
Breast-conserving in centrally located breast cancer patients confirmed safe by SEER based study
Introduction
Breast cancer is one of the most common cancers among women all over the world. Due to the promotion of early screening, the incidence of early breast cancer has increased. There are two types of surgical treatment for breast cancer, namely mastectomy and breast-conserving surgery (BCS). BCS is recommended for early-stage patients, which has little effect on the breast appearance. Some patients are not suitable for BCS, and conditions can be created through neoadjuvant chemotherapy. Patients who do not have the opportunity to undergo BCS should undergo mastectomy through which the breasts are completely cut. Concurrently, increasing numbers of patients are requesting that a satisfactory postoperative breast appearance is maintained. BCS has become increasingly important in the treatment of early breast cancer (1). Extensive medical research has confirmed the safety of BCS. A 20-year follow-up of the Danish randomized DBCG-82TM protocol (2) indicated that BCS in eligible patients is as effective as mastectomy regarding local tumor control, relapse-free survival (RFS) and overall survival (OS). A 10-year study in the Netherlands (3) with 37,207 patients has also confirmed the same OS of BCS and mastectomy. The results of the EORTC 10801 trial with a 20-year follow-up (4,5) were also the same.
Centrally located breast cancer is commonly defined as breast cancer within 2.0 cm from the nipple areola complex (NAC). For several reasons, BCS has not been considered suitable for breast cancer in the central region. Breast cancer in the central region is more likely to have multiple centers (6) which makes it difficult to resect completely. In addition, BCS in the central region which cannot retain the NAC may fail to achieve satisfactory cosmetic results. Moreover, previous studies have shown that the marginal positive rate and the probability of breast lymph node and axillary lymph node metastasis are higher in BCS for breast cancer in the central region. Thus, numerous randomized controlled trials, including NSABP B06, have specifically excluded central breast cancer from their research.
The oncological safety of breast-conservation in patients with centrally located breast cancer has elicited controversy for many years. In 2008, the American Radiological Society’s guidelines stipulated that tumors under the NAC are not contraindications for breast-conserving treatment as various studies have found that the 10-year survival rate and RFS rate of patients with early central breast cancer who underwent BCS were the same as those who received mastectomy (7,8). The 2013 National Comprehensive Cancer Network (NCCN) guidelines also recommended that breast cancer in the central region should not be contraindicated for BCS. Although some experts have reached an agreement on the safety of BCS in the central region, most of the related studies have been conducted retrospectively with a small sample size. In Haffty et al.’s study in 1995, 98 patients were enrolled, and 6 out of 88 patients who maintained NAC experienced a local recurrence (8). In 2020, Zhang et al. (9) have recently compared the safety of BCS and mastectomy in early-stage patients with centrally located breast cancer, finding that BCS is safe for well-selected, early-stage T1 or T2 central breast cancer which can only benefit a small group of specific patients.
The Surveillance, Epidemiology, and End Results (SEER) database is a free database containing cancer diagnosis, treatment, and survival data for approximately 30% of the U.S. population. It is a good tool to retrospectively analyze some clinical hypothesis but the results are only confined to the US.
Hence, our study aimed to evaluate the oncological safety of BCS in centrally located breast cancer patients based on the data from the SEER database. We retrospectively analyzed the data of 79,214 patients who had undergone BCS in 2012–2014 from the SEER database to evaluate the main demographic and clinical characteristics affecting prognosis. Our study provides a more in-depth and comprehensive understanding of the clinical features of breast-conservation in centrally located breast cancer patients and attempted to lay a theoretical foundation for surgical treatment.
We present the following article in accordance with the STROBE reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-21-914/rc).
Methods
Study design
The study aimed to use the SEER database to evaluate the oncological safety of BCS in centrally located breast cancer patients and provide a foundation for treatments in centrally located breast cancer patients.
