
Predictors of hyperkalemia after total parathyroidectomy in patients with drug-refractory secondary hyperparathyroidism
Introduction
End-stage renal disease (ESRD) requires long-term renal dialysis replacement therapy. Owing to recent advancements in medical science and technology, patients receiving dialysis treatment can achieve long-term survival. Secondary hyperparathyroidism (SHPT) has become one of the most frequent complications in patients with ESRD, presenting a higher risk of death due to the associated adverse consequences of metabolic disorders, bone disease, and cardiovascular disease (1). SHPT is mainly caused by hyperphosphatemia and vitamin D deficiency or resistance (2). Although there are pharmacological treatments, such as vitamin D preparations and calcium mimetics, for early SHPT, long-term pharmacological treatment often results in the development of drug resistance, and this drug-refractory SHPT requires surgical management. There are three types of parathyroidectomies (PTX): subtotal parathyroidectomy (SPTX), total parathyroidectomy (TPTX), and total parathyroidectomy with autologous transplantation (TPTX + AT). A Japanese study (3) showed a 41% reduction in cardiovascular mortality and a 34% reduction in overall mortality in patients who underwent PTX. Though surgical treatment of drug-refractory SHPT can be highly beneficial, it also has several risks, such as hypocalcemia, hypophosphatemia, and hyperkalemia (4,5), and electrolyte changes in patients during the perioperative period need to be closely monitored. Although we have extensive knowledge about hypocalcemia and hypophosphatemia, the mechanisms of hyperkalemia after TPTX are not clear. Some studies have shown that 25% to 80% of patients develop hyperkalemia after TPTX treatment (6,7). In contrast, the rate of cardiac arrest or in-hospital mortality in patients with hyperkalemic ESRD was 12.4% (8). This suggests that we do not have an adequate understanding of the changes in potassium homeostasis associated with TPTX.
The goal of this study was to investigate the primary preoperative clinical risk factors for predicting the risk of postoperative hyperkalemia after TPTX to assess patients’ indications for surgery more accurately. We present the following article in accordance with the STARD reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-27/rc).
Methods
General clinical data
This study was approved by the Ethics Committee of the Lishui Central Hospital, Zhejiang Province (No. 2021-277). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients and their family members signed an informed consent form. All bar 1 were included in the study.
We retrospectively analyzed a total of 149 eligible patients who had undergone TPTX with autotransplantation (TPTX + AT) for drug-refractory SHPT at Lishui Central Hospital from August 2015 to November 2021. The cohort included 71 males and 78 females, with a mean age of 49.66±9.74 years. Patients had received maintenance dialysis, including hemodialysis (n=85) and peritoneal dialysis (n=64), with a mean dialysis duration of 5.99±3.12 years (Table 1).
