Advances in imaging technologies for planning breast reconstruction
Introduction
Following mastectomy, the goal of breast reconstruction is to re-create a new breast mound that looks and feels natural, durable and can mature and change with the patient over time. Reconstruction is recognized to benefit the patient’s psychosexual wellbeing, recovery and psyche during breast cancer management and has become an important consideration for women after mastectomy for cancer or risk reduction surgery. Although implant based reconstruction is more widely performed, autologous reconstruction is seen as the preferable choice in some patients. However the procedure is perceived to be more complex, technically more challenging, has longer operative, recovery time, and higher earlier complication risk, particularly in microsurgical breast reconstruction.
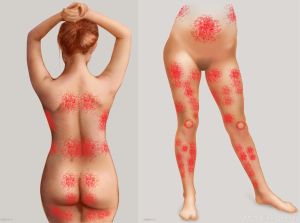
The success of free perforator-based flap transfer is reliant on a robust blood supply and inclusion of a dominant perforator that can support the flap. A big challenge in planning autologous breast reconstruction is to develop predictable, efficient and reproducible results. A better understanding of vascular anatomy is vital to the design and harvest of flaps. Knowledge of “hot spots” that represent high-density areas of dominant perforators and their potential vascular territories in the body creates a broad platform of possible donor sites (Figure 1). As the demand for perforator flaps has grown, surgeons have sought tools and technologies to enable them to perform these surgeries with more consistency and predictability (1). Computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) have played a valuable role in the advance of anatomical research of perforator flaps (2,3), and preoperative planning of patients in breast reconstruction, providing a three-dimensional (3D) representation of the vascular architecture. The adoption of sophisticated imaging technologies in clinical practice and research has propelled our knowledge of flap physiology, microcirculatory architecture, vascular territories, axiality of flow of dominant perforators and role of linking vessels within the soft tissue integument (2,4-14). This review describes the role of advanced imaging technologies in research and planning in breast reconstruction.
Imaging in vascular anatomical research
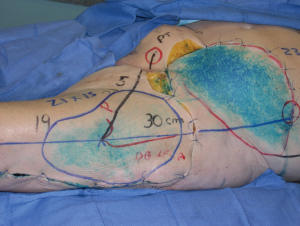
The curiosity in human anatomy and the vascular anatomy has spanned over the centuries (15). Through a comprehensive understanding of vascular territories of the skin and soft tissues (16-20) flaps can be tailored to optimize maximal axiality of blood flow from dominant perforators and inclusion of dominant linking vessels (4). Original injection techniques to study skin vascular territories included India ink, methylene blue, latex, and colored wax. Ink injection studies provide a two-dimensional (2D) surface estimation of potential vascular territories of the source vessel or individual perforator (Figure 2). The introduction of radiography and development of early contrast media allowed vascular anatomy to be visualized in a more detailed 2D format and measuring vessel caliber, perforator location, and determining boundaries of vascular territories on the basis of vascular branching within the subcutaneous tissue (20,21) However in the last decade, the use of CTA has elaborated the 3D vascular architectural network and understanding of the microcirculation in the dermis with ultra-high resolution and 0.3 mm slice thickness (Figure 3) (2,4,6,7,12,13,22-31). New imaging modalities have provided new knowledge in the dynamics of perforator vascular territories, the role of direct and indirect communications between perforators that are relevant to flap planning and harvest. This has been exemplified in anatomical studies of the lower abdominal wall delineating patterns of branching and vascular territories of the deep inferior epigastric artery perforators (DIEP) used in breast reconstruction (5,6,11,26,29).
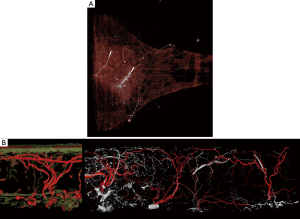
A step further into the understanding of perfusion territories in anatomical research is the incorporation of four-dimensional (4D) imaging using CTA (2,7,13). This has allowed a sequence of images to be collected and studied to appreciate the nature of vascular filling patterns from an individual perforator. In particular, 4D angiography can emphasize the location of dominant communicating direct linking vessels, axiality of their flow and impact on the overall vascular territory. The use of micro-CT in anatomical studies has been able to provide information on the contributions of the subdermal plexus on overall vascular territories of individual perforators on overall perfusion (Figure 3B) (12). Imaging technologies in research have attributed to the better understanding of the location of dominant perforators, patterns of their vascular territories in the 3D integument. This has facilitated development of new flaps or design modifications and considerations for planning in breast reconstruction. There is an evident circle of discovery and re-discovery in anatomical research that can optimize flap design.