Data source and patients
The data were downloaded from the SEER database through which we extracted the data for all cases that were diagnosed as breast cancer from 2012 to 2014. We excluded patients with no explicit type of basic characteristics. In total, 79,214 patients who have undergone BCS were included in this study, including patients with breast cancer in the central region (n=3,128) and outside the central region (n=76,086). As local recurrence data are unavailable in the SEER database, the primary outcome of our study was disease-specific survival (DSS) and OS. We defined DSS from the time of initial diagnosis to the time of disease-related death. The OS was determined based on the date of diagnosis to death from any cause. The SEER database is a database for free use, and a Data-Use Agreement for the SEER 1973–2015 Research Data File was completed. The original data in this study were downloaded from the SEER*Stat software version 8.3.6 in the client-server model (https://seer.cancer.gov/data/). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
To verify the results, patients who received BCS in different regions in Xiangya hospital from 2015–2016 were recruited.
The requirement for ethical approval and informed consent was waived by ethics committee of Xiangya Hospital, Central South University, because of the retrospective nature of the study.
Variables
The clinicopathological characteristics of patients before and after propensity score matching (PSM) were included in the analysis (Table 1): age at diagnosis, histologic type, grade, radiotherapy, chemotherapy, T and N stage based on the Derived American Joint Committee on Cancer (AJCC) stage Group (6th) (10,11), molecular subtype, estrogen receptor (ER) status, progesterone receptor (PR) status and human epidermal growth factor receptor 2 (HER2) status. Cases were divided into 2 subgroups: breast cancer in the central region and breast cancer not in the central region. Those whose primary sites of tumor were nipple and central portion of breast were defined as breast cancer in the central region according to the category in the SEER database, while the rest were classed as being in the noncentral region. Due to the data availability of the SEER, the extent of radiotherapy and chemotherapy is unknown.
Table 1
| Characteristics | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Noncentral | Central | Total | P | Noncentral | Central | Total | P | ||
| Age | <0.001 | 0.901 | |||||||
| 20–50 years | 11,090 (14.6) | 329 (10.5) | 11,419 (14.4) | 326 (10.4) | 329 (10.5) | 655 (10.5) | |||
| >50 years | 64,996 (85.4) | 2,799 (89.5) | 67,795 (85.6) | 2,801 (89.6) | 2,798 (89.5) | 5,599 (89.5) | |||
| Histologic | <0.001 | 0.826 | |||||||
| Ductal carcinoma | 59,161 (77.8) | 2,264 (72.4) | 6,1425 (77.5) | 2,268 (72.5) | 2,263 (72.4) | 4,531 (72.4) | |||
| Lobular carcinoma | 5,786 (7.6) | 262 (8.4) | 6,048 (7.6) | 272 (8.7) | 262 (8.4) | 534 (8.5) | |||
| Other | 11,139 (14.6) | 602 (19.2) | 11,741 (14.8) | 587 (18.8) | 602 (19.3) | 1,189 (19.0) | |||
