A cervical compartment syndrome impairs cerebral circulation in post-thyroidectomy hemorrhage: data from an animal model
Introduction
Post thyroidectomy hemorrhage is a rare but potential life-threatening complication (1-5). Although cases of hypoxemic brain damage and death are exceptional events, bleeding complications are associated with an increased risk for additional morbidity (2,5-7). Several risk factors for post thyroidectomy hemorrhage have been identified, however, the rates for bleeding complications in thyroid surgery of 0.3–4% have not changed over the past decades (2-10). Despite technical improvements still bleeding complications are relevant, not completely avoidable, and they remain unpredictable (2,5,6). Especially in the ongoing discussion about outpatient thyroid surgery, but also the increasing numbers of remote-access thyroid surgery procedures, post thyroidectomy hemorrhage is a dreaded complication (1,5).
Fighting old paradigms, we recently demonstrated the connection between an increased cervical compartment pressure (cCP) and respiratory failure (11). In a series of animal experiments with simulation of post thyroidectomy hemorrhage, respiratory drive was suppressed by the artificial increase of intra-cervical pressure, which was reversible with relief of this pressure (11). Due to endotracheal intubation, collapse or relevant obstruction of the airways was impossible (11). Harding et al. earlier disproved the hypothesis of external airway obstruction and suggested a multifactorial pathogenesis (12). Previously, we could demonstrate in ex-vivo human cadaver tracheas that pressure levels of more than 150 mmHg are required to cause relevant mechanical tracheal obstruction (13). We previously reported on four symptomatic patients with post thyroidectomy hemorrhage and increased cervical compartment pressure between 20 and 40 mmHg at time of revision surgery (14). Therefore, even in arterial hemorrhage, pressure levels of more than 100 mmHg are unlikely to be reached.
A deeper insight into the pathophysiology might allow to readjust the algorithm for clinical intervention and thus prevent serious complications associated with post thyroidectomy hemorrhage. Therefore, our aim was to examine the impact of an increased cervical compartment pressure during post thyroidectomy hemorrhage on cerebral vascular perfusion and cerebral oxygenation using our previously established animal model (11). We present the following article in accordance with the ARRIVE reporting checklist (15) (available at https://gs.amegroups.com/article/view/10.21037/gs-21-910/rc).
Methods
A series of experiments were performed on 6 German domestic pigs (age: 3.67±0.52 months, weight: 41.5±2.17 kg, 3 male/3 female) at the animal research site Bad Saarow, Germany. Due to the observational character of the study no assignment to groups, no control group or randomization was used. The experiments were supported by veterinarians ensuring appropriate and stress-free handling of the animals. All experiments were performed under general anesthesia induced with azaperone, ketamine and midazolam and maintained with midazolam and ketamine. Airways were secured by endotracheal intubation using a standard Magill tube with an internal diameter of 6 mm. The pigs breathed spontaneously.
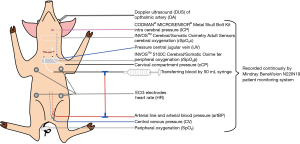
The experimental procedure followed a standardized protocol as previously described (11): standard monitoring was set up (Figure 1) and after thyroidectomy catheters were placed into the surgical site, thereafter, called the cervical compartment, for pressure measurement and for infusing blood into the neck (11). A catheter was placed into the internal jugular vein with its tip at the skull base for pressure monitoring. Due to arterial wall dissection during catheterization of both common carotid arteries in the first test animal, no further attempts were made in the remaining animals to avoid alterations in cerebral perfusion and oxygenation. This animal was excluded from analysis. Via a retroperitoneal approach, one central venous catheter was brought into the inferior vena cava for measurement of central venous pressure. A multi-lumen catheter was placed into the aorta abdominalis for blood pressure monitoring and to establish a direct connection to the neck using three-way stopcocks and an extension line with Luer Lock connection. Intracranial