
Prognostic significance of systemic immune-inflammation index and platelet-albumin-bilirubin grade in patients with pancreatic cancer undergoing radical surgery
Introduction
Pancreatic cancer (PC) is one of the leading causes of cancer-related mortality (1). The 5-year survival rate remains low at approximately 5% (2). Surgical resection is the only curative treatment for pancreatic ductal adenocarcinoma (PDAC); however, only 20% of patients have the chance for surgery (2).Carbohydrate antigen 19-9 (CA19-9) is the most commonly used biomarker for PDAC, with reliable sensitivity and specificity. However, a limitation of using elevated levels of CA19-9 for the diagnosis of PC is that CA19-9 also increases in other diseases, including biliary and gastrointestinal diseases (3). Therefore, we cannot reliably predict the outcome of patients with PC undergoing surgical resection, and it is important to develop reliable noninvasive prognostic indicators in such patients. Moreover, it is essential to establish precise and effective prognostic biomarkers to guide treatments for all patients with surgically resectable PC.
Many inflammatory and immune-related biomarkers have been proven to have predictive value for the prognosis of PC (4), such as neutrophil-to-lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR). The investigation of tumor driven inflammatory components is of great significance, and targeted inflammatory response pathways may become the cornerstone of cancer treatment. However, the value of known biomarkers in prognosis of PC is still controversial. The systemic immune-inflammation index (SII), a systemic inflammatory marker based on the counts of platelets (PLTs), neutrophils, and lymphocytes, has been linked to the outcomes of melanoma patients (5,6) and subsequently confirmed in patients with hepatocellular carcinoma (7-10) and lung cancer (5). High SII is associated with shorter overall survival (OS) (11,12). The platelet-albumin-bilirubin (PALBI) grade is a composite evaluation index based on liver function, which is associated with the poor outcome of patients with cirrhosis (13,14) and hepatocellular carcinoma (15). Patients with high PALBI grade have poor OS (13,16). Patients with pancreatic head cancer are prone to obstructive jaundice, which leads to abnormal liver function. Based on this, we explored the prognostic value of PALBI grade in PC patients. And there has been no relevant report regarding PALBI grade and the combination of SII and PALBI in patients with PC. Therefore, the purpose of this study was to analyze the prognostic ability of SII and PALBI grade in patients with surgically removed PC.
We present the following article in accordance with the REMARK reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-117/rc).
Methods
Patient selection
This retrospective study included patients with pathologically confirmed PC who had undergone radical surgery for the first time at the Affiliated Hospital of Qingdao University from August 2013 to December 2019 and followed up by December 2020. The inclusion criteria were as follows: (I) negative surgical margin; (II) the pathological type was PDAC; (III) complete data and information; The exclusion criteria were as follows: (I) unavailability to follow-up; or (II) simultaneous presence of other tumors. All patients were followed up carefully with hospital visits, telephone, or text messaging.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was registered in the clinicaltrials.gov (No. NCT05012540) and was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (approval No. QYFY WZLL 26364) and individual consent for this retrospective analysis was waived.
Data collection
The clinical data of patients with PC at admission were collected, including demographic data such as sex and age; whole blood cell counts; tumor location, size, lymph node metastasis (LNM), vascular invasion, and perineural invasion; and serum levels of CA19-9, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (Alb), and total bilirubin (TBIL). All laboratory parameters were assayed during routine workups before surgery. The NLR was calculated as the neutrophil/lymphocyte ratio, while the PLR was calculated as the PLT/lymphocyte ratio. SII was calculated as PLT count × neutrophil count/lymphocyte count. The PALBI grade was calculated as 2.02× log10 bilirubin −0.37× (log10 bilirubin) −0.04× Alb −3.48× log10 PLT +1.01× (log10 PLT). The main outcome was OS, which was defined as the time between surgery and death or the last follow-up. The secondary outcome was disease-free survival (DFS), which was defined as the first evidence of disease progression from the date of surgery.
Statistical analysis
The cutoff values for SII, PALBI grade, NLR, and PLR were confirmed by calculating the Youden index, which can maximize the sum of sensitivity and specificity (17). Univariate analysis of clinical baseline data was carried out by t-tests and chi-square tests in the SII groups and PALBI grade groups. Specifically, categorical data were analyzed using the chi-square test. For continuous normally distributed data, we used the t-test. Cox proportional hazards regression model was used to determine the prognostic factors associated with OS and DFS in univariate and multivariate analyses. The Kaplan-Meier (K-M) method was used to calculate the cumulative survival rate, and the log-rank test was used to evaluate the difference between groups. To adjust for the confounding effects of other clinical covariates, all variables were included in the multivariate Cox regression model, excluded PLT, NLR, PLR and PALBI grade due to multicollinearity. Death due to causes other than PC and survival before the end of the observation period were considered censored observations. The hazard ratio (HR) and 95% confidence interval (CI) were used to describe the relative effect. All tests were two-sided tests, and statistical significance was defined as P <0.05. SPSS (24.0) was used to analyze data.