| Grade | <0.001 | 0.140 | |||||||
| I | 21,798 (28.6) | 833 (26.6) | 22,631 (28.6) | 888 (28.4) | 833 (26.6) | 1,721 (27.5) | |||
| II | 33,719 (44.3) | 1,589 (50.8) | 35,308 (44.6) | 1,463 (46.8) | 1,589 (50.8) | 3,052 (48.8) | |||
| III | 20,425 (26.8) | 700 (22.4) | 21,125 (26.7) | 771 (24.7) | 699 (22.4) | 1,470 (23.5) | |||
| IV | 144 (0.2) | 6 (0.2) | 150 (0.2) | 5 (0.2) | 6 (0.2) | 11 (0.2) | |||
| Radiation | 0.093 | 0.085 | |||||||
| Non-radiation | 19,488 (25.6) | 843 (27.0) | 20,331 (25.7) | 903 (28.9) | 842 (26.9) | 1,745 (27.9) | |||
| Radiation | 56,598 (74.4) | 2,285 (73.0) | 58,883 (74.3) | 2,224 (71.1) | 2,285 (73.1) | 4,509 (72.1) | |||
| Chemotherapy | 0.001 | 0.955 | |||||||
| Nonchemotherapy | 52,012 (68.4) | 2,227 (71.2) | 54,239 (68.5) | 2,228 (71.3) | 2,226 (71.2) | 4,454 (71.2) | |||
| Chemotherapy | 24,074 (31.6) | 901 (28.8) | 24,975 (31.5) | 899 (28.7) | 901 (28.8) | 1,800 (28.8) | |||
| T stage | <0.001 | 0.001 | |||||||
| T1 | 56,202 (73.9) | 2,218 (70.9) | 58,420 (73.7) | 2,178 (69.7) | 2,218 (70.9) | 4,396 (70.3) | |||
| T2 | 18,314 (24.1) | 792 (25.3) | 19,106 (24.1) | 844 (27.0) | 792 (25.3) | 1,636 (26.2) | |||
| T3 | 1,260 (1.7) | 62 (2.0) | 1,322 (1.7) | 81 (2.6) | 62 (2.0) | 143 (2.3) | |||
| T4 | 310 (0.4) | 56 (1.8) | 366 (0.5) | 24 (0.8) | 55 (1.8) | 79 (1.3) | |||
| N stage | <0.001 | 0.209 | |||||||
| N0 | 61,462 (80.8) | 2,341 (74.8) | 63,803 (80.5) | 2,349 (75.1) | 2,340 (74.8) | 4,689 (75.0) | |||
| N1 | 12,275 (16.1) | 679 (21.7) | 12,954 (16.4) | 643 (20.6) | 679 (21.7) | 1,322 (21.1) | |||
| N2 | 1,700 (2.2) | 76 (2.4) | 1,776 (2.2) | 89 (2.8) | 76 (2.4) | 165 (2.6) | |||
| N3 | 649 (0.9) | 32 (1.0) | 681 (0.9) | 46 (1.5) | 32 (1.0) | 78 (1.2) | |||
| Molecular subtype | <0.001 | 0.559 | |||||||
| Luminal A | 60,015 (78.9) | 2,546 (81.4) | 62,561 (79.0) | 2,549 (81.5) | 2,546 (81.4) | 5,095 (81.5) | |||
| Luminal B | 6,451 (8.5) | 275 (8.8) | 6,726 (8.5) | 273 (8.7) | 274 (8.8) | 547 (8.7) | |||
| HER2 enriched | 2,201 (2.9) | 103 (3.3) | 2,304 (2.9) | 86 (2.8) | 103 (3.3) | 189 (3.0) | |||
| TNBC | 7,419 (9.8) | 204 (6.5) | 7,623 (9.6) | 219 (7.0) | 204 (6.5) | 423 (6.8) | |||
| ER | <0.001 | 0.934 | |||||||
| Negative | 10,319 (13.6) | 329 (10.5) | 10,648 (13.4) | 331 (10.6) | 329 (10.5) | 660 (10.6) | |||
| Positive | 65,767 (86.4) | 2,799 (89.5) | 68,566 (86.6) | 2,796 (89.4) | 2,798 (89.5) | 5,594 (89.4) | |||
| PR | 0.003 | 0.594 | |||||||
| Negative | 17,523 (23.0) | 649 (20.7) | 18,172 (22.9) | 631 (20.2) | 648 (20.7) | 1,279 (20.5) | |||
| Positive | 58,563 (77.0) | 2,479 (79.3) | 61,042 (77.1) | 2,496 (79.8) | 2,479 (79.3) | 4,975 (79.5) | |||
| HER2 | 0.219 | 0.480 | |||||||
| Negative | 67,434 (88.6) | 2,750 (87.9) | 70,184 (88.6) | 2,768 (88.5) | 2,750 (87.9) | 5,518 (88.2) | |||
| Positive | 8,652 (11.4) | 378 (12.1) | 9,030 (11.4) | 359 (11.5) | 377 (12.1) | 736 (11.8) | |||
Data are shown as number (percentage). PSM, propensity score matching; BCS, breast-conserving surgery; ER, estrogen receptor; PR, progesterone receptor; TNBC, triple-negative breast cancer.