Preoperative planning in breast reconstruction
Although the role of preoperative imaging can provide useful information, there is still a debate on whether it is necessary for all patients and its impact on improving outcomes. The harvest of perforator flaps is a complex procedure with a steep learning curve and little room for error therefore knowledge of location and anatomy of the underlying perforators can shorten the learning curve and increase the predictability and intraoperative decision-making to choose the appropriate technique in an individual patient (1,32). The location of dominant perforator hot spots is predictable, but there is a high degree of variability among patients and between individual sides of the same patient (11,33). Preoperative imaging can assist in selection of appropriate donor sites, flap design and determining an individual’s unique anatomy. There are different imaging modalities used in reconstructive surgery, with each having associated costs, time, availability, convenience, post-processing techniques, radiation exposure, and different levels of accuracy in mapping and interpretation. The use of handheld Doppler and color duplex ultrasonography have been traditionally used to detect the location of perforators and their characteristics of flow (34-37), and these techniques are less expensive and obviate the radiation exposure or need for contrast agents. However, these techniques have been associated with significant inter-observer variability, conducted over 45–60 minutes by highly experienced personnel, have high false positive rates, may be limited by difficulty in interpretation of findings, reproducibility, patient body habitus and only provides a 2D picture of the underlying vascular architecture (38). The use of CTA and MRA, which will be discussed, provides a global 3D map, objective findings, and reported as superior techniques in localization and characterization of perforator anatomy (Table 1) (10,39,40).
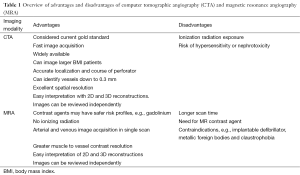
Full table
Computed tomographic angiography (CTA)
Masia et al. [2006] (41) and Alonso-Burgos et al. [2006] (42) were among the first to report the use of preoperative CTA in planning of free tissue transfer and today this has been widely adopted into clinical practice and considered to some extent as the gold standard for preoperative imaging (1,10,11,42-46). The CTA protocol is standardized and the technique has been previously described (41,47,48). Multi-detector row computed tomography (MDCT) has allowed for rapid large volume image acquisition that can be subsequently used to construct multidimensional images of small vessels. The images provide detailed representations of the source vessels, perforators and linking vessels within the subcutaneous tissue, and side branches within the muscle (Figure 4). The majority of published data is on its application in perforator mapping of the DIEP to examine perforator anatomy in terms of location, caliber, and course preoperatively (11,49). The major advantages of CTA for imaging vascular anatomy are that it eliminates inter-observer variability, it is non-invasive, highly reproducible, has a short scanning time (less than 5 minutes), high spatial resolution, visualization of vessels as small as 0.3 mm and easy interpretation of the imaging (46). A literature review by Chae et al. [2010] reported that most studies have sensitivity and specificity for perforator localization close to 100% (46). Post processing techniques, volume rendering and 3D reconstruction following CTA has optimized the visualization of vascular anatomy and course of the perforating branches and with greater concordance with intraoperative findings (50,51). Use of the maximum intensity projection technique can help visualize the pedicle in the coronal plane and axial plane to further depict the intramuscular course (42,50). Post processing and 3D digital reconstruction can be carried out using free or commercially available software and can facilitate the creation of the 3D global mapping of the vascular anatomy that can be easily interpreted by the surgeon.
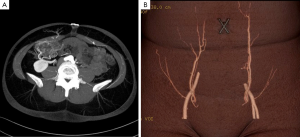
Although CTA can produce accurate information on perforator architecture, it does not provide physiological information on flow characteristics of perforators preoperatively or assessment of perfusion. It is debated whether CTA has beneficial effect on complications, flap failure rates, fat necrosis and overall patient outcomes. It has a valuable role in women who had previous abdominal surgery who could be a potential candidate for DIEP reconstruction, allowing assessment of scarring and availability of perforators (52). Chae et al. [2015] (46) they reported some studies have demonstrated that there is a beneficial effect on reducing operative time, length of hospital stay, intraoperative blood loss, and incidence of postoperative bulge in abdominal based flaps (38,40,44,53-57). The cost savings in reduced operative time could notably offset the costs incurred for the CT and 3D reconstruction (38), however larger prospective studies are required to advocate its true impact.
It is generally agreed that the role of preoperative CTA helped determine if a patient had suitable perforators, and based on caliber, course and characteristics of the perforators, it allowed the surgeon to develop a plan for perforator selection and anticipated dissection time (38). The use of a greater proportion of single dominant perforator flaps in DIEP reconstruction have been reported with the use of preoperative CTA by Ghattaura et al. [2010] (54). In their study there was an increase from 18% in the non-CTA group to 48% in the CTA group in the use of single perforator flaps, and an increase in use of medial row perforators from 31% in the non CTA group to 65% in the CTA group. This may have represented the increased confidence in the position, caliber and branching of perforators from preoperative findings, and if the dominant perforator was noted medially it saved time intra-operatively by obviating the need to review all perforators (54). Furthermore, preoperative imaging can help identify uncommon congenital absence or iatrogenic ligation of the deep inferior epigastric source vessels, aberrant communication of perforators with underlying source vessels, or absence of suitable DIEP perforators (45,56). Although CTA does not provide details on flow characteristics and perfusion, the ability to examine the linking vessels and branching pattern in the subcutaneous tissue and connection with adjacent perforator territories, may allow the surgeon to make inferences on the potential perfusion territories (45). Similarly, connections between the superficial and deep systems, and communicating vessels across the midline can be examined (58).
