Clinical applications of dynamic infrared thermography in plastic surgery: a systematic review
Introduction
Infrared radiation was discovered in 1800, by Sir William Herschel who measured the temperature of each visible light band and noticed that when the thermometer was placed beyond the red band of the visible spectrum, there was a further increase in temperature. Herschel called this invisible light “infrared” (1). In 1929, Hungarian physicist Kalman Tihanyi invented the first infrared-sensitive camera based on the idea that all objects emit a heat signal in the form of infrared radiation. An infrared thermographic camera can detect this radiation in a similar way to an ordinary camera that can detect visible light (2).
The use of infrared thermography (IRT) in medicine was adopted from the principle that increase in body temperature causes a higher amount of radiation emitted. Hence an increase in vascularity, which is a hallmark of many pathological changes such as inflammation or neoplasms with increased metabolic activity, leads to increase in temperature, which can be detected by an infrared thermographic camera. This can be observed in the form of static single images or noting the thermal recovery process in response to thermal stress; which is called dynamic infrared thermography (DIRT). This term was coined by De Weerd et al., to describe the form of IRT in which a thermal challenge (warm or cold) is applied to an area of interest, and the rate and pattern of temperature changes towards equilibrium is registered with an infrared camera. The analysis of the rate and pattern of rewarming is an indicator of the underlying skin and subcutaneous tissue perfusion (3). This paper aims to conduct a systematic review of the clinical applications of IRT imaging in plastic surgery.
Methods
A systematic review of scientific literature was undertaken. All prospective and retrospective studies including case reports, cohort studies, randomized control trials and clinical trials which analyze the applications of dynamic IRT in a medical setting, were included. All clinical and laboratory studies with human participants, cadavers and animals that underwent IRT imaging and computer software analysis were included in the review. Subgroup analyses were performed, with the use of DIRT divided into the following subgroups for analyses: to assess perforators in planning for flap reconstruction and wound closure; to monitor flaps post-operatively; to assess burn wound depth; to diagnose carpal tunnel syndrome (CTS); for other uses in plastic surgery. Inclusion criteria comprised all studies that use DIRT with the aforementioned endpoints. All animal, human and cadaveric studies that were published in English were included for analysis. Exclusion criteria comprised studies which analyzed temperature changes but did not use the IRT camera and studies analyzing the usefulness of IRT in diagnosing breast cancer.
The search strategy involved searching electronic databases Medline, OVID Old MEDLINE, EMBASE and Cochrane collaboration for all articles on the topic of DIRT published up till June 2012. The bibliographic references of the captured articles were examined in order to search for additional relevant citations. The search strategy was sensitive to texts and abstracts, with the keywords “thermography” and/or “infrared” and/or “DIRT”. Each potential paper was examined by two reviewers for adherence to inclusion/exclusion criteria. There were no conflicts of opinion between the two reviewers. All included studies were separated for a full reading, critical assessment and data extraction. The following data were gathered: general information on each study (author and publication year), type of study, types of participants, study location, subject number, study aim, type of infrared thermographic camera, type of analytical software and result. The data extracted by each publication were then separated according to clinical applications of the DIRT camera, and analyzed. The quality of each paper was assessed based on the Oxford levels of evidence scale as demonstrated in Table 1 (4).

Full table
Results
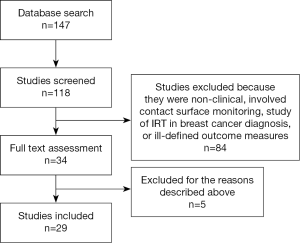
Electronic search using the prescribed criteria revealed 147 studies. After reading the titles and abstracts and eliminating duplicates, a total of 34 publications were chosen for a full reading and assessment in relation to inclusion and exclusion criteria (See Figure 1). Several studies were subsequently excluded. Bulstrode et al., 2002 assessed the use of surface thermography for post-operative monitoring of free flap reconstruction. This study was excluded as the study utilized surface temperature camera rather than digital infrared thermographic camera in the study (5). Rustemeyer et al., 2007 used an electronic contact thermometer to determine the reference value for surface temperature of the different facial regions. Again this study was excluded due to the use of a surface temperature camera (6). A study by May et al., 1985 was also excluded as they used thermocouple probe monitoring for free tissue transfer, replantation and revascularization procedure rather than digital infrared thermographic (DIRT) camera (7).