Table 1
| Covariate | Total (n=149) | Non-hyperkalemic group (n=124) | Hyperkalemic group (n=25) | P value |
|---|---|---|---|---|
| Age (years) | 49.66±9.74 | 49.44±9.57 | 50.76±10.69 | 0.537 |
| Gender | 0.201 | |||
| Male | 71 (47.65) | 62 (50.00) | 9 (36.00) | |
| Female | 78 (52.35) | 62 (50.00) | 16 (64.00) | |
| BMI (kg/m2) | 22.13±3.37 | 22.18±3.27 | 21.87±3.88 | 0.678 |
| SBP (mmHg) | 144.15±24.95 | 143.59±25.31 | 146.92±23.40 | 0.544 |
| DBP (mmHg) | 86.42±14.05 | 86.69±14.33 | 85.12±12.75 | 0.613 |
| HR (beats/min) | 87.09±12.61 | 87.18±12.60 | 86.68±12.89 | 0.858 |
| Dialysis duration (years) | 5.99±3.12 | 5.67±2.43 | 7.56±5.16 | 0.005* |
| SHPT duration (months) | 20.61±20.67 | 18.90±20.05 | 29.14±22.03 | 0.023* |
| Dialysis | <0.001* | |||
| Peritoneal dialysis | 64 (42.95) | 64 (51.61) | 0 (0.00) | |
| Hemodialysis | 85 (57.05) | 60 (48.39) | 25 (100.00) | |
| Diabetes | 0.333 | |||
| Yes | 11 (7.38) | 8 (6.45) | 3 (12.00) | |
| No | 138 (92.62) | 116 (93.55) | 22 (88.00) | |
| Hypertension | 0.183 | |||
| Yes | 131 (87.92) | 111 (89.52) | 20 (80.00) | |
| No | 18 (12.08) | 13 (10.48) | 5 (20.00) | |
| Preoperative UA (μmol/L) | 415.36±100.55 | 416.74±99.77 | 407.67±110.70 | 0.806 |
| Preoperative CH (mmol/L) | 4.46±1.15 | 4.47±1.17 | 4.41±1.04 | 0.821 |
| Preoperative TG (mmol/L) | 1.91±1.30 | 1.89±1.27 | 2.01±1.44 | 0.698 |
| Preoperative HDL (mmol/L) | 0.98±0.29 | 0.97±0.30 | 1.02±0.29 | 0.540 |
| Preoperative LDL (mmol/L) | 2.24±0.71 | 2.24±0.73 | 2.23±0.61 | 0.978 |
| Preoperative CT (pg/mL) | 11.02±18.14 | 10.30±16.12 | 14.64±26.41 | 0.356 |
| Preoperative ALP (U/L) | 268.40±256.98 | 246.30±234.22 | 380.75±334.63 | 0.019* |
| Preoperative ALB (g/L) | 36.28±3.63 | 36.09±3.60 | 37.22±3.72 | 0.155 |
| Preoperative Na+ (mmol/L) | 139.78±2.78 | 139.74±2.92 | 140.00±2.00 | 0.666 |
| Preoperative Cl− (mmol/L) | 101.01±3.98 | 100.87±4.19 | 101.71±2.69 | 0.338 |
| Preoperative K+ (mmol/L) | 4.24±0.59 | 4.16±0.57 | 4.65±0.53 | <0.001* |
| Postoperative K+ (mmol/L) | 4.47±0.88 | 4.16±0.58 | 5.98±0.44 | <0.001* |
| Preoperative Ca2+ (mmol/L) | 2.38±0.18 | 2.37±0.18 | 2.46±0.16 | 0.025* |
| Postoperative Ca2+ (mmol/L) | 2.13±0.41 | 2.12±0.39 | 2.14±0.52 | 0.846 |
| Preoperative P (mmol/L) | 2.13±0.56 | 2.10±0.55 | 2.27±0.56 | 0.165 |
| Postoperative P (mmol/L) | 1.35±0.38 | 1.36±0.37 | 1.31±0.41 | 0.510 |
| Preoperative iPTH (pg/mL) | 1,563.63±665.10 | 1,534.40±665.88 | 1,708.63±655.10 | 0.233 |
| Postoperative iPTH (pg/mL) | 26.40±50.45 | 26.83±54.16 | 24.13±23.28 | 0.815 |
| Operation time (min) | 87.33±28.04 | 85.70±28.50 | 95.40±24.58 | 0.115 |
Values are presented as mean ± standard deviation or number (percentage). *, P<0.05. ALB, albumin; ALP, alkaline phosphatase; BMI, body mass index; Ca, serum calcium; CH, cholesterol; Cl, serum chloride; CT, calcitonin; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HR, heart rate; iPTH, intact parathyroid hormone; K, serum potassium; LDL, low-density lipoprotein; Na, serum sodium; P, phosphorous; SBP, systolic blood pressure; SHPT, secondary hyperparathyroidism; TG, triglyceride; UA, uric acid.