Although CTA has superseded Doppler ultrasound (US) in many institutions, it is not universally considered as a first-line preoperative imaging choice (59,60). Agreement between color Doppler US and CTA has been reported to be good. Cina et al. [2010] (60) reported in a study of 45 patients who had both CTA and Color Doppler US, both modalities were accurate in perforator mapping and had their associated strengths and weaknesses in assessing artery calibers and estimating the intramuscular courses as previously described. However there are conflicting comparative studies between Doppler US and CTA in terms of determining the superiority of perforator location of one modality over another, but many are limited by sample size and may come down to the quality and type of information that is desired from preoperative study (39,40,47,59,60).
Although CTA is associated with a higher cost than US techniques, it is still considered as affordable and currently than MRA studies. As previously described, the use of CTA in preoperative planning has been associated with a reduction in operative time, length of hospital stay and complications which can offset the increased cost of CTA studies when compared to US (46). For example, Rozen et al. [2009] have previously reported a cost analysis study of CTA in DIEP flap surgery comparing the total expense of a CTA study at their institution (US $490), cost benefits of reduced operative time and length of hospital stay, with an overall calculated cost saving of US $3,410 per case, which was considered a positive motivator for hospital systems to support this imaging modality in preoperative imaging (61). It is important that if CTA is to be incorporated into the preoperative planning of microsurgical breast reconstruction that a dedicated protocol is established within the institution specific to the device being used. Similarly there is valuable role in collaborating with radiology colleagues to create a reporting procedure to highlight crucial and relevant details specific to DIEP flap surgery. Radiation exposure has been a limitation or sometimes concern for preoperative planning, however the advancement of CT protocols which have been developed for more focused exams has limited the radiation exposure on average around 5mSv per scan, which is equivalent to two abdominal radiographs and lower than a standard abdominal CT (30,41,48,62,63). Sensitivity to iodinated contrast materials or its nephrotoxicity can be the main limitation associated with CTA in selected patients with known allergies or renal impairment.
Several dose-reduction techniques including tube current modulation, reduced tube voltage, use of higher pitch and noise filters have been implemented in the past, and these have shown variable degrees of success due to depth of radiation penetration and image distortion (64). Advances in CTA technology continues to provide more sophisticated equipment. This may include increasing number of detector rows, higher resolution imaging, faster image acquisition, higher temporal resolution to minimize motion artifacts, 4D scanning at half the radiation dose, refinements in the dual energy properties for better tissue differentiation, and the possibility to acquire high quality imaging with concentrations of contrast media. This reduces the overall exposure of the patient to potential sensitivities and radiation exposure and has significant clinical implications for preoperative planning of breast reconstruction and reducing any risks to the patient. As reconstructive algorithms evolve it has been shown that these techniques, such as adaptive statistical iterative reconstruction protocols that have been incorporated into commercial CT scanners, can now achieve reproduce high quality comparable images without amplification of noise in the imaging (64). Other developments include 3D perforator anatomy models for the patient from preoperative imaging. Real-time projection guidance tools for intraoperative perforator identification have also been investigated, but require further detailed studies (51,56,65). Hummelink et al. [2015] reported the use of a handheld projection tool intra-operatively to map the perforators and anatomical course of the vessel to the source DIEA from the preoperative CTA directly onto the abdomen. This new tool was to demonstrate the combination of a minimalist operative room compatible projection system and CTA reconstructed images. A comparison was made with localization with a handheld Doppler, demonstrating better localization, and with a potential benefit in patients with a high BMI >30 (65).
Magnetic resonance angiography (MRA)
In 1994, Ahn et al. first described the use of MR imaging for delineating perforators of the lower abdomen for TRAM flap selection without contrast (66). The use of contrast-enhanced MR angiography (CE-MRA) protocol in examination of the DIEA vascular anatomy is a relatively newer imaging modality and represents the next generation in preoperative DIEP planning. The protocol has been previously described by Chernyak (47,67,68). Rozen et al. [2009] reported a low specificity with MRA (50%) despite a high sensitivity (100%), which suggested that it was still inferior to preoperative CTA (69). However significant advances have been seen in imaging acquisition, processing, contrast agents and the scanners themselves. MRA has notable advantages over CTA as it obviates the exposure to ionizing radiation but can still provide high imaging quality and accurate localization of perforators with high concordance with intraoperative findings (67,70,71). Chernyak et al. demonstrated a 97% correlation with intraoperative findings on assessment of 21 patients, 30 DIEP flaps, in which 27 out of 33 were raised on a single perforator (67). The utility of MRA has been applied in the preoperative assessment of gluteal and upper thigh flaps to accurately determine the location of dominant perforators (72). Although stronger field strength 3.0 T scanners are becoming increasingly more commonplace, with superior spatial resolution, contrast enhancement and lower motion artifact (46), Vasile et al. used 1.5 T hardware for improved image quality and represent a scanner that is currently more widely available (72). MRA is considered to have lower spatial resolution but higher contrast resolution, permitting a better delineation of intramuscular course of perforators (73). There are ongoing developments for better spatial resolution using newer contrast agents and algorithms to gain high quality imaging even at 1.5 T, for example Verslius et al. [2013] reviewed images captured in the equilibrium phase to get high spatial resolution of smaller DIEA perforators together with use of newer blood pool contrast agent (74).