pressure was monitored using a CODMAN® MICROSENSOR® (DePuy Synthes, USA) after borehole trepanation through the frontal bone (Cranial Access Kit, Johnson & Johnson, USA). Cerebral oxygenation was measured using transcranial near-infrared spectroscopy (NIRS; INVOS™ 5100C Cerebral/Somatic Oximeter). Near-infrared spectroscopy quantifies the ratio between oxygenated and total hemoglobin as an unselective signal of arterial perfusion and venous drainage as well as the microcirculation of the cerebral cortex (16,17). INVOSTM adhesive electrodes (INVOS™ Cerebral/Somatic Oximetry Adult Sensors, Medtronic, USA) were placed at the parietal bone and the lower ventral neck for direct comparison of cerebral versus somatic (peripheral) oxygenation. All parameters including arterial blood pressure, heart rate, intracranial pressure, central venous pressure, cerebral and peripheral oxygenation (rSO2c and rSO2p), peripheral oxygen saturation (SpO2), pressure in the internal jugular vein and the cervical compartment pressure, were recorded continuously with a Mindray BeneVision N22|N19 patient monitoring system (Mindray, China). Doppler ultrasound of ophthalmic artery was performed in all animals to evaluate cerebral perfusion. Peak systolic (PSV), mean (MV) and end-diastolic flow velocities (EDV) were measured using curved volume probes (SMT medical GmbH, Germany). Pulsatility (PI) and resistive indices (RI) were calculated (18-20). The experimental setup is shown schematically in Figure 1. Heparin 5.000IE were applied in all animals at the start of manipulations on the blood vessels. Ringer’s solution was infused to avoid hypovolemia during post thyroidectomy hemorrhage simulation (Braun Melsungen AG, Germany).
First, post thyroidectomy hemorrhage was simulated by transferring blood into the cervical compartment using the established direct connection between the catheters in the aorta abdominalis and the neck to increase the cervical compartment pressure (11). Blood was slowly pumped artificially from the aorta to the neck using a 50 mL syringe until apnea occurred. Apnea was defined clinically as a loss of respiratory function for at least 30 sec followed by peripheral hypoxemia. After apnea occurred, cervical compartment pressure was reduced by extracting blood from the cervical compartment. When respiration recovered and vital signs remained stable at baseline levels, the experiment was repeated. Primary endpoint was the difference in cerebral oxygenation (rSO2c) recorded by near-infrared spectroscopy between baseline and increased cervical compartment pressure levels. The sample size calculation was based on a 10% difference in cerebral oxygenation due to the increased pressure in the cervical compartment. Furthermore, the cerebral perfusion (Doppler ultrasound), venous congestion in the internal jugular vein and peripheral oxygenation were quantified.
Second, spontaneous arterial bleeding from the carotid arteries into the cervical compartment was induced. Five animals from the first experimental series and one which did not have previous intervention were used. The spontaneous increase in cervical compartment pressure and the course of vital signs were recorded without further manipulations. Then all animals were sacrificed.
Statistical analysis
For data management and statistical analysis SPSS Statistics (Version 26, IBM, USA) and Prism 8 (GraphPad Software, USA) were used. Median (range) were calculated. Vital signs and parameters of cerebral blood flow and oxygen saturation was analyzed for different events during the experiments: at baseline, with increased pressure, at maximum cervical compartment pressure. Friedmann’s ANOVA for repeated measures (FA) was used with Bonferroni correction for multiple comparisons and Wilcoxon signed-rank test (Wilcoxon) for comparison of related metric data between groups as normal distribution could not be assumed. Pearson’s correlation was used to examine the relationship between variables and Kaplan-Meier diagrams as well as log rank test to compare differences in time between cerebral and peripheral oxygenation. P values <0.05 were considered statistically significant.
Ethical statement
Experiments were performed under a project license (No. 2347-5-2018) granted by the federal state government of Brandenburg, in compliance with EU Directive 2010/63/EU and German national guidelines for the care and use of animals.