Included studies
After full reading and analysis, a total of 29 publications met the inclusion criteria. The papers were then separate into categories for systemic review according to the application of DIRT.
Use of dynamic infrared thermography (DIRT) in assessing and mapping perforators in pre-operative flap reconstructions
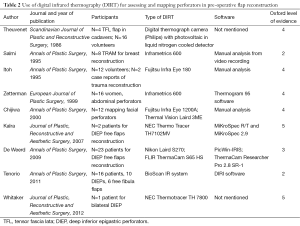
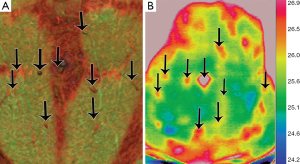
After literature review, nine publications were analyzed. These included seven prospective studies and two case reports. All these studies use a similar type of digital infrared thermographic (DIRT) camera and analytical software, as described in Table 2. Theuvenet et al. used the DIRT camera to evaluate cadavers for the marking of perforators in various areas that could be harvested as free flaps. The skin and subcutis were dissected from the underlying fascia and muscle; and 31 out of 36 perforators that were detected by DIRT, were located. A further 16 living volunteers had their perforators analyzed after cooling, exsanguination and use of tourniquet. After the tourniquet was released, hot spots were detected with DIRT (8). Five publications (Itoh 1995, Salmi 1995, Zetterman 2000, Kalra 2007 and De Weerd 2009) use DIRT camera to detect perforators in abdominal free flaps (9-13). Chijiwa et al. used IRT to create a facial map of perforators to enable planning of flaps of the face (14). In all the studies, the subjects’ skin was cooled and allowed to rewarm while the thermal images were recorded. Two publications (Salmi 1995 and Kalra 2007) further analyze deep inferior epigastric perforators (DIEP) intra-operatively, both report a decrease in temperature of 3.4±1.05 °C of the whole flap from the reference point (P<0.05) detected by DIRT, after ligation of the pedicles (10,12). De Weerd et al. also observed the rate and pattern of rewarming of the hot spots to represent quality of the perforators. A hot spot (reperfusion) that produces a rapid rewarming for a larger area is preferable for flap reconstruction (13). Tenorio et al. conducted a trial comparing DIRT with handheld Doppler on 16 patients with surgical dissection confirming the perforator location, as the reference standard. They found that location matched within a distance of 0-15 mm in 67% of patients. They concluded that while Doppler located perforators in the deeper level, where they exited the muscular fascia; thermography detected their location beneath the skin and hence both techniques were complementary. Using a thermographic map obtained by DIRT the Doppler flow examination time could be shortened and the Doppler was useful to recognize arterial versus venous flow (15). These were all well presented photographically (see Figures 2,3).
Full table
A total of 108 patients were included in the studies. Although many of the studies were self-described as prospective clinical trials, as there were no established reference standards, control groups or randomization in the processes described, or in some cases, the lack of critical appraisal and sensitivity analysis, they were classified as evidence level 3 to 4. The case reports with expert opinions were classified as level 5 evidence. As Salmi et al. and Tenorio et al.’s studies included the use of a reference standard, in the form of an exploratory cohort study; it was classified as evidence level 2.
Use of dynamic infrared thermography (DIRT) in post-operative monitoring of flap reconstruction
A total of five publications were included in the study. There were three randomized controlled trials and two prospective clinical studies as shown in Table 3. All of the randomized controlled trials were conducted using laboratory animal experiments with two publications using DIRT to determine the end point (flap necrosis and reperfusion state) for the given interventions. Tenorio et al., found that after raising a pedicled island flap and ligating the arterial supply, DIRT detected a change in temperature from red to green with the disappearance of the hot spot representing the pedicle within 20 minutes, well before any macroscopic changes were visible. Whereas, in venous occlusion there was a brief increase followed by a steady decrease of the infrared emission observed. In contrast to the arterial ligation group, macroscopic signs of venous congestion were obvious, at the same time as detectable by DIRT (15). This was a validating cohort study with a clear reference standard and hence was classified as 2 level of evidence.