Results
Clinicopathological characteristics
A total of 214 patients undergoing PC surgery were enrolled. The average age of the patients was 60.29 [standard deviation (SD) 9.353] years, and 128 (59.8%) of them were men. According to the tumor location, the pancreatic head cancer for 65.4% (n=140). Patients with yellow skin or sclera were identified as having jaundice. Based on receiver operating characteristic (ROC) analysis, the patients were divided into high and low SII groups by 705 as the cutoff, and into high and low PALBI grade groups by −5.6 as the cutoff. There were 57 and 159 patients in the high SII group and the high PALBI group, respectively. The demographic and clinical data of the patients are shown in Table 1.
Table 1
| Factors | All | SII | PALBI | |||||
|---|---|---|---|---|---|---|---|---|
| High | Low | P | High | Low | P | |||
| N | 214 | 57 | 157 | – | 159 | 55 | – | |
| Age (years), mean (±SD) | 60.29 (±9.353) | 0.107 | 0.340 | |||||
| Sex, n (%) | 0.976 | 0.005 | ||||||
| Male | 128 (59.8) | 34 | 94 | 104 | 24 | |||
| Female | 86 (40.2) | 23 | 63 | 55 | 31 | |||
| Jaundice, n (%) | 0.003 | <0.001 | ||||||
| Yes | 85 (39.7) | 32 | 53 | 85 | 0 | |||
| No | 129 (60.3) | 25 | 104 | 74 | 55 | |||
| CA19-9 (U/mL) | 0.917 | 0.053 | ||||||
| ≤114 | 78 (36.4) | 14 | 64 | 52 | 26 | |||
| >114 | 136 (63.6) | 43 | 93 | 107 | 29 | |||
| Alb (g/L) | <0.001 | 0.021 | ||||||
| ≥30 | 205 (95.8) | 50 | 155 | 55 | 150 | |||
| <30 | 9 (4.2) | 7 | 2 | 9 | 0 | |||
| TBIL (μmol/L) | <0.001 | <0.001 | ||||||
| 0–35 | 120 (56.1) | 19 | 101 | 65 | 55 | |||
| 36–200 | 44 (20.6) | 13 | 31 | 44 | 0 | |||
| >200 | 50 (23.4) | 25 | 25 | 50 | 0 | |||
| ALT (U/L) | 0.084 | <0.001 | ||||||
| 0–100 | 133 (62.1) | 30 | 103 | 79 | 54 | |||
| >100 | 81 (37.9) | 27 | 54 | 80 | 1 | |||
| AST (U/L) | 0.002 | <0.001 | ||||||
| 0–80 | 150 (70.1) | 31 | 119 | 95 | 55 | |||
| >80 | 64 (29.9) | 38 | 26 | 64 | 0 | |||
| CRP (mg/L) | 0.109 | 0.357 | ||||||
| ≤0.5 | 117 (54.7) | 26 | 91 | 84 | 33 | |||
| >0.5 | 97 (45.3) | 31 | 66 | 75 | 22 | |||
| Pancreatitis | 0.915 | 0.384 | ||||||
| Yes | 8 (3.7) | 2 | 6 | 7 | 1 | |||
| No | 206 (96.3) | 55 | 151 | 152 | 54 | |||
| Abdominal pain | 0.003 | 0.988 | ||||||
| Yes | 183 (85.5) | 42 | 141 | 136 | 47 | |||
| No | 31 (14.5) | 15 | 16 | 23 | 8 | |||
| Diabetes | 0.778 | 0.226 | ||||||
| Yes | 46 (21.5) | 13 | 33 | 31 | 15 | |||
| No | 168 (78.5) | 44 | 124 | 128 | 40 | |||
| Perineural invasion | 0.165 | 0.204 | ||||||
| Yes | 179 (83.6) | 128 | 51 | 43 | 136 | |||
| No | 35 (16.4) | 29 | 6 | 12 | 23 | |||
| Lymph node metastasis | 0.001 | 0.883 | ||||||
| Yes | 103 (48.1) | 65 | 38 | 26 | 77 | |||
| No | 111 (51.9) | 92 | 19 | 29 | 82 | |||
| Vascular invasion | 0.037 | 0.447 | ||||||
| Yes | 95 (44.4) | 63 | 32 | 22 | 73 | |||
| No | 119 (55.6) | 94 | 25 | 33 | 86 | |||
| Tumor location | 0.160 | <0.001 | ||||||
| Head | 140 (65.4) | 43 | 97 | 120 | 20 | |||
| Body | 54 (25.2) | 11 | 43 | 29 | 25 | |||
| Tail | 20 (9.3) | 3 | 17 | 10 | 10 | |||
| Tumor size | 0.614 | 0.054 | ||||||
| ≤2 cm | 27 (12.6) | 7 | 20 | 24 | 3 | |||