Results
Overall, n=12 experiments with simulation of post thyroidectomy hemorrhage into the cervical compartment could be performed in n=5 animals (n=2.4/animal). In two animals one experiment, in two animals three experiments and in one animal four experiments could be performed. The cervical compartment pressure was artificially increased over time reaching the level of the diastolic arterial blood pressure of 50.0 (40.0–55.0) mmHg (P=0.024) and further on a maximum level of 70.0 (42.0–90.0) mmHg (P<0.001) with significant difference in FA (P<0.001). A pressure dependent apnea could be induced in all experiments (n=12/12; 100%) (Figure 2A). There was no significant difference in baseline vital signs (heart rate: P=0.068; mean arterial blood pressure: P=0.066), cervical compartment pressure (P=0.102), peak systolic flow velocity (P=0.138) and peripheral (P=0.786) or cerebral oxygenation (P=0.180) at beginning of the experiments between first and repeated experiments (Wilcoxon).

Cerebral oxygenation
Regarding the primary endpoint, there was a significant decrease of cerebral oxygenation in all experiments (FA: P<0.001) with 24.2% (9.5–34.4%) decrease of cerebral oxygenation at time of apnea compared to baseline values (P=0.043). The minimal median value for cerebral oxygenation was 38.0% (35.0–51.0%), according to a 39.8% (19.0–44.6%) decrease compared to baseline values (P<0.001). In case of recovery (66.7%; n=8/12), cerebral oxygenation returned to baseline levels. During the time the cerebral oxygenation decreased up to 5% (P=0.043) and 10% (P<0.001), peripheral oxygenation was stable like the other vital signs heart rate, arterial blood pressure, intra cranial pressure and central venous pressure (FA: P<0.001) (Figure 2B; Table 1).
Table 1
| Baseline | −5% | cCP = diastolic artBP | −10% | Maximum cCP | Apnea | |
|---|---|---|---|---|---|---|
| cCP | ||||||
| Value (mmHg) | 0 (0–5.0) | 50.0 (40.0–55.0)* | 70.0 (42.0–70.0)* | |||
| Δ time (min:s) | 0:00 | 02:50 (0:50–10:40) | 06:30 (1:45–15:08) | 07:02 (1:35–12:55) | ||
| rSO2c | ||||||
| Value (%) | 63.5 (56.0–74.0) | 60.3 (53.0–70.0) | 57.5 (50.0–67.0)* | 43.5 (35.0–64.0) | 47.0 (38.0–65.0) | |
| Δ time (min:s) | 2:40 (1:00–10:25) | 3:42 (1:20–11:00) | ||||
| SpO2 | ||||||
| Value (%) | 100.0 (98.0–100.0) | 95.0 (93.0–95.0)* | 90.0 (89.0–91.0)* | 99.5 (74.0–100.0) | 93.0 (79.0–99.0)* | |
| Δ time (min:s) | 07:02 (1:35–14:30) | 07:30 (1:50–16:50) | ||||
| rSO2p | ||||||
| Value (%) | 73.0 (60.0–85.0) | 69.4 (57.0–81.0)* | 66.0 (54.0–77.0)* | 71.5 (62.0–89.0) | 70.0 (52.0–83.0)* | |
| Δ time (min:s) | 06:13 (1:35–16.45) | 07:17 (1:55–17:05) |
All P values are corrected by Bonferroni correction for multiple comparisons. *, significant at P<0.05 Friedmann test (ANOVA for repeated measures). cCP, cervical compartment pressure; rSO2c, central oxygenation; SpO2p, peripheral oxygen saturation; rSO2, peripheral oxygenation; Δ (delta), difference; artBP, arterial blood pressure.
Peripheral oxygenation and apnea
The decrease of peripheral oxygenation occurred with a significant delay compared to the cerebral oxygenation. While the cerebral oxygenation decreased simultaneously to the increasing cervical compartment pressure, the peripheral oxygenation decreased with a delay of 180 (15–715) sec and 185 (15–625) sec compared to cerebral oxygenation (log rank: P=0.002/P=0.004) (Figure 2C). Apnea occurred about 200 (−5 to 365) sec after a 10% decrease of the cerebral, but 35 (−35 to 385) sec before a 10% decrease of the peripheral oxygenation (Table 1).