The imaging of the venous system has received greater interest over the years and can be considered as an important component to pre-surgical DIEP flap planning. Schaverien et al. [2011] (70) has reported that CE-MRA from their experience that there was a highly significant relationship between pre-surgical information from CE-MRA on the connections between perforator vena comitantes and the superficial inferior epigastric vein (SIEV) and the incidence of diffuse venous congestion (P<0.0001) (75). MR post processing and analysis is similar to that of CTA (47), although it would always be beneficial to work closely with the radiology colleagues to develop and optimize the reconstruction and reporting protocols to facilitate interpretation. There is little evidence in the published literature to make inferences on the role of MRA on clinical outcomes in microsurgical breast reconstruction.
CE-MRA with gadolinium has a better safety risk profile, lower hypersensitivity compared to iodinating contrast media used in CTA, and associated with a rare syndrome of nephrogenic systemic fibrosis in patients with severe renal impairment (70). Some of the advances in MRA have been the introduction of newer contrast agents and scanners with higher field strength for better quality image acquisition. Examples of novel contrast agents include the use of extracellular contrast agents such as gadobenate dimeglumine, and newer blood pool contrast agents such as gadofosveset trisodium that have produced super quality images due to higher relaxivity and longer imaging window (46,76). Gadofosveset trisodium reversibly binds to serum albumin with a high fraction providing greater contrast enhancement (46). The lack of ionization radiation exposure also permits the safe assessment of multiple potential donor sites within the same study using Gadofosveset trisodium (76). Masia et al. [2010] (77) have developed protocols with non-contrast MRA and obtained clear images of the perforator anatomy that had 100% correlation with intraoperative findings.
Limitations with MRA in preoperative planning in breast reconstruction include the higher costs compared to CTA, and limitation in detecting perforators less than 0.8 mm in diameter, susceptibility to motion artifact and the requirement for the patient to breath-hold for longer periods, which may not be feasible in some patients (46). MRA is contraindicated in severe obesity, patients with implanted defibrillators, implanted metallic devices or foreign bodies. It is a relative contraindication in patients with artificial heart valves, patients who cannot lay still, or have severe anxiety or claustrophobia. Currently the higher cost, availability and timing for the examination, is a major drawback to the adoption of MRA in breast reconstruction. Actual scanning time required for CE-MRA is around 20–30 seconds, compared to MD-CTA which is less than 20 seconds (70), although total examination time may be up to 40 minutes. The key focus areas in advancement and innovation for MRA imaging are based on the scanner technology, contrast agents and algorithms for image acquisition. As technology and contrast agents become increasingly sophisticated, there will be an increase in image quality, reduced acquisition times and reduced susceptibility to motion artifact. As MRA becomes more accessible it may become considered as a first line imaging modality for preoperative planning in the near future, in particular for younger patients, iodine hypersensitivity or renal impairment (46).
Laser-assisted indocyanine green fluorescence angiography (LA-ICGFA)
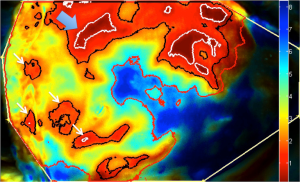
Fluorescent angiography has been used in other specialties but its utility in reconstructive surgery is relatively new and still developing. Following the injection of indocyanine green through peripheral injection, the cutaneous vascularity is captured within 15 seconds to 2.5 minutes using infrared energy to excite the ICG, and recorded with inbuilt video and analysis software algorithms to generate quantitative data (1,46). It has a short half-life of 3–4 minutes and excreted in the bile, which makes repeat measurements possible intra-operatively. Its role in preoperative imaging is still limited and the information it can provide is limited to a few millimeters deep from the skin (78). LA-ICGFA can characterize flow and perfusion in flaps and tissue and its use has focused on intraoperative assessment of flap perfusion, patency of the anastomosis, and on occasion postoperative monitoring (79-82). New quantitative algorithms may have the potential of identifying cutaneous perforators in a 2D map using ingress/ egress calculations per pixel to create timing maps to locate dominant perforators (Figure 5). However the 3D mapping and detailed knowledge of underlying anatomy is absent. This technology is considered better at assessing flap physiology and perfusion used intra-operatively as an adjunct to clinical judgment, and in conjunction with other preoperative imaging technologies to improve predictability and reduced complications in breast reconstruction. It can be used intra-operatively to assess DIEP tissue perfusion following liposuction (83). Its main impact has been in early identification of mastectomy skin necrosis reducing its subsequent complications, but this also has an impact on breast reconstruction planning (80,84).