Full table
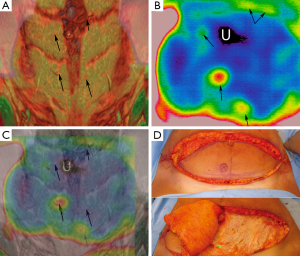
Two publications (Wolff 1995 and Shejbal 2011) used DIRT to assess the flap survival and perfusion and to compare the use of vasoactive drugs (capsaicin, methyl prednisolone and mitomycin) in improving survival of random pattern skin flaps and different types of venous flaps (16,17). Both studies used DIRT to determine perfusion status with Wolff’s study using isotope perfusion as a comparison (18). In view of this comparison study, it has been classified as level 2 evidence. Although Shejbal’s study found that DIRT was accurate in determining flap viability, DIRT was a tool used in the trial and was not the focus of the trial itself and hence, for the purposes of this review, was classified as evidence level 4. An application in digital replantation has also been assessed (unpublished), as shown in Figure 4.
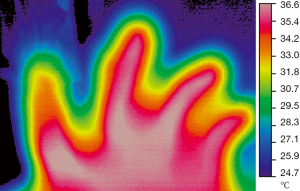
Use of dynamic infrared thermography (DIRT) in burns
In 1974 Hackett demonstrated that diminished blood flow to deep dermal and full thickness burns can be identified by thermography (19). Several authors have since then, discussed the use of thermography to accurately assess the depth of burns, especially since studies have shown that clinical acumen is only 60–75% accurate, even with experienced burn surgeons (20). In a study of 30 patients with burns, Zhu et al. concluded that IRT was an effective method of burn depth measurement as long as the temperature difference was calibrated in relation to a symmetrical normal part of the body. This study was classified as level 4 due to the absence of a good reference standard (21). Renkielska et al. used DIRT to analyze burn depth in determining treatment options, operative versus conservative management. They used animal models, assessing 23 wounds of different carefully calculated depths and the tissue was histologically assessed to determine the degree of dermis injury. To improve on the existing studies that used static thermograms, they made it dynamic using a thermal challenge in the form of heating by optical irradiation. The results of DIRT assessment corresponded exactly with the histological evaluation and hence they concluded that DIRT is an effective method of burn wound discrimination and early burn treatment planning (22). A subsequent review done at the John Hopkins Burns Centre in USA in 2006 (Devgan et al.) concluded that although there were reports of high accuracy in burn depth assessment with thermography, it was limited by the confounding effects of ambient heat loss and sensitive timing (23). In 2008, Monstrey et al. reviewed the various techniques used for burn depth assessment, including IRT and concluded that, laser Doppler imaging was the most accurate in predicting wound outcome, based on published evidence so far (20). The studies and their findings are summarized in Table 4.
Full table
Use of dynamic infrared thermography (DIRT) in carpal tunnel syndrome (CTS)
In 1987, Herrick’s study of 90 patients with inadequately applied reference standards (and hence evidence level 4) proposed that thermography is probably useful in diagnosing CTS (24). This was followed by So et al. in 1989 who studied the use of IRT in diagnosing patients with entrapment neuropathies, as part of a level 2 study. They studied 20 healthy volunteers and 37 patients but found the sensitivity of thermography far below that of nerve conduction studies and concluded that thermography was not useful for this purpose (25). These findings were also confirmed by Reilly et al. in the same year, in their study with 23 patients (26). However, a randomized controlled trial with blinding, performed by Tchou in 1992, concluded that for diagnosing CTS thermography had a specificity of 98−100% (27). However these results have not been duplicated since then. In 2008, Jesensek Papez et al. conducted a study into the use of IRT for diagnosing CTS, with better technology using artificial neural networks. They studied 502 images of hands (from 132 healthy, 119 affected patients) and demonstrated that IRT was able to correctly classify 72.2% of the hands. They used nerve conduction studies as the reference standard. They concluded that IRT could be useful as a screening method in populations with high ergonomic risk factors for CTS, but was inadequate as a diagnostic tool where severity level is required to determine further management (28). Table 5 summarizes this review.