| ≤4 and >2 cm | 137 (64.0) | 34 | 103 | 103 | 34 | |||
| >4 cm | 50 (23.4) | 16 | 34 | 32 | 18 | |||
| Operative method | 0.012 | <0.001 | ||||||
| PPPD | 140 (65.4) | 53 | 87 | 120 | 20 | |||
| Distal pancreatectomy | 74 (34.6) | 15 | 59 | 39 | 35 | |||
P refers to the difference between the high and low groups. Alb, albumin; ALT, alanine transaminase; AST, aspartate transaminase CA19-9, carbohydrate antigen 19-9; CRP, C-reactive protein; PALBI grade, platelet-albumin-bilirubin grade; PPPD, pylorus-preserving pancreaticoduodenectomy; SD, standard deviation; SII, systemic immune-inflammation index; TBIL, total bilirubin.
There were differences in the presence of jaundice (P=0.003), Alb level (P<0.001), AST level (P=0.002), TBIL level (P<0.001), and abdominal pain (P=0.003) between the high SII group and the low SII group. Similarly, the presence of jaundice (P<0.001), Alb level (P=0.021), ALT level (P<0.001), AST level (P<0.001), TBIL (P<0.001), and tumor location (P<0.001) of the high PALBI grade group were significantly different from those of the low PALBI group. Using OS as an endpoint, the area under the ROC curve (AUC) of SII was 0.525 (95% CI: 0.435–0.615) (Figure 1A), the AUC of PALBI grade was 0.461 (95% CI: 0.374–0.548) (Figure 1B), the AUC of PLR was 0.499 (95% CI: 0.410–0.588) (Figure 1C), and the AUC of NLR was 0.524 (95% CI: 0.433–0.616) (Figure 1D). There were no obvious differences in discrimination ability between SII, PALBI grade, NLR, and PLR regarding OS.
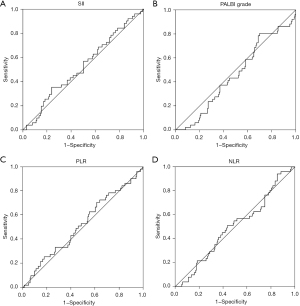
Systemic inflammatory markers are associated with OS
As shown in Table 2, univariate analysis identified ALT (HR, 1.926; P=0.028), AST (HR, 1.994; P=0.028), high PLT (HR, 2.312; P=0.044), tumor size (HR, 0.276; P=0.036), LNM (HR, 3.285; P=0.001), vascular invasion (HR, 2.520; P=0.003), PLR (HR, 2.277; P=0.009), NLR (HR, 4.045; P=0.020), and high SII (HR, 2.995; P<0.001) as factors affecting OS. In the multivariate analysis, SII (HR, 2.843; P=0.002), LNM (HR, 2.874; P=0.010), vascular invasion (HR, 2.502; P=0.007), and larger tumor size (HR, 0.215; P=0.026) were independently associated with OS. In the entire cohort, PALBI grade was not an independent risk factor of OS.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | 1.089 (0.619–1.915) | 0.768 | 1.197 (0.590–2.427) | 0.618 | |
| Jaundice (yes vs. no) | 1.711 (0.950–3.084) | 0.074 | 0.648 (0.156–2.695) | 0.551 | |
| CA19-9 (≤114 vs. >114 U/mL) | 1.000 (0.999–1.001) | 0.809 | 0.822 (0.426–1.588) | 0.560 | |
| Alb (≥30 vs. <30 g/L) | 3.601 (0.466–27.833) | 0.219 | 2.866 (0.276–29.724) | 0.378 | |
| TBIL (μmol/L) | |||||
| 0–35 vs. 36–200 | 1.930 (0.988–3.770) | 0.054 | 2.653 (0.595–11.831) | 0.201 | |
| 0–35 vs. >200 | 1.855 (0.825–4.169) | 0.135 | 2.983 (0.611–14.574) | 0.177 | |
| ALT (0–100 vs. >100 U/L) | 1.926 (1.072–3.458) | 0.028 | 1.304 (0.272–6.240) | 0.740 | |
| AST (0–80 vs. >80 U/L) | 1.994 (1.078–3.687) | 0.028 | 1.149 (0.316–4.181) | 0.833 | |
| PLT (109/L) (>300 vs. ≤300) | 2.312 (1.024–5.219) | 0.044 | NA | ||
| Tumor location | |||||