Cerebral perfusion: doppler ultrasound of ophthalmic artery
Doppler ultrasound measurements of the ophthalmic artery were performed in all experiments (n=12). With increased cervical compartment pressure there was a steady decrease of PSV, PI and RI (Figure 3A). PSV reached its minimum value at a maximum cervical compartment pressure and equally low levels at time of apnea. Only the difference for PSV at maximum cervical compartment pressure compared to baseline value was significant (P=0.045; FA: P=0.042). MV and EDV values did not relevantly change over the course of the experiments. Before apnea occurred, there was a constant decrease in PSV, PI and RI until minimum values at time of maximum cervical compartment pressure have been reached and a consecutive decrease of rSO2c (Figure 3B; Table 2).

Table 2
| Baseline | rSO2c 5% drop | cCP = diastolic artBP | rSO2c 10% drop | Maximum cCP | Apnea | |
|---|---|---|---|---|---|---|
| Time (min:s) | 0:00 | 2:40 (1:00–10:25) | 02:50 (0:50–10:40) | 3:42 (1:20–11:00) | 06:30 (1:45–15:08) | 07:02 (1:35–12:55) |
| PSV (cm/s) | 36.8 (28.1–60.9) | 35.4 (29.2–51.4) | 33.9 (21.3–44.7) | 33.4 (20.8–44.6) | 28.0 (18.4–43.2) | 27.8 (17.2–43.5) |
| EDV (cm/s) | 12.1 (10.8–13.5) | 11.8 (10.7–13.4) | 11.8 (10.4–14.1) | 11.7 (10.6–14.3) | 11.6 (10.1–13.2) | 11.7 (10.4–13.6) |
| MV (cm/s) | 23.7 (18.4–37.09 | 24.4 (21.2–31.7) | 24.1 (16.0–28.3) | 23.9 (17.1–28.2) | 19.2 (14.2–35.8) | 20.4 (13.8–27.5) |
| PI | 0.98 (0.71–1.32) | 1.03 (0.76–1.24) | 1.02 (0.58–1.16) | 1.01 (0.56–1.16) | 0.77 (0.45–1.26) | 0.75 (0.43–1.14) |
| RI | 0.66 (0.52–0.79) | 0.68 (0.55–0.77) | 0.67 (0.55–0.77) | 0.67 (0.43–0.74) | 0.55 (0.36–0.77) | 0.54 (0.35–0.73) |
All P values are corrected by Bonferroni correction for multiple comparisons. *, significant at P<0.05 Friedmann test (ANOVA for repeated measures). cCP, cervical compartment pressure; rSO2c, central oxygenation; artBP, arterial blood pressure; PSV, peak systolic flow velocity; MV, mean flow velocity; EDV, end diastolic flow velocity; RI, resistive index; PI, pulsatility index; OA, ophthalmic artery.
Spontaneous hemorrhage into the cervical compartment
Spontaneous hemorrhage into the cervical compartment was simulated in n=6 animals. Starting at baseline values of 2.0 (0–6.0) mmHg cervical compartment pressure increased after inducing the arterial hemorrhage reaching the level of the diastolic arterial blood pressure of 39.0 (31.0–45.0) mmHg [cCP 39.0 (33.0–45.0) mmHg] by a median experimental duration of 406 (290–566) sec. A maximum cervical compartment pressure of 56.0 (35.0–72.0) mmHg was reached similar to the mean arterial blood pressure level of 53.50 (38.0–74.0) mmHg. Vital signs showed a physiologic reaction of hypovolemia with intermittent decrease of arterial blood pressure and increase of heart rate. With compensation of hypovolemia the vital signs returned to baseline levels (Figure 4).

Discussion
Simulating post thyroidectomy hemorrhage, we can demonstrate a significant decrease of cerebral oxygenation with increasing levels of cervical compartment pressure. Moreover, these pathophysiological events followed a clear temporal sequence: at first, the pressure level in the internal jugular vein increased followed by a decrease of the PSV in the ophthalmic artery, both leading to a decrease in cerebral oxygenation. These findings suggest a progressive impairment of venous drainage and arterial perfusion of the brain causing cerebral hypoxemia. With further increasing cervical compartment pressure, a maximum decrease of cerebral perfusion and oxygenation was reached, finally causing apnea followed by a significant decrease of peripheral oxygenation. At the time of apnea, a 18.6% decrease in PSV and a corresponding 24.2% decrease of the cerebral oxygenation was measured. Beyond any doubt, cerebral oxygenation is important to maintain cerebral global functions.