Full table
Use of dynamic infrared thermography (DIRT) for hemangiomas
There is only level five evidence (Saxena et al. in 2008), where they used DIRT to document and plan further treatment for 102 children with cutaneous haemangiomas and other vascular malformations after conservative management, cryotherapy and laser therapy (29).
Use of dynamic infrared thermography (DIRT) in melanoma
Identifying melanomas is one of the oldest applications of infrared thermal imaging in medicine as studied by Maillard and Hessler in 1969 (30). Recent studies have shown that temperature changes are different in benign and malignant pigmented cutaneous lesions, as discussed by Centigul et al., but these promising results are yet to be confirmed with larger sample groups (31). At present, there is only level five evidence (Santa Cruz et al. 2009), suggesting the possibility of the usefulness of DIRT, in melanoma (32).
Use of dynamic infrared thermography (DIRT) in detecting breast neoplasms
Over 800 peer-reviewed studies have been published on the use of breast thermography to diagnose breast cancer, and there are over 110 review articles on this topic. Lack of standardization of technique and continual evolution in the technology and equipment used, makes a comprehensive review of the subject extremely difficult, and attempting a systematic review of this topic is beyond the remit of this paper. A recent systematic review by Fitzgerald et al. published in March 2012, using QUADAS criteria concluded that, as most of the studies were of average quality, there is insufficient evidence to support thermography or to show that it provides benefit to patients as an adjunctive tool to mammography (33). With the advent of the use of texture features and support vector machine, as suggested by Acharya et al. (34) or using three-dimensional close-actual-breast model with a numerical grid and tetrahedral elements, as suggested by Ng et al. (35) the sensitivity and specificity of thermography as a screening tool is likely to increase. DIRT may be a useful adjunct to existing methods of breast cancer diagnosis, but at present still cannot replace mammography or ultrasound.
Discussion
The objective of this systematic review is to analyze the clinical applications of DIRT in plastic surgery. In planning flap reconstruction, it is crucial to be able to identify a suitable perforator. Currently there is a need for a non-invasive, inexpensive, sensitive, accurate bedside device with minimal or no adverse effect. Doppler ultrasound yields a low sensitivity and is operator dependent for both identifying and interpretation of results. Cutaneous perforators can be mapped by magnetic resonance imaging (MRI), computer tomographic angiography (CTA) and digital subtraction angiography (DSA) but these diagnostic imaging techniques are expensive, are not suitable for intra-operative or post-operative monitoring, and may be associated with high radiation dose, risk of contrast extravasation and nephrotoxicity. The average CTA involves a separate hospital visit with a CT appointment taking up to 30 minutes, requiring the skills of a radiographer, sophisticated expensive equipment and a radiologist to verify the report, where as infrared thermal photography can be performed by the surgeon, in the preliminary clinic visit, using simpler less expensive equipment and usually takes only 10 minutes to perform (see Figure 5) (36).

While the need for such a device is evident, the data does not offer DIRT as a definitive improvement over current techniques in a holistic manner. While its benefits have been demonstrated, at best the data suggests that DIRT is a useful adjunct to current techniques.
DIRT can be used as an alternative or collaborate with other diagnostic interventions to map and characterize perforators in designing cutaneous and musculocutaneous flaps, by the presence of hot spots. It has a similar sensitivity and specificity in detecting cutaneous perforators as CTA and tissue dissection (10,13). However while the CTA provides information on all the perforators in the area, giving a precise location less than 1 mm, vessel calibre and additionally gives the exact course of the vessel helping surgeons choose the perforator that requires the least intramuscular dissection, DIRT can only identify perforators that are more than 1 mm in diameter, with a location precision of less than 1 cm (36). The short course of the perforator from the source vessel to the skin explains the rapid rewarming of the skin at the hot spot. DIRT provides a qualitative assessment of perforators to the flap which can provide additional information when combined with CTA to choose the best flap for reconstruction.