| Head vs. body | 1.155 (0.621–2.147) | 0.649 | 1.825 (0.369–9.021) | 0.461 | |
| Head vs. tail | 0.447 (0.13–1.488) | 0.189 | 1.040 (0.161–6.704) | 0.967 | |
| Tumor size (cm) | |||||
| ≤2 vs. ≤4 and >2 | 0.659 (0.301–1.442) | 0.297 | 0.513 (0.200–1.320) | 0.167 | |
| ≤2 vs. >4 | 0.276 (0.083–0.919) | 0.036 | 0.215 (0.055–0.834) | 0.026 | |
| Operative method | 1.010 (0.560–1.820) | 0.974 | 1.365 (0.251–7.425) | 0.719 | |
| Diabetes (yes vs. no) | 1.121 (0.591–2.126) | 0.727 | 0.818 (0.391–1.709) | 0.593 | |
| Perineural invasion (yes vs. no) | 1.489 (0.753-2.941) | 0.252 | 1.046 (0.476–2.303) | 0.910 | |
| Lymph node metastasis (yes vs. no) | 3.285 (1.652–6.530) | 0.001 | 2.874 (1.286–6.423) | 0.010 | |
| Vascular invasion (yes vs. no) | 2.520 (1.377–4.614) | 0.003 | 2.502 (1.282–4.883) | 0.007 | |
| SII (≥705 vs. <705) | 2.995 (1.658–5.412) | 0.000 | 2.843 (1.446–5.588) | 0.002 | |
| PALBI grade (≥−5.6 vs. <−5.6) | 1.889 (0.937–3.808) | 0.075 | NA | ||
| PLR (≤127 vs. >127) | 2.277 (1.224–4.236) | 0.009 | NA | ||
| NLR (≤1.35 vs. >1.35) | 4.045 (1.246–13.133) | 0.020 | NA | ||
NA indicates not included in the multivariate analysis model due to interference of these indices with the SII. Alb, albumin; ALT, alanine transaminase; AST, aspartate transaminase; CA19-9, carbohydrate antigen 19-9; CI, confidence interval; HR, hazard ratio; NLR, neutrophil/lymphocyte ratio; OS, overall survival; PALBI grade, platelet-albumin-bilirubin grade; PLR, platelet/lymphocyte ratio; PLT, platelet; SII, systemic immune-inflammation index; TBIL, total bilirubin.
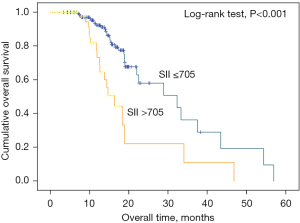
Figure 2 shows the K-M cumulative OS curves of the entire cohort. Shorter OS was significantly associated with a higher SII. The median OS was 31.5 and 19.9 months for patients with SII <705 and SII ≥705, respectively.
Systemic inflammatory markers are associated with DFS
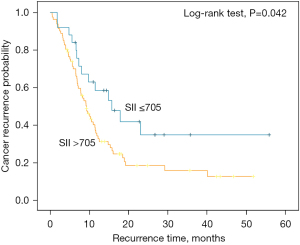
In Table 3, high TBIL (HR, 0.476; P=0.022), tumor location (HR, 2.655; P=0.003), tumor size (≤2 vs. ≤4 and >2 cm) (HR, 3.444; P=0.018), bigger tumor size (≤2 vs. >4 cm) (HR, 5.973; P=0.001), diabetes (HR, 1.985; P=0.004), and operative method (HR, 1.765; P=0.034) were prognostic factors for DFS as determined by univariate analysis. Moreover, LNM (HR, 2.345; P<0.001), perineural invasion (HR, 2.578; P=0.006), and SII (HR, 0.550; P=0.045) were significantly associated with DFS. Multivariate analysis identified larger tumor size (HR, 5.600; P=0.008), diabetes (HR, 2.228; P=0.013), and TBIL (HR, 0.295; P=0.028) as independent factors affecting DFS. In addition, LNM (HR, 2.565; P=0.001), perineural invasion (HR, 3.925; P=0.001), and SII (HR, 0.235; P<0.001) were also independent factors for DFS in PC patients who underwent radical surgery. There were no significant associations between PALBI grade and DFS via the multivariate analysis (Table 3). K-M analysis indicated that the high SII group was related to poor DFS (log-rank test, P=0.042) (Figure 3). The PALBI grade was not an independent risk factor of DFS in all patients according to the univariate analysis.