Inclusion and exclusion criteria
Drug-refractory SHPT was resistant to drug therapy, due to reduced expression of vitamin D and calcium-sensitive receptors, although medication still results in obviously elevated intact parathyroid hormone. The criteria for inclusion were the same as in our previous study (2): (I) level of intact parathyroid hormone higher than 800 pg/mL; (II) hyperphosphatemia or hypercalcemia that was not able to be controlled by drug therapy; (III) hyperplastic paracrine glands as suggested by ultrasonography or other imaging evidence, and (IV) significant clinical symptoms, such as skeletal pain, skeletal deformities, osteoporosis, uncorrectable anemia, and pruritus of the skin.
Patients were excluded if they met any of the following criteria: (I) primary hyperparathyroidism; (II) total non-PTX with forearm autotransplantation; (III) reoperation for parathyroid hyperplasia, or (IV) the presence of contraindications to general anesthesia (Figure 1).
Surgical procedure
All patients were anesthetized by combined intravenous-inhalational general anesthesia and underwent surgical treatment performed by the same trained group of surgeons. All parathyroid glands found were removed, and a relatively normal portion without proliferative nodules of the parathyroid gland was transplanted to the brachioradialis muscle of the forearm.
Demographic information, including gender, age, and body mass index (BMI), clinical data, including dialysis modality, duration of the procedure, and previous underlying diseases, were recorded for all patients. Preoperative blood tests were performed to monitor levels of hemoglobin, fasting blood glucose, alkaline phosphatase, serum sodium, serum calcium, serum potassium, serum chloride, serum phosphorus, and intact parathyroid hormone levels. Hyperkalemia was defined as a serum potassium level >5.3 mmol/L. If preoperative hyperkalemia was diagnosed, treatment was administered to reduce serum potassium levels ≤5.3 mmo/L. Levels of intact parathyroid hormone, serum potassium, serum calcium, and serum phosphorous were measured in the morning after surgery and again on the third postoperative day before dialysis treatment. Perioperative arterial blood gases were collected at least twice, first after the removal of 2 parathyroid glands and then after the removal of all the parathyroid glands. If potassium levels were >5.3 mmol/L, they were decreased through the intravenous administration of either high glucose and insulin (50% dextrose 20 mL + insulin 10 U) or, at the anesthesiologist’s discretion, calcium gluconate for hyperkalemia. Arterial blood gases were repeated 15 to 20 minutes after administering the potassium-lowering drugs to reassess potassium levels, and if necessary, emergency hemodialysis was arranged to treat the hyperkalemia further.
Statistical analysis
Our study was conducted using the R statistical software package (R V.3.4.3) (https://www.r-project.org/) and EmpowerStats® (http://www.empowerstats.com, X&Y solutions, Inc., Boston, MA, USA) for statistical analysis. Continuous variables are represented as mean ± standard deviation (SD). Categorical variables are expressed as numbers (percentages). Normally and non-normally distributed continuous variables were performed using t-test and Kruskal Wallis rank-sum test. Fisher’s exact probability test was used to compare categorical variables. Univariate logistic analyses were performed to assess the relationship between variables. Predictive parameters for postoperative hyperkalemia were calculated based on receiver operating characteristic (ROC) curves. A two-tailed P value <0.05 was considered statistically significant.
Results
A total of 149 patients with drug-refractory SHPT treated with TPTX + AT were analyzed. Demographic data for the group are shown in Table 1. Patients’ mean postoperative levels of intact parathyroid hormone (26.40±50.45 pg/mL) were significantly lower than preoperative levels (1,563.63±665.10 pg/mL) (P<0.05), while mean postoperative potassium levels were 4.47±0.88 mmol/L, with a maximum of 7.09 mmol/L. Of the 149 patients, 25 (16.78%) had hyperkalemia on postoperative day 1, and mean postoperative potassium levels were considerably higher than in the preoperative period (P<0.05), with a significant decrease on postoperative day 3 (P<0.05) (Table 2).