With the introduction of free style reconstruction flaps comes a need for a safe and reliable method of perforator mapping. Wei et al., described “free style” flaps when a flap is raised after a perforator was identified in an unfamiliar region, flap harvest can still be done, by surgically exploring the region for vessels of suitable size (37). This had led to an increase in the number of flaps available. Based on the evidence published in literature thus far, DIRT may provide an accurate, inexpensive method that is useful in identifying perforators for flap planning.
Flap and re-implantation monitoring, as well as assessing ischaemic limbs is still heavily reliant on clinical evaluation, which in itself, is dependent on the experience and knowledge of the medical staff, in recognizing tissue that is vascularly compromised. Early and accurate recognition of perfusion failure is critical. If surgical revision is performed within the first hour after detection of venous or arterial occlusion, the salvage can reach 68–70% and the salvage rates decrease thereafter (38). Ideally, the monitoring system needs to be non-invasive, free of side-effects, reliable, rapid, repeatable, accurate, painless and easy to operate. With the developments in computer analytical software and technology, DIRT equipment has become smaller and can accurately analyze, with finer detail. This has allowed DIRT to be used intra-operatively and on the ward as a post-operative monitoring device. The studies that used DIRT to monitor flaps showed that the flap temperature increased by 1.4±0.18 °C, following completion of anastomosis. They also noted the temperature in the free flap was generally higher than the tissue in its original position, which may indicate decrease in vascular resistance due to it being a non-innervated flap (10,18). De Weerd et al., found that DIRT had a higher sensitivity in detecting early vascular flow in flap reconstruction than arterial Doppler ultrasound. Thus, DIRT joins the plethora of methods currently in use to monitor flap perfusion, which include near-infrared spectroscopy, implantable Doppler probes, colour Duplex sonography, laser Doppler flowmetry and microdialysis. Although a validating cohort study with absolute reference standards or a comparison study with current flap monitoring techniques, is pending, it is safe to say that literature points to the usefulness of DIRT for postoperative flap monitoring.
In burns, differentiating between superficial dermal and deep dermal burns is difficult even for experienced practitioners. But this differentiation is significant in the option of treatment prescribed, as superficial dermal wounds can be treated conservatively and will heal within 3 weeks, whereas deep dermal burns should ideally be excised and grafted. Various techniques have been used to accurately identify the depth of a burn wound, with the reference standard being the histological diagnosis or the clinical appearance of the wound on follow-up. Although thermography benefits from relative technical ease and validity, it is limited by the confounding effects of ambient heat loss and sensitive timing. Evaporative loss of heat to the environment causes wounds to be interpreted as falsely deep, introducing systematic error to this technique. In addition, accuracy is compromised if wounds begin to granulate, so optimal results occur when thermography is done within 3 days of sustaining the burn injury (39,40). Existing literature at present, points to laser Doppler flowmetry as the ideal method for burn depth assessment, with higher accuracy and specificity.
Clinical signs and symptoms alone are not often enough to diagnose CTS. There is a large amount of evidence to support the use of nerve conduction studies as the gold standard for the diagnosis of CTS. Despite Tchou’s remarkable results in their randomized controlled trial in 1992, the most recent large review, conducted in 2008 by Jesensek Papez et al., confirmed that although IRT was useful in identifying severe cases of CTS, nerve conduction studies were still the only reliable gold standard (28). Despite improvement in technology and research, IRT is still considered fairly unreliable and hence should not be used as a diagnostic test for CTS.
Over the last 20 years, there have been calls for increased standardization of procedures associated with IRT from patient preparation and body positioning for image recording, to evaluation of thermal imaging. A total of 24 body positions and 90 regions of interest have been defined to construct a clinical database of reference thermograms. This has shown to have a significant influence on the accuracy of measurements obtained from thermal images (41). Currently, there is no system or a consistent value which can be used to standardize a thermal profile (‘thermoprofile’) of the human body.