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | 0.871 (0.543–1.396) | 0. 565 | 0.818 (0.482–1.390) | 0.458 | |
| Jaundice (yes vs. no) | 0.638 (0.395–1.030) | 0.066 | 0.595 (0.167–2.117) | 0.595 | |
| CA19-9 (≤114 vs. >114 U/mL) | 1.115 (0.697–1.782) | 0.650 | 0.790 (0.454–1.377) | 0.406 | |
| Alb (≥30 vs. <30 g/L) | 3.894 (0.540–28.098) | 0.178 | 3.527 (0.411–30.274) | 0.250 | |
| TBIL (μmol/L) | |||||
| 0–35 vs. 36–200 | 0.476 (0.252–0.900) | 0.022 | 0.295 (0.099–0.875) | 0.028 | |
| 0–35 vs. >200 | 0.661 (0.373–1.170) | 0.155 | 0.793 (0.227–2.774) | 0.716 | |
| ALT (0–100 vs. >100 U/L) | 0.661 (0.410–1.065) | 0.089 | 3.227 (0.994–10.479) | 0.051 | |
| AST (0–80 vs. >80 U/L) | 0.616 (0.366–1.039) | 0.069 | 1.061 (0.421–2.673) | 0.900 | |
| PLT (109/L) (>300 vs. ≤300) | 0.563 (0.227–1.399) | 0.216 | NA | ||
| Tumor location | |||||
| Head vs. body | 1.564 (0.917–2.666) | 0.100 | 1.446 (0.314–6.659) | 0.636 | |
| Head vs. tail | 2.655 (1.384–5.093) | 0.003 | 1.922 (0.389–9.485) | 0.423 | |
| Tumor size (cm) | |||||
| ≤2 vs. ≤4 and >2 | 3.444 (1.242–9.552) | 0.018 | 2.442 (0.803–7.427) | 0.116 | |
| ≤2 vs. >4 | 5.973 (2.001–17.831) | 0.001 | 5.600 (1.571–19.960) | 0.008 | |
| Operative methods | 1.765 (1.042–2.990) | 0.034 | 1.148 (0.257–5.131) | 0.856 | |
| Diabetes (yes vs. no) | 1.985 (1.245–3.164) | 0.004 | 2.228 (1.182–4.199) | 0.013 | |
| Perineural invasion (yes vs. no) | 2.578 (1.320–5.038) | 0.006 | 3.925 (1.773–8.689) | 0.001 | |
| Lymph node metastasis (yes vs. no) | 2.345 (1.480–3.717) | 0.000 | 2.565 (1.453–4.526) | 0.001 | |
| Vascular invasion (yes vs. no) | 1.085 (0.671–1.756) | 0.739 | 1.065 (0.606–1.870) | 0.828 | |
| SII (≥705 vs. <705) | 0.550 (0.307–0.987) | 0.045 | 0.235 (0.110–0.502) | 0.000 | |
| PALBI (≥−5.6 vs. <−5.6) | 0.727 (0.430–1.231) | 0.235 | NA | ||
| PLR (≤127 vs. >127) | 0.517 (0.324–0.825) | 0.006 | NA | ||
| NLR (≤1.35 vs. >1.35) | 0.741 (0.375–1.448) | 0.380 | NA | ||
NA indicates not included in the multivariate analysis model due to interference of these indices with the SII. Alb, albumin; ALT, alanine transaminase; AST, aspartate transaminase; CA19-9, carbohydrate antigen 19-9; CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NLR, neutrophil/lymphocyte ratio; PALBI grade, platelet-albumin-bilirubin grade; PLR, platelet/lymphocyte ratio; PLT, platelet; SII, systemic immune-inflammation index; TBIL, total bilirubin.
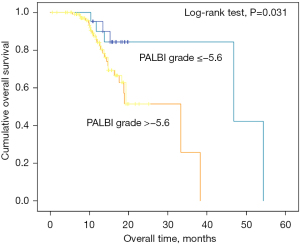
PALBI grade is associated with pancreatic head cancer
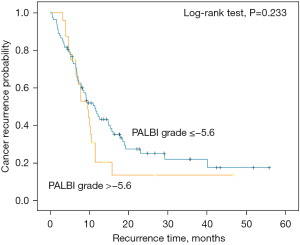
The PALBI grade was also associated with tumor location, as patients with pancreatic head cancer were more often characterized by increased TBIL and decreased Alb. This may be the reason that PALBI was not a significant prognostic factor for all the PC patients. Therefore, we verified whether PALBI grade is related to the prognosis of pancreatic head cancer. There were 140 patients who had tumor location at the pancreatic head. According to the K-M curve, we found that in patients with pancreatic head cancer, high PALBI grade was associated with shorter OS (Figure 4). The median OS was 44.4 and 25.2 months for patients with pancreatic head cancer respectively.