Near-infrared spectroscopy technology can detect regional changes in cerebral oxygenation and has proven to reliably measure the cerebral oxygenation and is also well established in experimental animal studies (16,17,21). Baseline levels for cerebral oxygenation are comparable to our data (53–67%) (17,21-23). Decreases in cerebral oxygenation of 20% and more have been detected and considered to be relevant in brain ischemia-reperfusion models (17,22,23). In our data, a comparable 24.2% decrease of the cerebral oxygenation occurred with strong correlation to an increased cervical compartment pressure and decreased cerebral perfusion.
Doppler ultrasound examination of ophthalmic vessels is a common and reliable technique, used to assess the cerebral perfusion as it reflects the terminal perfusion area of the internal carotid arteries (24-26). Experimental studies already demonstrate a correlation between cerebral arterial perfusion quantified by doppler ultrasound and levels of cerebral oxygenation (17,21-23). Compared to series on healthy controls our experiments have comparable baseline characteristics (18,19,24,26). There is a well described correlation of cerebral perfusion through the internal carotid arteries and flow velocities in the ophthalmic and central retinal arteries (20,27). Heßler et al. detected a 30% decrease of PSV of the central retinal artery in carotid artery stenoses of more than 70% (20). The relevant decrease of PSV in our data started at the time the cervical compartment pressure equaled the diastolic arterial blood pressure. PSV was at minimum level at maximum cervical compartment pressure and the time of apnea (−18.6%). Therefore, a relevant decrease in cerebral perfusion can be assumed, and seems to be the key mechanism of post thyroidectomy hemorrhage related major morbidity and mortality (20,27). This mechanism is consistent with other data showing a significant decrease in cerebral blood flow even wearing a necktie (28). Additionally, the venous drainage must be impaired because of the simultaneously increased pressure in the internal jugular vein.
The increased cervical compartment pressure almost reaching the mean arterial blood pressure is not sufficient for a relevant airway compression as reported previously (13). Therefore, a compartment syndrome could explain the mechanism for the decrease in cerebral oxygenation due an increasing cervical compartment pressure. If untreated, this cervical compartment syndrome can lead to potentially lethal complications like hypoxic brain damage and death (2-7).
A compartment syndrome is defined by an increased pressure in a defined anatomic space because of injury, trauma or hematoma formation (29,30). It causes decreased tissue oxygenation and alterations in cell metabolism with risk for cellular death (29), which can affect adjacent compartments as well (30). A compartment pressure equaling the diastolic arterial blood pressure level is critical because of relevant impairment of microvascular flow due to impairment of venous drainage. When exceeding this threshold level, even the arterial inflow is reduced (29,30). Pressure levels above the diastolic arterial blood pressure can be reached easily in the cervical compartment as demonstrated by our data on spontaneous cervical hemorrhage. Due to the correlation of increased pressure and impairment of perfusion and function in any compartment syndrome, the diagnosis of a compartment syndrome is made either clinically or by well-established single or repeated invasive pressure measurements (29,30). Novel concepts like continuous pressure monitoring might be a much faster and more reliable parameter (14) as the only rational therapy of post thyroidectomy hemorrhage and any other compartment syndrome is a release of the increased pressure by re-opening the wound and/or surgical intervention as fast as possible (1-4,6,11,14,29,30).