Conclusions
DIRT gives indirect information on tissue perfusion. From attention deficit hyperactivity disorder (42) to complex regional pain syndrome, the use of IRT as a diagnostic tool continues to expand. With the advantages of improved technology in the form of smaller, portable cameras with quick set up and simple data capture, along with standardization protocols set in place for uniformity and repeatability of data capture, the use of a non-invasive tool to diagnose as well as assess response to treatment for various clinical conditions, is extremely appealing. In plastic surgery, DIRT can be used to identify perforators in planning flap reconstruction, intraoperatively to assess perfusion and post operatively for flap monitoring. Ambient heat loss and the time sensitivity of wound assessment preclude the use of DIRT for burn depth analysis. So also, there is not enough evidence to support the use of DIRT for the diagnosis of CTS. It is clear from all the studies to date that current DIRT technology does not give the best sensitivity or specificity. Rather, it provides additional information which should be used in conjunction with existing diagnostic modalities and clinical assessment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ring EF. The discovery of infrared radiation in 1800. Imaging Sci J 2000;48:1-8.
- Ring EF. The historical development of thermal imaging in medicine. Rheumatology (Oxford) 2004;43:800-2. [PubMed]
- de Weerd L, Mercer JB, Weum S. Dynamic infrared thermography. Clin Plast Surg 2011;38:277-92. [PubMed]
- Patsopoulos NA, Analatos AA, Ioannidis JP. Relative citation impact of various study designs in the health sciences. JAMA 2005;293:2362-6. [PubMed]
- Bulstrode NW, Wilson GR, Inglis MS. No-touch free-flap temperature monitoring. Br J Plast Surg 2002;55:174. [PubMed]
- Rustemeyer J, Radtke J, Bremerich A. Thermography and thermoregulation of the face. Head Face Med 2007;3:17. [PubMed]
- May JW Jr, Halls MJ. Thermocouple probe monitoring for free tissue transfer, replantation, and revascularization procedures. Clin Plast Surg 1985;12:197-207. [PubMed]
- Theuvenet WJ, Koeyers GF, Borghouts MH. Thermographic assessment of perforating arteries. A preoperative screening method for fasciocutaneous and musculocutaneous flaps. Scand J Plast Reconstr Surg 1986;20:25-9. [PubMed]
- Itoh Y, Arai K. Use of recovery-enhanced thermography to localize cutaneous perforators. Ann Plast Surg 1995;34:507-11. [PubMed]
- Salmi AM, Tukiainen E, Asko-Seljavaara S. Thermographic mapping of perforators and skin blood flow in the free transverse rectus abdominis musculocutaneous flap. Ann Plast Surg 1995;35:159-64. [PubMed]
- Zetterman E, Salmi AM, Suominen S, et al. Effect of cooling and warming on thermographic imaging of the perforating vessels of the abdomen. Eur J Plast Surg 1999;22:58-61.
- Kalra S, Dancey A, Waters R. Intraoperative selection of dominant perforator vessel in DIEP free flaps based on perfusion strength using digital infrared thermography - a pilot study. J Plast Reconstr Aesthet Surg 2007;60:1365-8. [PubMed]
- de Weerd L, Weum S, Mercer JB. The value of dynamic infrared thermography (DIRT) in perforatorselection and planning of free DIEP flaps. Ann Plast Surg 2009;63:274-9. [PubMed]
- Chijiwa T, Arai K, Miyazaki N, et al. Making of a facial perforator map by thermography. Ann Plast Surg 2000;44:596-600. [PubMed]
- Tenorio X, Mahajan AL, Wettstein R, et al. Early detection of flap failure using a new thermographic device. J Surg Res 2009;151:15-21. [PubMed]
- Wolff KD, Telzrow T, Rudolph KH, et al. Isotope perfusion and infrared thermography of arterialised, venous flow-through and pedicled venous flaps. Br J Plast Surg 1995;48:61-70. [PubMed]
- Shejbal D, Drvis P, Bedekovic V. Thermography-measured effect of capsaicin, methylprednisolone and mitomycin on the survival of random skin flaps in rats. Skin Res Technol 2012;18:157-61. [PubMed]
