopsy Result Predict the Need for an Axillary Lymph Node Dissection in Clinically Node-Negative Breast Cancer Patients in the ACOSOG Z0011 Era? Ann Surg Oncol 2016;23:1123-8. [Crossref] [PubMed]