We also analyzed the association between the PALBI grade and DFS (Figure 5), and the results showed that there was no obvious difference between the 2 groups (log-rank test, P=0.233).
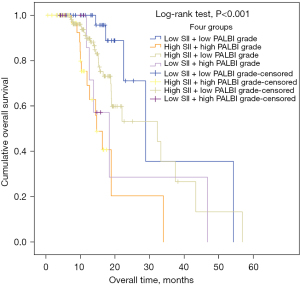
The prognostic value of SII combined with PALBI grade
To further assess the prognostic value of SII combined with PALBI grade in PC, we divided the total samples into 4 groups as follows: high SII + high PALBI grade, low SII + low PALBI grade, low SII + high PALBI grade, and high SII + low PALBI grade. Through the pairwise comparisons of different groups, we found that the combination of high SII and high PALBI grade had stronger predictive value for poor prognosis (log-rank test, P<0.001) (Figure 6).
Discussion
The prognosis of PC is poor because of its increasing incidence, high degree of malignancy, rapid development, and strong invasiveness (18). Chronic inflammation may increase the risk of PC, and PC-associated inflammatory infiltrate in the tumor microenvironment further enhances tumor growth and metastasis (19). The relationship between SII and the prognosis of PC may be due to thrombocytopenia, neutrophilia, and lymphocytopenia. This indicates that the inflammatory state is elevated and the immune system response is reduced. Cancer inflammation has a negative impact on survival (20,21). Neutrophil count has been shown to negatively correlate with the prognosis of some tumors (22-25). The increase in neutrophil count is stimulated by inflammatory cytokines to promote neutrophil phagocytic and bactericidal effects, which provides the “fuel that feeds the flames” of tumor growth and metastasis (25,26). Several studies have confirmed the prognostic value of PLT count in lung cancer, gynecologic malignancies, melanoma, and hepatocellular carcinoma (27-32). PLTs have been suggested to mediate antigen presentation by dendritic cells to T cells (33) and form heterotypic aggregates with neutrophils (34). Several recent studies have indicated that inflammation markers have prognostic value for OS and DFS in patients with cancer (35-38).
According to our results, high SII is an independent factor of poor prognosis in patients who underwent radical surgery. In several malignant tumors including PC, elevated SII before surgery plays a key role in prognostic evaluation (5,39-42). Our study further analyzed the prognostic value of the SII and identified the cutoff value at 705. Serum Alb has generally been used to assess nutritional status and function of visceral protein synthesis. Low levels of serum Alb have also been determined to be an independent factor of poor survival in patients with PC (43). Alb synthesized in the liver can reflect the nutritional status of the body. When nutritional status is poor or liver function is abnormal, the Alb based PALBI grade will increase. Bilirubin has immunomodulatory effects, such as inhibiting T cell activity (44). A retrospective multicenter cohort study showed that bilirubin levels can affect the prognostic ability of the SII in terms of cancer-specific survival and recurrence in resectable PC (43). Liver damage can also cause bilirubin secretion and imbalance, leading to increased circulating bilirubin levels (45). The PALBI grade calculation involves the levels of bilirubin and Alb. A study has shown that higher PALBI grade is related to shorter OS in patients with hepatocellular carcinoma (43). There have been no studies, however, on the relationship between PALBI grade and PC. In our study, we found that PALBI grade was not an independent risk factor for all patients with pancreatic cancer undergoing radical surgery. We also verified whether PALBI grade is related to the prognosis of pancreatic head cancer by performing a subgroup analysis of patients with pancreatic head cancer, and analyzed the K-M curve between PALBI grade and the OS of these patients. The results showed that a high PALBI grade was associated with a shorter OS in patients. In addition, in order to further clarify the prognostic value of the combination of the 2 indictors, we divided all patients into 4 groups by cutoff points. Through pairwise comparisons between the groups, we found that the combination of high SII and high PALBI grade indicated poor prognosis. The prognostic utility of the combination of SII and PALBI grade should be evaluated in further studies.