Taken together with the observed delay of about 3 minutes between the decrease of cerebral versus peripheral oxygenation in our animal model, beyond evident clinical signs like cervical swelling and discomfort, the commonly used standard monitoring can only detect the late sign of severe post thyroidectomy hemorrhage. Especially with the ongoing discussion about outpatient thyroid surgery and increasing numbers of remote access surgical procedures in thyroid surgery, a reliable remote patient surveillance will be necessary to maintain patients’ safety. We previously demonstrated that postoperative continuous cervical pressure monitoring might be feasible and a reliable tool to increase patients’ safety in thyroid surgery (14). Based on our findings and previous published data, the severity of post thyroidectomy hemorrhage depends on the type and dynamics of the underlaying bleeding, but most post-thyroidectomy hemorrhages occur due to arterial and less often to venous bleeding sources (2-4,6,11,14). In any kind of bleeding, an expending hematoma with progressive impairment of cerebral and cervical venous drainage can occur causing symptoms like swelling, difficulty in swallowing and laryngeal edema. Only in cases of arterial hemorrhage the cervical compartment pressure may reach levels of the mean arterial blood pressure, causing a dangerous impairment of cerebral arterial perfusion, which possibly causes apnea, global hypoxemia, and death, if not treated in time. In some cases, hematoma formation can be observed without acute clinical relevance, which might have to be evacuated in further postoperative course. Therefore, we suggest a clinical classification for post-thyroidectomy hemorrhage, considering the possible severity and risks associated with this hemorrhagic event. This classification must be evaluated regarding its clinical relevance in further studies (Table 3).
Table 3
| Grading | Intra-operative finding of bleeding source | Clinical implication |
|---|---|---|
| I | Superficial hematoma: hematoma/bleeding superficial to the strap muscle | No need for urgent surgical intervention, low risk for bleeding and surgery associated morbidity |
| II | Hematoma without active bleeding in former thyroid space (deep to the strap muscle) | Planned (early elective) surgical revision, moderate risk for surgery-associated morbidity |
| III | Urgent surgical therapy of acute post-thyroidectomy hemorrhage with hematoma / bleeding in former thyroid space (deep to the strap muscle)… | |
| A | Diffuse bleeding without clear venous or arterial bleeding source | Possible impairment of cervical drainage: moderate risk for severe morbidity |
| B | Venous bleeding source | Slowly progressing increase of cervical compartment pressure with impairment of cervical/cerebral drainage: moderate risk for severe morbidity |
| C | Arterial or combined with venous bleeding source | Rapidly progressing increase of cervical compartment pressure with impairment of cerebral perfusion and cervical/cerebral drainage: high risk for severe morbidity and mortality |
| IV | Any bleeding source | Death due to post-thyroidectomy hemorrhage |
Limitations of our work are that the data is obtained from an animal model. But as post thyroidectomy hemorrhage is a rare and potentially life-threatening complication, comparable data cannot be acquired from prospective clinical trials. However, the pathophysiology seems to correlate well between the chosen model in pigs and human pathophysiology in studies dealing with cerebral ischemia evaluating cerebral oxygenation (17,22,23). In our experimental setting with by intubation secured airways we cannot address a laryngo-pharyngeal mucosal edema, which can be caused by the impaired cervical venous and lymphatic drainage due to the elevated cervical compartment pressure (12). In our data we only could demonstrate the relevant increase in pressure of the internal jugular vein as a correlate to this impaired venous drainage. This laryngo-pharyngeal edema is of upmost clinical relevance as it may complicate intubation attempts to secure the patient’s airway for revision surgery and even lead to the need for a tracheostomy (6,12). Furthermore, it might result in prolonged intubation and ventilation therapy (12).
The number of animals and experiments were limited for ethical reasons. However, results were reproducible and conclusive. We did not detect relevant differences in the baseline vital signs or outcome measures between first and repeated experiments in the animals. The number of experiments differed between the animals, as we only repeated the experiments in an animal if respiratory function recovered and the vital signs as well as outcome measures like cervical compartment pressure and oxygenation returned to baseline values. We used doppler ultrasound measurements of the ophthalmic artery instead of arterial catheterization of the internal carotid arteries which had the advantage of not causing at least partial obstruction and therefore alterations of these primary outcome parameters. The cerebral perfusion and oxygenation underlie complex mechanisms of autoregulation, but within arterial blood pressure levels of 50 to 150 mmHg cerebral perfusion remains constant (16,21-23,26). Nevertheless, there are additional biochemical and physiological factors like changes in CO2 or O2 concentrations as well as pH levels that can cause unpredictable changes in cerebral perfusion, which we cannot address with our data (21-23). So far, the exact amount of oxygen depletion required for a centrally caused apnea is not known, but a relevant decrease of cerebral arterial perfusion and oxygenation can be assumed to have impact on the cerebral global function including the respiratory center in the brainstem. As the cerebral arterial perfusion decreased before a decrease of cerebral oxygenation could be observed, these observations appear to be coherent.