Dynamic infrared thermography (DIRT)
The use of thermal imaging to assess the cutaneous circulation was introduced in the 1980s but its application in preoperative planning of perforator flaps is more novel (85-88). There is limited evidence on the efficacy and comparison of this imaging modality as a tool for preoperative perforator selection in breast reconstruction. The technique is based on surface cooling followed by a period of rewarming, and during the rewarming phase, the cutaneous perfusion is analyzed with an infrared camera and hot spots correlate with perforator location. This technology is non-invasive and requires no contrast agents, however the accuracy of the this technology has been compared with Doppler (85) and findings were dependent on the pattern of rewarming. This technology, although non-invasive and with low patient risk profile, can only provide moderate and variable data on perforator location through a 2D map. Compared to the 3D architectural mapping from CTA and MRA, it is considered an inferior preoperative imaging choice. It is not widely available and although it may be associated with lower costs, there is a dearth of evidence to support its utility for preoperative planning and limited evidence intra-operatively that would support decision-making in breast reconstruction (89).
Conclusions
Technology is rapidly progressing and continues to challenge and exceed expectations, while maintaining considerations for patient safety and accessibility. In our experience the review of comprehensive imaging such as CTA and MRA can highlight other dominant perforators at nearby potential donor sites and facilitate the surgeon in considerations for all feasible options for autologous breast reconstruction, but do not provide physiological information of flow characteristics. The development of some of these technologies serve as an aid to assist to reduce the learning curve, potentially decrease surgery time and operative stress and may translate to improved clinical outcomes. Any imaging technique should aspire to have the lowest risk of harm to the patient, acquisition of the highest quality images providing the greatest amount of information, and be performed within the short duration and minimal burden to the patient. Current imaging modalities should still be considered as an adjunct and not a replacement for clinical judgment in planning and intra-operative decision-making in breast reconstruction. The evolution of technology has facilitated the ability to improve the predictability and reproducibility of outcomes in autologous breast reconstruction (1) and translate to improved patient outcomes and efficiency, although large prospective clinical data is still required in this area (1,38,43,51,54,55,70,73,79,80,90-92).
Acknowledgements
Dr. AT Mohan received financial support from the Blond Research Fellowship Royal College of Surgeons of England for a research fellowship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nahabedian MY. Overview of perforator imaging and flap perfusion technologies. Clin Plast Surg 2011;38:165-74. [PubMed]
- Saint-Cyr M, Schaverien M, Arbique G, et al. Three- and four-dimensional computed tomographic angiography and venography for the investigation of the vascular anatomy and perfusion of perforator flaps. Plast Reconstr Surg 2008;121:772-80. [PubMed]
- Tregaskiss AP, Goodwin AN, Bright LD, et al. Three-dimensional CT angiography: a new technique for imaging microvascular anatomy. Clin Anat 2007;20:116-23. [PubMed]
- Saint-Cyr M, Wong C, Schaverien M, et al. The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg 2009;124:1529-44. [PubMed]
- Wong C, Saint-Cyr M, Mojallal A, et al. Perforasomes of the DIEP flap: vascular anatomy of the lateral versus medial row perforators and clinical implications. Plast Reconstr Surg 2010;125:772-82. [PubMed]
- Schaverien M, Saint-Cyr M, Arbique G, et al. Arterial and venous anatomies of the deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg 2008;121:1909-19. [PubMed]
- Wong C, Saint-Cyr M, Rasko Y, et al. Three- and four-dimensional arterial and venous perforasomes of the internal mammary artery perforator flap. Plast Reconstr Surg 2009;124:1759-69. [PubMed]
- Xue J, Arbique G, Hatef D, et al. Four-dimensional vascular tree reconstruction using moving grid deformation. Acad Radiol 2007;14:1540-53. [PubMed]
- Sur YJ, Morsy M, Mohan AT, et al. Three-dimensional computed tomographic angiographic study of the inter-perforator flow of the lower leg. Plast Reconstr Surg 2016. [Epub ahead of print]. [PubMed]
- Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the DIEA: a cadaveric study. Plast Reconstr Surg 2008;122:363-9. [PubMed]
- Rozen WM, Ashton MW, Grinsell D. The branching pattern of the deep inferior epigastric artery revisited in-vivo: a new classification based on CT angiography. Clin Anat 2010;23:87-92. [PubMed]
- Laungani AT, Van Alphen N, Christner JA, et al. Three-dimensional CT angiography assessment of the impact of the dermis and the subdermal plexus in DIEP flap perfusion. J Plast Reconstr Aesthet Surg 2015;68:525-30. [PubMed]
- Chan JW, Wong C, Ward K, et al. Three- and four-dimensional computed tomographic angiography studies of the supraclavicular artery island flap. Plast Reconstr Surg 2010;125:525-31. [PubMed]
- Saint-Cyr M. Assessing perforator architecture. Clin Plast Surg 2011;38:175-202. [PubMed]
- Bergeron L, Tang M, Morris SF. A review of vascular injection techniques for the study of perforator flaps. Plast Reconstr Surg 2006;117:2050-7. [PubMed]
- Manchot C. The cutaneous arteries of the human body. 18th ed. New York: Springer-Verlag, 1983.