Statistical methods
Statistical analysis was performed by SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The PSM was used to eliminate the effects of non-random statistics. We used 1:1 nearest-neighbor matching, setting the caliper as 0.02 to balance the baseline covariates within the groups (12). The 79,214 patients’ characteristics between central and noncentral groups were compared by chi-square test. The DSS and OS survival curve using the Kaplan-Meier method was used to compare the survival difference in breast conservation in the central region of breast cancer patients. The Cox proportional hazards regression model on univariate and multivariate analysis was performed for prognostic variables. The same statistical analysis was done using the data from our own hospital. A corresponding 95% confidence interval (CI) was calculated, and the statistical significance level was set at P<0.05.
Results
In total, 79,214 patients who have undergone BCS were included in this study, with breast cancer in the central region (n=3,128) and outside the central region (n=76,086).
Baseline characteristics of patients before and after PSM
We analyzed the data of 79,214 patients from the SEER database who had undergone BCS in 2012–2014. The group was stratified by breast cancer region (Table 1), including patients with breast cancer in the central region (n=3,128) and not in the central region (n=76,086). After PSM, a total of 6,254 patients (central 31,27 vs. noncentral 3,127) were matched and the covariates were properly balanced between the 2 groups. The baseline characteristics of the patients before and after PSM are summarized in Table 1. Notable differences were detected in T stage (P<0.05).
Survival of patients who received BCS in central region compared with noncentral region
To evaluate the survival of patients who received BCS in different regions, we calculated the DSS and OS through Kaplan-Meier survival curves with the log-rank test (Figures 1,2). The mean DSS of breast cancer patients was 58.1 months in the central region and 58.0 months in noncentral region (P>0.05), and the mean OS was the same 58.0 months (P>0.05) indicating there was no difference between the central and noncentral group. Patients who underwent BCS in the central region and non-central region had relevantly the same DSS and OS, which suggested that BCS in centrally located breast cancer patients is equally safe as BCS in noncentral breast cancer patients.
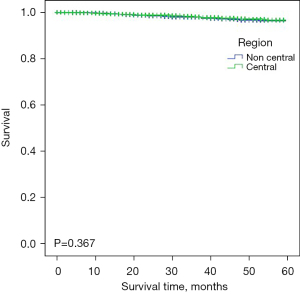
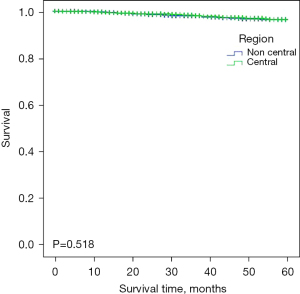
Prognostic factors associated with DSS and OS
We analyzed the prognostic factors in patients who underwent BCS in the central region using Cox proportional hazards regression model for both univariate and multivariate analyses of DSS and OS (Table 2). After selection by univariate Cox regression analysis of each variable, several covariates were put in the multivariate Cox regression analysis and forest plot (Figure 3). Radiation, chemotherapy, T stage, N stage, molecular subtype, ER, and PR were independent prognostic factors for patients. Radiation, chemotherapy, lower T stage, lower N stage, ER+, and PR+ indicated a better DSS and OS.