This study has some strengths and limitations. This was a retrospective observational study including 214 patients undergoing pancreatic surgery. This study is the first to explore the impact of PALBI grade on PC patients. In terms of limitations, this was a single-center study and thus lacks representativeness. Moreover, it was limited by its retrospective study design and limited scope of analyzed outcomes. Therefore, large-scale multicenter studies are required in the future, using more representative samples with higher statistical power.
Conclusions
In summary, this study showed that preoperative high SII is an independent predictor of OS and DFS for PC patients who underwent radical surgery. Higher PALBI grade is associated with shorter OS in patients with pancreatic head cancer. The combination of high SII and high PALBI grade is even more informative in predicting prognosis. SII and PALBI grade can be calculated easily from routine blood indicators, which is practical and easy-to-use and may help clinicians guide the treatment of patients with surgically resectable PC.
Acknowledgments
Funding: This work was supported by Shandong Provincial Medicine and Health Science and Technology Development Plan (grant No. 202003030683). This study was also supported by grant from the National Natural Science Foundation of Shandong Province (No. ZR202103040311).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-117/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-117/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-117/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (approval No. QYFY WZLL 26364) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takehara M, Sato Y, Kimura T, et al. Cancer-associated adipocytes promote pancreatic cancer progression through SAA1 expression. Cancer Sci 2020;111:2883-94. [Crossref] [PubMed]
- Jomrich G, Gruber ES, Winkler D, et al. Systemic Immune-Inflammation Index (SII) Predicts Poor Survival in Pancreatic Cancer Patients Undergoing Resection. J Gastrointest Surg 2020;24:610-8. [Crossref] [PubMed]
- Luo G, Jin K, Cheng H, et al. Carbohydrate antigen 19-9 as a prognostic biomarker in pancreatic neuroendocrine tumors. Oncol Lett 2017;14:6795-800. [Crossref] [PubMed]
- van Wijk L, de Klein GW, Kanters MA, et al. The ultimate preoperative C-reactive protein-to-albumin ratio is a prognostic factor for survival after pancreatic cancer resection. Eur J Med Res 2020;25:46. [Crossref] [PubMed]
- Yang R, Chang Q, Meng X, et al. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer 2018;9:3295-302. [Crossref] [PubMed]
- Yu J, Wu X, Yu H, et al. Systemic Immune-Inflammation Index and Circulating T-Cell Immune Index Predict Outcomes in High-Risk Acral Melanoma Patients Treated with High-Dose Interferon. Transl Oncol 2017;10:719-25. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Yang J, Bao Y, Chen W, et al. Nomogram Based on Systemic Immune Inflammation Index and Prognostic Nutrition Index Predicts Recurrence of Hepatocellular Carcinoma After Surgery. Front Oncol 2020;10:551668. [Crossref] [PubMed]
- Wang D, Hu X, Xiao L, et al. Prognostic Nutritional Index and Systemic Immune-Inflammation Index Predict the Prognosis of Patients with HCC. J Gastrointest Surg 2021;25:421-7. [Crossref] [PubMed]
- Zhao LY, Yang DD, Ma XK, et al. The Prognostic Value of aspartate aminotransferase to lymphocyte ratio and systemic immune-inflammation index for Overall Survival of Hepatocellular Carcinoma Patients Treated with palliative Treatments. J Cancer 2019;10:2299-311. [Crossref] [PubMed]
- Shui Y, Li M, Su J, et al. Prognostic and clinicopathological significance of systemic immune-inflammation index in pancreatic cancer: a meta-analysis of 2,365 patients. Aging (Albany NY) 2021;13:20585-97. [Crossref] [PubMed]
- Bittoni A, Pecci F, Mentrasti G, et al. Systemic immune-inflammation index: a prognostic tiebreaker among all in advanced pancreatic cancer. Ann Transl Med 2021;9:251. [Crossref] [PubMed]
- Oikonomou T, Goulis L, Doumtsis P, et al. ALBI and PALBI Grades Are Associated with the Outcome of Patients with Stable Decompensated Cirrhosis. Ann Hepatol 2019;18:126-36. [Crossref] [PubMed]
- Božin T, Mustapić S, Bokun T, et al. Albi Score as a Predictor of Survival in Patients with Compensated Cirrhosis Resected for Hepatocellular Carcinoma: Exploratory Evaluation in Relationship to Palbi and Meld Liver Function Scores. Acta Clin Croat 2018;57:292-300. [Crossref] [PubMed]