- Cormack, GC, Lamberty BG. The Arterial Anatomy of Skin Flaps. Edinburgh: Churchill Livingstone, 1994.
- Salmon M. Artères de la peau. Paris: Masson, 1936.
- Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg 2003;30:331-42. v. [PubMed]
- Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987;40:113-41. [PubMed]
- Cormack GC, Lamberty BG. Cadaver studies of correlation between vessel size and anatomical territory of cutaneous supply. Br J Plast Surg 1986;39:300-6. [PubMed]
- Schaverien M, Wong C, Bailey S, et al. Thoracodorsal artery perforator flap and Latissimus dorsi myocutaneous flap--anatomical study of the constant skin paddle perforator locations. J Plast Reconstr Aesthet Surg 2010;63:2123-7. [PubMed]
- Villa M, Saint-Cyr M, Wong C, et al. Extended vertical rectus abdominis myocutaneous flap for pelvic reconstruction: three-dimensional and four-dimensional computed tomography angiographic perfusion study and clinical outcome analysis. Plast Reconstr Surg 2011;127:200-9. [PubMed]
- Saint-Cyr M, Schaverien M, Wong C, et al. The extended anterolateral thigh flap: anatomical basis and clinical experience. Plast Reconstr Surg 2009;123:1245-55. [PubMed]
- Colohan S, Wong C, Lakhiani C, et al. The free descending branch muscle-sparing latissimus dorsi flap: vascular anatomy and clinical applications. Plast Reconstr Surg 2012;130:776e-787e. [PubMed]
- Bailey SH, Saint-Cyr M, Wong C, et al. The single dominant medial row perforator DIEP flap in breast reconstruction: three-dimensional perforasome and clinical results. Plast Reconstr Surg 2010;126:739-51. [PubMed]
- Schaverien M, Saint-Cyr M. Perforators of the lower leg: analysis of perforator locations and clinical application for pedicled perforator flaps. Plast Reconstr Surg 2008;122:161-70. [PubMed]
- Rozen WM, Chubb D, Stella DL, et al. Evaluating anatomical research in surgery: a prospective comparison of cadaveric and living anatomical studies of the abdominal wall. ANZ J Surg 2009;79:913-7. [PubMed]
- Rozen WM, Ashton MW. The venous anatomy of the abdominal wall for Deep Inferior Epigastric Artery (DIEP) flaps in breast reconstruction. Gland Surg 2012;1:92-110. [PubMed]
- Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the deep inferior epigastric artery: a blinded, prospective cohort study. Plast Reconstr Surg 2008;122:1003-9. [PubMed]
- Aho JM, Laungani AT, Herbig KS, et al. Lumbar and thoracic perforators: vascular anatomy and clinical implications. Plast Reconstr Surg 2014;134:635e-45e. [PubMed]
- Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg 2009;124:752-64. [PubMed]
- Rozen WM, Chubb D, Whitaker IS, et al. Deep inferior epigastric perforators do not correlate between sides of the body: the role for preoperative imaging. J Plast Reconstr Aesthet Surg 2010;63:e842-3. [PubMed]
- Giunta RE, Geisweid A, Feller AM. The value of preoperative Doppler sonography for planning free perforator flaps. Plast Reconstr Surg 2000;105:2381-6. [PubMed]
- Blondeel PN, Beyens G, Verhaeghe R, et al. Doppler flowmetry in the planning of perforator flaps. Br J Plast Surg 1998;51:202-9. [PubMed]
- Berg WA, Chang BW, DeJong MR, et al. Color Doppler flow mapping of abdominal wall perforating arteries for transverse rectus abdominis myocutaneous flap in breast reconstruction: method and preliminary results. Radiology 1994;192:447-50. [PubMed]
- Chang BW, Luethke R, Berg WA, et al. Two-dimensional color Doppler imaging for precision preoperative mapping and size determination of TRAM flap perforators. Plast Reconstr Surg 1994;93:197-200. [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [PubMed]
- Scott JR, Liu D, Said H, et al. Computed tomographic angiography in planning abdomen-based microsurgical breast reconstruction: a comparison with color duplex ultrasound. Plast Reconstr Surg 2010;125:446-53. [PubMed]
- Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16. [PubMed]
- Masia J, Clavero JA, Larrañaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [PubMed]
- Alonso-Burgos A, García-Tutor E, Bastarrika G, et al. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg 2006;59:585-93. [PubMed]
- Masia J, Kosutic D, Clavero JA, et al. Preoperative computed tomographic angiogram for deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg 2010;26:21-8. [PubMed]
- Casey WJ 3rd, Chew RT, Rebecca AM, et al. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast Reconstr Surg 2009;123:1148-55. [PubMed]
- Casey WJ 3rd, Rebecca AM, Kreymerman PA, et al. Computed tomographic angiography: assessing outcomes. Clin Plast Surg 2011;38:241-52. [PubMed]
- Chae MP, Hunter-Smith DJ, Rozen WM. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland Surg 2015;4:164-78. [PubMed]
- Aubry S, Pauchot J, Kastler A, et al. Preoperative imaging in the planning of deep inferior epigastric artery perforator flap surgery. Skeletal Radiol 2013;42:319-27. [PubMed]