Table 2
| Covariate | Serum potassium levels, mean ± standard deviation | P value |
|---|---|---|
| Preoperative potassium | 4.24±0.59 | 0.002 |
| Postoperative potassium | 4.47±0.88 | 0.011 |
| 3-day postoperative potassium | 4.23±0.71 | – |
Comparison of the factors associated with postoperative hyperkalemia (Table 1) suggested that there were no differences in gender, age, BMI, blood pressure, and heart rate between the hyperkalemic and non-hyperkalemic groups. However, the average dialysis duration was 7.56±5.16 years in the hyperkalemic group, which was significantly longer than 5.67±2.43 years in the non-hyperkalemic group (P=0.005). In addition, the duration of SHPT was 29.14±22.03 years in the hyperkalemic group, which was also significantly longer than 18.90±20.05 years in the non-hyperkalemic group (P=0.023). Preoperative alkaline phosphatase levels were higher in the hyperkalemic group (380.75±334.63 U/L) than in the non-hyperkalemic group (246.30±234.22 U/L) (P=0.019), as were preoperative serum calcium levels (2.46±0.16 vs. 2.37±0.18 mmol/L, respectively) (P=0.025). Preoperative serum potassium levels were also significantly higher in the hyperkalemic group than in the non-hyperkalemic group (4.65±0.53 vs. 4.16±0.57 mmol/L, respectively) (P<0.001). We also observed that postoperative hyperkalemia was only seen in patients who had hemodialysis, while in those who had peritoneal dialysis, no postoperative hyperkalemia occurred. Further analysis included univariate logistic regression analysis for dialysis duration, SHPT duration, preoperative alkaline phosphatase levels, preoperative serum potassium levels, and preoperative serum calcium levels. As shown in Table 3 dialysis duration was associated with postoperative hyperkalemia, and increased probability of hyperkalemia with increased number of years on dialysis [odds ratio (OR) 1.18, 95% confidence interval (CI): 1.03, 1.35, P=0.014]. Preoperative serum potassium levels were also associated with postoperative risk of hyperkalemia. Increased serum potassium levels and increased risk of hyperkalemia (OR 4.95, 95% CI: 2.05, 11.96, P<0.001), as were as preoperative serum calcium levels (OR 16.17, 95% CI: 1.36, 191.58, P=0.027). SHPT duration and alkaline phosphatase levels proved to be independent variables with no significant influence on the incidence of postoperative hyperkalemia.
Table 3
| Covariate | OR (95% CI) | P value |
|---|---|---|
| Dialysis duration (years) | 1.18 (1.03, 1.35) | 0.014 |
| SHPT duration (months) | 1.02 (1.00, 1.04) | 0.030 |
| Preoperative ALP (U/L) | 1.00 (1.00, 1.00) | 0.027 |
| Preoperative K+ (mmol/L) | 4.95 (2.05, 11.96) | <0.001 |
| Preoperative Ca2+ (mmol/L) | 16.17 (1.36, 191.58) | 0.027 |
ALP, alkaline phosphatase; Ca, serum calcium; CI, confidence interval; K, serum potassium; OR, odds ratio; SHPT, secondary hyperparathyroidism.
ROC curves (Figure 2) showed that the optimum cutoff point for preoperative serum potassium levels was 4.57 mmol/L, with a sensitivity of 64%, specificity of 75%, and area under the curve (AUC) of 0.732. The optimum cutoff point for dialysis duration was 8.5 years, with a sensitivity of 36%, specificity of 88.71%, and AUC of 0.596. For preoperative serum calcium levels, the optimum cutoff point was 2.31 mmol/L, with a sensitivity of 92%, specificity of 37.9%, and AUC of 0.636. The risk of postoperative hyperkalemia was lower in patients with dialysis duration of less than 8.5 years than in those with longer dialysis duration (OR 0.47, 95% CI: 0.04, 0.90, P=0.005). In addition, the risk of postoperative hyperkalemia was lower in patients with preoperative blood calcium levels of less than 2.31 mmol/L (OR 0.52, 95% CI: 0.09, 0.95, P=0.025) and those with preoperative blood potassium levels below 4.57 mmol/L (OR 0.88, 95% CI: 0.44, 1.32, P<0.001) (Table 4).