- de Weerd L, Miland AO, Mercer JB. Perfusion dynamics of free DIEP and SIEA flaps during the first postoperative week monitored with dynamic infrared thermography. Ann Plast Surg 2009;62:42-7. [PubMed]
- Hackett ME. The use of thermography in the assessment of depth of burn and blood supply of flaps, with preliminary reports on its use in Dupuytren's contracture and treatment of varicose ulcers. Br J Plast Surg 1974;27:311-7. [PubMed]
- Monstrey S, Hoeksema H, Verbelen J, et al. Assessment of burn depth and burn wound healing potential. Burns 2008;34:761-9. [PubMed]
- Zhu WP, Xin XR. Study on the distribution pattern of skin temperature in normal Chinese and detection of the depth of early burn wound by infrared thermography. Ann N Y Acad Sci 1999;888:300-13. [PubMed]
- Renkielska A, Nowakowski A, Kaczmarek M, et al. Burn depths evaluation based on active dynamic IR thermal imaging--a preliminary study. Burns 2006;32:867-75. [PubMed]
- Devgan L, Bhat S, Aylward S, et al. Modalities for the assessment of burn wound depth. J Burns Wounds 2006;5:e2. [PubMed]
- Herrick RT, Herrick SK. Thermography in the detection of carpal tunnel syndrome and other compressive neuropathies. J Hand Surg Am 1987;12:943-9. [PubMed]
- So YT, Olney RK, Aminoff MJ. Evaluation of thermography in the diagnosis of selected entrapment neuropathies. Neurology 1989;39:1-5. [PubMed]
- Reilly PA, Clarke AK, Ring EF. Thermography in carpal tunnel syndrome (CTS). Br J Rheumatol 1989;28:553-4. [PubMed]
- Tchou S, Costich JF, Burgess RC, et al. Thermographic observations in unilateral carpal tunnel syndrome: report of 61 cases. J Hand Surg Am 1992;17:631-7. [PubMed]
- Jesensek Papez B, Palfy M, Mertik M, et al. Infrared thermography based on artificial intelligence as a screening method for carpal tunnel syndrome diagnosis. J Int Med Res 2009;37:779-90. [PubMed]
- Saxena AK, Willital GH. Infrared thermography: experience from a decade of pediatric imaging. Eur J Pediatr 2008;167:757-64. [PubMed]
- Maillard GF, Hessler C. Thermography of malignant melanoma. Preliminary report. Dermatologica 1969;139:353-8. [PubMed]
- Centigul MP, Herman C. Quantification of the thermal signature of a melanoma lesion. Int J Therm Sci 2011;50:421-31.
- Santa Cruz GA, González SJ, Bertotti J, et al. First application of dynamic infrared imaging in boron neutron capture therapy for cutaneous malignant melanoma. Med Phys 2009;36:4519-29. [PubMed]
- Fitzgerald A, Berentson-Shaw J. Thermography as a screening and diagnostic tool: a systematic review. N Z Med J 2012;125:80-91. [PubMed]
- Acharya UR, Ng EY, Tan JH, et al. Thermography based breast cancer detection using texture features and Support Vector Machine. J Med Syst 2012;36:1503-10. [PubMed]
- Ng EY, Sudharsan NM. Computer simulation in conjunction with medical thermography as an adjunct tool for early detection of breast cancer. BMC Cancer 2004;4:17. [PubMed]
- Whitaker IS, Lie KH, Rozen WM, et al. Dynamic infrared thermography for the preoperative planning of microsurgical breast reconstruction: a comparison with CTA. J Plast Reconstr Aesthet Surg 2012;65:130-2. [PubMed]
- Wei FC, Mardini S. Flaps and reconstructive surgery. Elsevier Health Sciences, 2009.
- Brown JS, Devine JC, Magennis P, et al. Factors that influence the outcome of salvage in free tissue transfer. Br J Oral Maxillofac Surg 2003;41:16-20. [PubMed]
- Anselmo V, Zawacki B. Infrared photography as a diagnostic tool for the burn ward. Proc Soc Photo-Optical. Instr Eng 1973;8:181.
- Liddington MI, Shakespeare PG. Timing of the thermographic assessment of burns. Burns 1996;22:26-8. [PubMed]
- Ammer K. The Glamorgan protocol for recording and evaluation of thermal images of the human body. Thermol Int 2008;18:125-44.
- Coben R, Myers TE. Sensitivity and specificity of long wave infrared imaging for attention-deficit/hyperactivity disorder. J Atten Disord 2009;13:56-65. [PubMed]