- Phillips TJ, Stella DL, Rozen WM, et al. Abdominal wall CT angiography: a detailed account of a newly established preoperative imaging technique. Radiology 2008;249:32-44. [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. Planning and optimising DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconstr Aesthet Surg 2010;63:289-97. [PubMed]
- Pacifico MD, See MS, Cavale N, et al. Preoperative planning for DIEP breast reconstruction: early experience of the use of computerised tomography angiography with VoNavix 3D software for perforator navigation. J Plast Reconstr Aesthet Surg 2009;62:1464-9. [PubMed]
- Gacto-Sánchez P, Sicilia-Castro D, Gómez-Cía T, et al. Computed tomographic angiography with VirSSPA three-dimensional software for perforator navigation improves perioperative outcomes in DIEP flap breast reconstruction. Plast Reconstr Surg 2010;125:24-31. [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. The effect of anterior abdominal wall scars on the vascular anatomy of the abdominal wall: A cadaveric and clinical study with clinical implications. Clin Anat 2009;22:815-22. [PubMed]
- Uppal RS, Casaer B, Van Landuyt K, et al. The efficacy of preoperative mapping of perforators in reducing operative times and complications in perforator flap breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:859-64. [PubMed]
- Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg 2010;63:1597-601. [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [PubMed]
- Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization -- better for the patient, the surgeon, and the hospital. Clin Radiol 2013;68:131-8. [PubMed]
- Minqiang X, Lanhua M, Jie L, et al. The value of multidetector-row CT angiography for pre-operative planning of breast reconstruction with deep inferior epigastric arterial perforator flaps. Br J Radiol 2010;83:40-3. [PubMed]
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [PubMed]
- Keys KA, Louie O, Said HK, et al. Clinical utility of CT angiography in DIEP breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:e61-5. [PubMed]
- Cina A, Salgarello M, Barone-Adesi L, et al. Planning breast reconstruction with deep inferior epigastric artery perforating vessels: multidetector CT angiography versus color Doppler US. Radiology 2010;255:979-87. [PubMed]
- Rozen WM, Ashton MW, Whitaker IS, et al. The financial implications of computed tomographic angiography in DIEP flap surgery: a cost analysis. Microsurgery 2009;29:168-9. [PubMed]
- Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol 2013;23:2333-43. [PubMed]
- Rozen WM, Whitaker IS, Stella DL, et al. The radiation exposure of Computed Tomographic Angiography (CTA) in DIEP flap planning: low dose but high impact. J Plast Reconstr Aesthet Surg 2009;62:e654-5. [PubMed]
- Niumsawatt V, Debrotwir AN, Rozen WM. Reducing radiation dose without compromising image quality in preoperative perforator flap imaging with CTA using ASIR technology. Int Surg 2014;99:485-91. [PubMed]
- Hummelink S, Hameeteman M, Hoogeveen Y, et al. Preliminary results using a newly developed projection method to visualize vascular anatomy prior to DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:390-4. [PubMed]
- Ahn CY, Narayanan K, Shaw WW. In vivo anatomic study of cutaneous perforators in free flaps using magnetic resonance imaging. J Reconstr Microsurg 1994;10:157-63. [PubMed]
- Chernyak V, Rozenblit AM, Greenspun DT, et al. Breast reconstruction with deep inferior epigastric artery perforator flap: 3.0-T gadolinium-enhanced MR imaging for preoperative localization of abdominal wall perforators. Radiology 2009;250:417-24. [PubMed]
- Mathes DW, Neligan PC. Preoperative imaging techniques for perforator selection in abdomen-based microsurgical breast reconstruction. Clin Plast Surg 2010;37:581-91. xi. [PubMed]
- Rozen WM, Stella DL, Bowden J, et al. Advances in the pre-operative planning of deep inferior epigastric artery perforator flaps: magnetic resonance angiography. Microsurgery 2009;29:119-23. [PubMed]
- Schaverien MV, Ludman CN, Neil-Dwyer J, et al. Contrast-enhanced magnetic resonance angiography for preoperative imaging of deep inferior epigastric artery perforator flaps: advantages and disadvantages compared with computed tomography angiography: a United Kingdom perspective. Ann Plast Surg 2011;67:671-4. [PubMed]
- Greenspun D, Vasile J, Levine JL, et al. Anatomic imaging of abdominal perforator flaps without ionizing radiation: seeing is believing with magnetic resonance imaging angiography. J Reconstr Microsurg 2010;26:37-44. [PubMed]
- Vasile JV, Newman T, Rusch DG, et al. Anatomic imaging of gluteal perforator flaps without ionizing radiation: seeing is believing with magnetic resonance angiography. J Reconstr Microsurg 2010;26:45-57. [PubMed]
- Pauchot J, Aubry S, Kastler A, et al. Preoperative imaging for deep inferior epigastric perforator flaps: a comparative study of computed tomographic angiography and magnetic resonance angiography. Eur J Plast Surg 2012;35:795-801.