- Sonohara F, Yamada S, Tanaka N, et al. Comparison of non-invasive liver reserve and fibrosis models: Implications for surgery and prognosis for hepatocellular carcinoma. Hepatol Res 2019;49:1305-15. [Crossref] [PubMed]
- Ho CHM, Chiang CL, Lee FAS, et al. Comparison of platelet-albumin-bilirubin (PALBI), albumin-bilirubin (ALBI), and child-pugh (CP) score for predicting of survival in advanced hcc patients receiving radiotherapy (RT). Oncotarget 2018;9:28818-29. [Crossref] [PubMed]
- YOUDEN WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Tang X, Zhang M, Sun L, et al. The Biological Function Delineated Across Pan-Cancer Levels Through lncRNA-Based Prognostic Risk Assessment Factors for Pancreatic Cancer. Front Cell Dev Biol 2021;9:694652. [Crossref] [PubMed]
- Padoan A, Plebani M, Basso D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int J Mol Sci 2019;20:676. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Ocana A, Nieto-Jiménez C, Pandiella A, et al. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer 2017;16:137. [Crossref] [PubMed]
- Li QQ, Lu ZH, Yang L, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev 2014;15:945-50. [Crossref] [PubMed]
- Komura N, Mabuchi S, Yokoi E, et al. Comparison of clinical utility between neutrophil count and neutrophil-lymphocyte ratio in patients with ovarian cancer: a single institutional experience and a literature review. Int J Clin Oncol 2018;23:104-13. [Crossref] [PubMed]
- Yoon CI, Park S, Cha YJ, et al. Associations between absolute neutrophil count and lymphocyte-predominant breast cancer. Breast 2020;50:141-8. [Crossref] [PubMed]
- Zhu X, Chen Y, Cui Y. Absolute Neutrophil Count and Mean Platelet Volume in the Blood as Biomarkers to Detect Lung Cancer. Dis Markers 2020;2020:1371964. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Sasaki K, Kawai K, Tsuno NH, et al. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg 2012;36:192-200. [Crossref] [PubMed]
- Yuan Y, Zhong H, Ye L, et al. Prognostic value of pretreatment platelet counts in lung cancer: a systematic review and meta-analysis. BMC Pulm Med 2020;20:96. [Crossref] [PubMed]
- Scheiner B, Kirstein M, Popp S, et al. Association of Platelet Count and Mean Platelet Volume with Overall Survival in Patients with Cirrhosis and Unresectable Hepatocellular Carcinoma. Liver Cancer 2019;8:203-17. [Crossref] [PubMed]
- Menczer J. Preoperative elevated platelet count and thrombocytosis in gynecologic malignancies. Arch Gynecol Obstet 2017;295:9-15. [Crossref] [PubMed]
- Rachidi S MD. Platelet count correlates with stage and predicts survival in melanoma. Platelets 2019;30:1042-6. [Crossref] [PubMed]
- Yang W, Chen YY, Bi C, et al. Predictive and prognostic values of preoperative platelet parameters in patients with gynecological tumors. J Clin Lab Anal 2020;34:e23295. [Crossref] [PubMed]
- Audia S, Mahévas M, Samson M, et al. Pathogenesis of immune thrombocytopenia. Autoimmun Rev 2017;16:620-32. [Crossref] [PubMed]
- Blair P, Rex S, Vitseva O, et al. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res 2009;104:346-54. [Crossref] [PubMed]
- Kay J, Thadhani E, Samson L, et al. Inflammation-induced DNA damage, mutations and cancer. DNA Repair (Amst) 2019;83:102673. [Crossref] [PubMed]
- Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol 2020;119:199-245. [Crossref] [PubMed]
- Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol 2020;15:123-47. [Crossref] [PubMed]
- Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med 2019;18:121-6. [Crossref] [PubMed]
- Zhang Y, Xiao G, Wang R. Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis. Cancer Manag Res 2019;11:4185-200. [Crossref] [PubMed]
- Huang H, Liu Q, Zhu L, et al. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci Rep 2019;9:3284. [Crossref] [PubMed]
- Zhang W, Wang R, Ma W, et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Transl Med 2019;7:431. [Crossref] [PubMed]
- Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal 2019;33:e22964. [Crossref] [PubMed]
- Aziz MH, Sideras K, Aziz NA, et al. The Systemic-immune-inflammation Index Independently Predicts Survival and Recurrence in Resectable Pancreatic Cancer and its Prognostic Value Depends on Bilirubin Levels: A Retrospective Multicenter Cohort Study. Ann Surg 2019;270:139-46. [Crossref] [PubMed]
- Liu Y, Li P, Lu J, et al. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J Immunol 2008;181:1887-97. [Crossref] [PubMed]
- Zhang TN, Yin RH, Wang LW. The prognostic and predictive value of the albumin-bilirubin score in advanced pancreatic cancer. Medicine (Baltimore) 2020;99:e20654. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