Conclusions
In post thyroidectomy hemorrhage we are dealing with a cerebral dysfunction due to lack of cerebral oxygenation based on a cervical compartment syndrome. Obvious clinical and pathologic vital signs can only be late signs for post thyroidectomy hemorrhage. Due to the correlation of increased pressure and impairment of perfusion and function, new strategies for postoperative patients’ surveillance are necessary to avoid major morbidity (6,14).
Acknowledgments
Funding: This work was supported by Johnson & Johnson Medical GmbH (Ethicon Germany), Medtronic Germany and Mindray Medical Germany (technical support) and ISAR-M Medical Technology (Germany) (financial support to the Institute for Surgical Research Oberbayern).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-21-910/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-910/dss
Peer Review File: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-910/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-21-910/coif). All authors report that this work was supported by Johnson & Johnson Medical GmbH (Ethicon Germany), Medtronic Germany and Mindray Medical Germany (technical support) and ISAR-M Medical Technology (Germany) (financial support to the Institute for Surgical Research Oberbayern) to carry out these animal experiments. SS and HMS are minority owners of ISAR-M Medical Technology (Germany). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2347-5-2018) granted by the federal state government of Brandenburg in compliance with EU Directive 2010/63/EU and German national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hermann M, Gschwandtner E, Schneider M, et al. Modern thyroid surgery - the surgeon's endocrine-surgical understanding and his responsibility for the extent of surgery and complication rate. Wien Med Wochenschr 2020;170:379-91. [Crossref] [PubMed]
- Lorenz K, Sekulla C, Kern J, et al. Management of postoperative hemorrhage following thyroid surgery. Chirurg 2015;86:17-23. [Crossref] [PubMed]
- Promberger R, Ott J, Kober F, et al. Risk factors for postoperative bleeding after thyroid surgery. Br J Surg 2012;99:373-9. [Crossref] [PubMed]
- Maneck M, Dotzenrath C, Dralle H, et al. Case volume and complications after thyroid gland surgery in Germany: an analysis of routine data from 48,387 AOK patients. Chirurg 2021;92:40-8. [Crossref] [PubMed]
- Doran HE, Wiseman SM, Palazzo FF, et al. Post-thyroidectomy bleeding: analysis of risk factors from a national registry. Br J Surg 2021;108:851-7. [Crossref] [PubMed]
- Weiss A, Parina RP, Tang JA, et al. Outcomes of thyroidectomy from a large California state database. Am J Surg 2015;210:1170-6; discussion 1176-7. [Crossref] [PubMed]
- Mahoney RC, Vossler JD, Woodruff SL, et al. Predictors and Consequences of Hematoma After Thyroidectomy: An American College of Surgeons National Surgical Quality Improvement Program Database Analysis. J Surg Res 2021;260:481-7. [Crossref] [PubMed]
- Aspinall S, Oweis D, Chadwick D. Effect of surgeons' annual operative volume on the risk of permanent Hypoparathyroidism, recurrent laryngeal nerve palsy and Haematoma following thyroidectomy: analysis of United Kingdom registry of endocrine and thyroid surgery (UKRETS). Langenbecks Arch Surg 2019;404:421-30. [Crossref] [PubMed]
- Oltmann SC, Alhefdhi AY, Rajaei MH, et al. Antiplatelet and Anticoagulant Medications Significantly Increase the Risk of Postoperative Hematoma: Review of over 4500 Thyroid and Parathyroid Procedures. Ann Surg Oncol 2016;23:2874-82. [Crossref] [PubMed]
- Raggio BS, Barton BM, Kandil E, et al. Association of Continued Preoperative Aspirin Use and Bleeding Complications in Patients Undergoing Thyroid Surgery. JAMA Otolaryngol Head Neck Surg 2018;144:335-41. [Crossref] [PubMed]