- Versluis B, Tuinder S, Boetes C, et al. Equilibrium-phase high spatial resolution contrast-enhanced MR angiography at 1.5T in preoperative imaging for perforator flap breast reconstruction. PLoS One 2013;8:e71286. [PubMed]
- Schaverien MV, Ludman CN, Neil-Dwyer J, et al. Relationship between venous congestion and intraflap venous anatomy in DIEP flaps using contrast-enhanced magnetic resonance angiography. Plast Reconstr Surg 2010;126:385-92. g. [PubMed]
- Agrawal MD, Thimmappa ND, Vasile JV, et al. Autologous breast reconstruction: preoperative magnetic resonance angiography for perforator flap vessel mapping. J Reconstr Microsurg 2015;31:1-11. [PubMed]
- Masia J, Kosutic D, Cervelli D, et al. In search of the ideal method in perforator mapping: noncontrast magnetic resonance imaging. J Reconstr Microsurg 2010;26:29-35. [PubMed]
- Pestana IA, Zenn MR. Correlation between abdominal perforator vessels identified with preoperative CT angiography and intraoperative fluorescent angiography in the microsurgical breast reconstruction patient. Ann Plast Surg 2014;72:S144-9. [PubMed]
- Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg 2010;125:1065-73. [PubMed]
- Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthet Surg J 2014;34:61-5. [PubMed]
- Holm C, Dornseifer U, Sturtz G, et al. Sensitivity and specificity of ICG angiography in free flap reexploration. J Reconstr Microsurg 2010;26:311-6. [PubMed]
- Yamaguchi S, De Lorenzi F, Petit JY, et al. The "perfusion map" of the unipedicled TRAM flap to reduce postoperative partial necrosis. Ann Plast Surg 2004;53:205-9. [PubMed]
- Casey WJ 3rd, Connolly KA, Nanda A, et al. Indocyanine green laser angiography improves deep inferior epigastric perforator flap outcomes following abdominal suction lipectomy. Plast Reconstr Surg 2015;135:491e-497e. [PubMed]
- Wapnir I, Dua M, Kieryn A, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol 2014;21:100-6. [PubMed]
- de Weerd L, Weum S, Mercer JB. The value of dynamic infrared thermography (DIRT) in perforatorselection and planning of free DIEP flaps. Ann Plast Surg 2009;63:274-9. [PubMed]
- Itoh Y, Arai K. Use of recovery-enhanced thermography to localize cutaneous perforators. Ann Plast Surg 1995;34:507-11. [PubMed]
- Salmi AM, Tukiainen E, Asko-Seljavaara S. Thermographic mapping of perforators and skin blood flow in the free transverse rectus abdominis musculocutaneous flap. Ann Plast Surg 1995;35:159-64. [PubMed]
- Zetterman E, Salmi AM, Suominen S, et al. Effect of cooling and warming on thermographic imaging of the perforating vessels of the abdomen. Eur J Plast Surg 1999;22:58-61.
- Chubb DP, Taylor GI, Ashton MW. True and 'choke' anastomoses between perforator angiosomes: part II. dynamic thermographic identification. Plast Reconstr Surg 2013;132:1457-64. [PubMed]
- Tong WM, Dixon R, Ekis H, et al. The impact of preoperative CT angiography on breast reconstruction with abdominal perforator flaps. Ann Plast Surg 2012;68:525-30. [PubMed]
- Zang M, Yu S, Xu L, et al. Intercostal artery perforator propeller flap for reconstruction of trunk defects following sarcoma resection. J Plast Reconstr Aesthet Surg 2015;68:822-9. [PubMed]
- Liu DZ, Mathes DW, Zenn MR, et al. The application of indocyanine green fluorescence angiography in plastic surgery. J Reconstr Microsurg 2011;27:355-64. [PubMed]