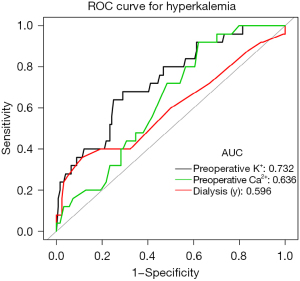
Table 4
| Covariate | Non-hyperkalemic group (n=124) | Hyperkalemic group (n=25) | P value | OR | 95% CI |
|---|---|---|---|---|---|
| Dialysis duration >8.5 years | 0.005 | 0.47 | 0.04, 0.90 | ||
| No | 110 | 16 | |||
| Yes | 14 | 9 | |||
| Preoperative calcium >2.305 mmol/L | 0.025 | 0.52 | 0.09, 0.95 | ||
| No | 47 | 2 | |||
| Yes | 77 | 23 | |||
| Preoperative potassium >4.565 mmol/L | <0.001 | 0.88 | 0.44, 1.32 | ||
| No | 93 | 9 | |||
| Yes | 31 | 16 | |||
AT, autotransplantation; CI, confidence interval; OR, odds ratio; SHPT, secondary hyperparathyroidism; TPTX, total parathyroidectomy.
Discussion
As cases of SHPT increase, so drug-refractory cases gradually become more common, requiring treatment with TPTX + AT. Successful PTX usually rapidly reduces intact parathyroid hormone levels, relieving symptoms and reducing mortality.
High levels of intact parathyroid hormone have been shown to affect multiple organ functions in patients with ESRD (9), inducing harmful conditions such as vascular calcification (10), electrolyte disturbances (11), inhibition of immunoglobulin production (12), and inhibition of erythropoietin synthesis (13). Electrolyte disorders, especially hyperkalemia, are particularly acute conditions. Hyperkalemia is currently defined as a higher-than-normal range of serum potassium levels, with various arbitrary cutoff values used to indicate different levels of severity (14). Despite there being no single cutoff value for defining hyperkalemia, many papers studying SHPT and hyperkalemia have used serum potassium levels >5.3 mmol/L to define hyperkalemia (15-17). Therefore, this criterion was also used to define hyperkalemia in our study.
A review of the findings of Yang et al. (5) found an increased incidence of hyperkalemia after PTX in young male patients with ESRD, and they reported a total of 32 patients undergoing PTX for SHPT, of whom 8 (25%) became hyperkalemic during the procedure. Song et al. (7) reported a total of 90 patients, of whom 24.4% developed postoperative hyperkalemia, and found that the incidence of postoperative hyperkalemia was higher in males and younger patients, though the differences were not statistically significant. However, the results of their study did not suggest a correlation between postoperative hyperkalemia and age or gender.
Szeto et al. (18) showed that hypokalemia was more common in patients on peritoneal dialysis, they suggesting that lower normal blood potassium levels in patients on peritoneal dialysis may be due to the more sustained and adequate clearance of blood potassium by peritoneal dialysis compared to hemodialysis. There were no patients on peritoneal dialysis among the 8 hyperkalemic patients in the study by Yang et al. (5), consistent with the results of the present study.
Even with the best of our preoperative preparations, we can see that postoperative hyperkalemia can still occur after PTX + AT, and the prevention of postoperative hyperkalemia places a higher demand on our patient management. Bures et al. (19) suggested that early postoperative dialysis treatment with assessment of low risk of postoperative complications may be useful in reducing the incidence of postoperative hyperkalemia.