- Schopf S, von Ahnen T, von Ahnen M, et al. New insights into the pathophysiology of postoperative hemorrhage in thyroid surgery: An experimental study in a porcine model. Surgery 2018;164:518-24. [Crossref] [PubMed]
- Harding J, Sebag F, Sierra M, et al. Thyroid surgery: postoperative hematoma--prevention and treatment. Langenbecks Arch Surg 2006;391:169-73. [Crossref] [PubMed]
- von Ahnen T, von Ahnen M, Wirth U, et al. Pathophysiology of airway obstruction caused by wound hematoma after thyroidectomy: an ex vivo study. Eur Surg 2015;47:123-6. [Crossref]
- von Ahnen T, von Ahnen M, Militz S, et al. Compartment Pressure Monitoring After Thyroid Surgery: A Possible Method to Detect a Rebleeding. World J Surg 2017;41:2290-7. [Crossref] [PubMed]
- Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol 2020;18:e3000410. [Crossref] [PubMed]
- Olsen KS, Svendsen LB, Larsen FS. Validation of transcranial near-infrared spectroscopy for evaluation of cerebral blood flow autoregulation. J Neurosurg Anesthesiol 1996;8:280-5. [Crossref] [PubMed]
- Allen BS, Ko Y, Buckberg GD, et al. Studies of isolated global brain ischaemia: I. A new large animal model of global brain ischaemia and its baseline perfusion studies. Eur J Cardiothorac Surg 2012;41:1138-46. [Crossref] [PubMed]
- Wiącek MP, Modrzejewska M, Zaborski D. Age-related changes in retrobulbar circulation: a literature review. Int Ophthalmol 2020;40:493-501. [Crossref] [PubMed]
- Modrzejewska M, Siesky B, Amireskandari A, et al. Parameters characterizing age-dependent retrobulbar circulation in healthy subjects measured by color Doppler ultrasonography. Curr Eye Res 2015;40:729-36. [Crossref] [PubMed]
- Heßler H, Zimmermann H, Oberwahrenbrock T, et al. No Evidence for Retinal Damage Evolving from Reduced Retinal Blood Flow in Carotid Artery Disease. Biomed Res Int 2015;2015:604028. [Crossref] [PubMed]
- Moerman A, De Hert S. Recent advances in cerebral oximetry. Assessment of cerebral autoregulation with near-infrared spectroscopy: myth or reality? F1000Res 2017;6:1615. [Crossref] [PubMed]
- Booth EA, Dukatz C, Sood BG, et al. Near-infrared spectroscopy monitoring of cerebral oxygen during assisted ventilation. Surg Neurol Int 2011;2:65. [Crossref] [PubMed]
- Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 2007;38:2818-25. [Crossref] [PubMed]
- Sorrentino P, Tarantino G, Conca P, et al. Abnormally high resistive index of central retinal artery by ultrasound color Doppler in patients with viral chronic liver disease: correlation with worsening liver staging. Ultrasound Med Biol 2004;30:599-604. [Crossref] [PubMed]
- Steigerwalt RD Jr, Cesarone MR, Laurora G, et al. Doppler ultrasonography of the central retinal artery by duplex scanning. Retina 1996;16:513-7. [Crossref] [PubMed]
- Aikimbaev K, Guvenc B, Canataroglu A, et al. Value of duplex and color doppler ultrasonography in the evaluation of orbital vascular flow and resistance in sickle cell disease. Am J Hematol 2001;67:163-7. [Crossref] [PubMed]
- Cohn EJ Jr, Sandager GP, Benjamin ME, et al. Assessment of ocular perfusion after carotid endarterectomy with color-flow duplex scanning. J Vasc Surg 1999;29:665-71. [Crossref] [PubMed]
- Lüddecke R, Lindner T, Forstenpointner J, et al. Should you stop wearing neckties?-wearing a tight necktie reduces cerebral blood flow. Neuroradiology 2018;60:861-4. [Crossref] [PubMed]
- Konstantakos EK, Dalstrom DJ, Nelles ME, et al. Diagnosis and management of extremity compartment syndromes: an orthopaedic perspective. Am Surg 2007;73:1199-209. [Crossref] [PubMed]
- Malbrain ML, Roberts DJ, Sugrue M, et al. The polycompartment syndrome: a concise state-of-the-art review. Anaesthesiol Intensive Ther 2014;46:433-50. [Crossref] [PubMed]