Previous research had shown that approximately 1/3 of patients receiving maintenance hemodialysis develop hyperkalemia. The prevalence of hyperkalemia among patients receiving prolonged dialysis is 2.4 times higher than among those on shorter courses of dialysis (20), with a consequent increase in the risk of emergency room visits, hospitalization, and sudden death due to malignant arrhythmias. Among the 149 participants receiving dialysis in this study, there were 25 cases of hyperkalemia after TPTX + AT, representing an incidence of 16.78%, consistent with previous reports of a 14.8–66.7% incidence of hyperkalemia after PTX (5,15,21). We suggest that a possible cause of hyperkalemia after TPTX + AT is that the procedure promotes osteoblast activity and decreases osteoclast activity, leading to postoperative short-term changes in bone metabolic markers and hungry bone syndrome (HBS) (6,22). Levels of intact parathyroid hormone reduce dramatically shortly after surgery, and a large number of calcium ions enter the bones, causing a rapid decrease in the calcium content in the extracellular fluid. The sodium-calcium exchanger increases intracellular sodium ions through the membrane barrier action (23) and affects the activity of the sodium-potassium pump, promoting sodium efflux and potassium influx (16,24) and thus leading to higher extracellular potassium content. The inability of the kidneys to metabolize and remove excess potassium ions from the extracellular fluid in patients receiving dialysis is the primary cause of hyperkalemia after PTX. Consistent with our study, high preoperative blood potassium levels tend to lead to postoperative hyperkalemia because of the accumulation of potassium ions in the blood. In contrast, hyperkalemia due to hypercalcemia, which has not been reported in the literature, might be the result of a combination of changes in intact parathyroid hormone levels during PTX, as postulated earlier.
ROC curve analysis revealed that preoperative blood potassium and blood calcium levels, and dialysis duration, are the 3 significant indicators for postoperative hyperkalemia, with preoperative blood potassium levels having a balanced sensitivity and specificity, preoperative blood calcium levels having a high sensitivity but low specificity, and dialysis duration having a low sensitivity but high specificity. According to our study, patients with preoperative potassium levels >4.57 mmol/L are at higher risk for hyperkalemia, and perioperative blood gas analysis is recommended to determine potassium concentrations and promptly administer glucose and insulin therapy if hyperkalemia occurs. This critical value for the prognosis of postoperative hyperkalemia can help prevent complications, such as life-threatening arrhythmias, arising from postoperative hyperkalemia.
This study had certain limitations. It was a single-center retrospective study with a relatively small sample size, and it lacked data on patient medication and diet regimes, which need to be studied further.
Conclusions
This study reviewed the occurrence of hyperkalemia after TPTX + AT in patients with drug-refractory SHPT and evaluated how multiple perioperative factors impacted postoperative hyperkalemia. Results showed that preoperative serum potassium and serum calcium levels and the duration of dialysis treatment could predict postoperative hyperkalemia. Delayed surgery, emergency care, or medication should be considered for patients with preoperative blood potassium levels >4.57 mmol/L. This strategy would be a simple and effective way to reduce the postoperative risk of patients.
Acknowledgments
Funding: This study was supported by the Medical and Health Research Program of Zhejiang Province (No. 2021435899 and No. 2022ZH090); Lishui Municipal Science and Technology Program (grant No. 2019SJZC07).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-27/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-27/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-27/coif). All authors report grants from Health Commission of Zhejiang Province, Lishui Science and Technology Bureau, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Lishui Central Hospital, Zhejiang Province (No. 2021-277). All patients and their family members signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xu Y, Evans M, Soro M, et al. Secondary hyperparathyroidism and adverse health outcomes in adults with chronic kidney disease. Clin Kidney J 2021;14:2213-20. [Crossref] [PubMed]
- Zhu L, Cheng F, Zhu X, et al. Safety and effectiveness of reoperation for persistent or recurrent drug refractory secondary hyperparathyroidism. Gland Surg 2020;9:401-8. [Crossref] [PubMed]
- Komaba H, Taniguchi M, Wada A, et al. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int 2015;88:350-9. [Crossref] [PubMed]
- Koshkelashvili N, Lai JY. IMAGES IN CLINICAL MEDICINE. Hyperkalemia after Missed Hemodialysis. N Engl J Med 2016;374:2268. [Crossref] [PubMed]
- Yang YL, Lu HF, Chung KC, et al. Young age, male sex, and end-stage renal disease with secondary hyperparathyroidism as risk factors for intraoperative hyperkalemia during parathyroidectomy. J Clin Anesth 2015;27:195-200. [Crossref] [PubMed]
- Cruz DN, Perazella MA. Biochemical aberrations in a dialysis patient following parathyroidectomy. Am J Kidney Dis 1997;29:759-62. [Crossref] [PubMed]
- Song YH, Cai GY, Xiao YF, et al. Can we predict who will develop postoperative hyperkalaemia after parathyroidectomy in dialysis patients with secondary hyperparathyroidism? BMC Nephrol 2019;20:225. [Crossref] [PubMed]
- Singh T, Alagasundaramoorthy S, Gregory A, et al. Low dialysis potassium bath is associated with lower mortality in end-stage renal disease patients admitted to hospital with severe hyperkalemia. Clin Kidney J 2021;14:2059-63. [Crossref] [PubMed]
- Massry SG, Smogorzewski M. Mechanisms through which parathyroid hormone mediates its deleterious effects on organ function in uremia. Semin Nephrol 1994;14:219-31. [PubMed]
- Salam S, Gallagher O, Gossiel F, et al. Vascular calcification relationship to vascular biomarkers and bone metabolism in advanced chronic kidney disease. Bone 2021;143:115699. [Crossref] [PubMed]
- Fouque D, Roth H, Pelletier S, et al. Control of mineral metabolism and bone disease in haemodialysis patients: which optimal targets? Nephrol Dial Transplant 2013;28:360-7. [Crossref] [PubMed]
- Gaciong Z, Alexiewicz JM, Linker-Israeli M, et al. Inhibition of immunoglobulin production by parathyroid hormone. Implications in chronic renal failure. Kidney Int 1991;40:96-106. [Crossref] [PubMed]
- Brancaccio D, Cozzolino M, Gallieni M. Hyperparathyroidism and anemia in uremic subjects: a combined therapeutic approach. J Am Soc Nephrol 2004;15:S21-4. [Crossref] [PubMed]
- Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 2014;10:653-62. [Crossref] [PubMed]
- Li S, Liu S, Chen Q, et al. Clinical predictor of postoperative hyperkalemia after parathyroidectomy in patients with hemodialysis. Int J Surg 2018;53:1-4. [Crossref] [PubMed]
- Zou Y, Zhang L, Zhou H, et al. Risk factors of hyperkalemia after total parathyroidectomy in patients with secondary hyperparathyroidism. Ren Fail 2020;42:1029-31. [Crossref] [PubMed]
- Bajaj Y, Roberts S, Simon D, et al. Intra-operative hyperkalemia: a serious but under recognised complication of renal parathyroidectomy - a prospective study: how we do it. Clin Otolaryngol 2011;36:69-72. [Crossref] [PubMed]
- Szeto CC, Chow KM, Kwan BC, et al. Hypokalemia in Chinese peritoneal dialysis patients: prevalence and prognostic implication. Am J Kidney Dis 2005;46:128-35. [Crossref] [PubMed]
- Bures C, Uluk Y, Besmens M, et al. Hyperkalemia Following Parathyroidectomy in Patients with Renal Hyperparathyroidism-New Thresholds for Urgent Perioperative Dialysis. J Clin Med 2022;11:409. [Crossref] [PubMed]
- Palmer BF, Clegg DJ. Gastrointestinal potassium binding in hemodialysis. Kidney Int 2020;98:1095-7. [Crossref] [PubMed]
- Yang G, Wang J, Sun J, et al. Perioperative hyperkalemia in hemodialysis patients undergoing parathyroidectomy for renal hyperparathyroidism. Intern Emerg Med 2019;14:1065-71. [Crossref] [PubMed]
- Ge Y, Yang G, Wang N, et al. Bone metabolism markers and hungry bone syndrome after parathyroidectomy in dialysis patients with secondary hyperparathyroidism. Int Urol Nephrol 2019;51:1443-9. [Crossref] [PubMed]
- Gonzalez-Serratos H, Hilgemann DW, Rozycka M, et al. Na-Ca exchange studies in sarcolemmal skeletal muscle. Ann N Y Acad Sci 1996;779:556-60. [Crossref] [PubMed]
- Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 2003;83:1269-324. [Crossref] [PubMed]
(English Language Editor: LJ. Roberts)