Table 2
| Category | DSS | OS | |||||
|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | ||
| Grade | |||||||
| I | Reference | Reference | |||||
| II | 0.549 | 0.848 | 0.494–1.455 | 0.489 | 0.830 | 0.489–1.409 | |
| III | 0.208 | 1.446 | 0.814–2.570 | 0.225 | 1.419 | 0.807–2.496 | |
| IV | 0.809 | 1.218 | 0.247–5.999 | 0.814 | 1.209 | 0.248–5.888 | |
| Radiation | |||||||
| Non-radiation | Reference | Reference | |||||
| Radiation | <0.001 | 0.333 | 0.236–0.468 | <0.001 | 0.349 | 0.249–0.489 | |
| Chemotherapy | |||||||
| Non-chemotherapy | Reference | Reference | |||||
| Chemotherapy | 0.032 | 0.653 | 0.442–0.964 | 0.025 | 0.642 | 0.437–0.945 | |
| T stage | |||||||
| T1 | Reference | Reference | |||||
| T2 | <0.001 | 3.003 | 2.008–4.491 | <0.001 | 2.917 | 1.961–4.339 | |
| T3 | <0.001 | 5.032 | 2.678–9.457 | <0.001 | 4.825 | 2.577–9.036 | |
| T4 | <0.001 | 5.763 | 2.534–13.109 | <0.001 | 5.483 | 2.415–12.451 | |
| N stage | |||||||
| N0 | Reference | Reference | |||||
| N1 | 0.001 | 2.021 | 1.354–3.018 | <0.001 | 2.059 | 1.384–3.063 | |
| N2 | <0.001 | 3.100 | 1.678–5.728 | <0.001 | 3.361 | 1.848–6.112 | |
| N3 | <0.001 | 6.713 | 3.446–13.076 | <0.001 | 6.768 | 3.483–13.151 | |
| Molecular subtype | |||||||
| Luminal A | 0.411 | 1.735 | 0.466–6.452 | 0.444 | 1.670 | 0.450–6.199 | |
| Luminal B | 0.313 | 1.959 | 0.530–7.242 | 0.350 | 1.866 | 0.505–6.895 | |
| HER2 enriched | 0.021 | 0.402 | 0.186–0.870 | 0.034 | 0.452 | 0.216–0.943 | |
| TNBC | Reference | Reference | |||||
| ER | |||||||
| Negative | Reference | Reference | |||||
| Positive | 0.021 | 0.248 | 0.076–0.813 | 0.024 | 0.256 | 0.078–0.838 | |
| PR | |||||||
| Negative | Reference | Reference | |||||
| Positive | 0.002 | 0.460 | 0.283–0.748 | 0.002 | 0.471 | 0.290–0.764 | |
HR with 95% CI for death in the OS and DSS of patients. P values of the Cox proportional hazard regression are reported. PSM, propensity score matching; BCS, breast-conserving surgery; ER, estrogen receptor; PR, progesterone receptor; TNBC, triple-negative breast cancer; OS, overall survival; DSS, disease-specific survival; HR, hazard ratio; CI, confidence interval.
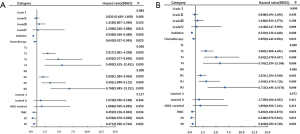
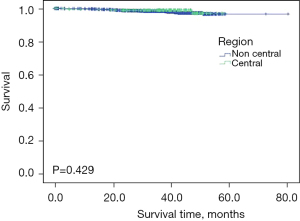
Result verification
The baseline characteristics of the patients in our hospital are summarized in Table 3. Besides T stage (P<0.05), there were no notable differences in other characteristics between the noncentral group and central group. We then calculated the OS through Kaplan-Meier survival curves with the log-rank test (Figure 4). The mean OS of breast cancer patients was 57.2 months in the central region and 78.2 months in noncentral region (P>0.05), indicating there was no difference between the central and noncentral group.
Table 3
| Characteristics | Noncentral, n (%) | Central, n (%) | P value |
|---|---|---|---|
| Age | 0.707 | ||
| 20–50 years | 858 (59.25) | 128 (57.92) | |
| >50 years | 590 (40.75) | 93 (42.08) | |
| Histologic type | 0.376 | ||
| Ductal carcinoma | 1326 (91.57) | 197 (89.14) | |
| Lobular carcinoma | 29 (2.00) | 4 (1.81) | |
| Other | 93 (6.42) | 20 (9.05) | |
| Grade | 0.537 | ||
| I | 38 (2.62) | 6 (2.71) | |
| II | 663 (45.79) | 110 (49.77) | |
| III | 546 (37.71) | 72 (32.58) | |
| IV | 201 (13.88) | 33 (14.93) | |
| Radiation | 0.819 | ||
| Non-radiation | 179 (12.36) | 25 (11.31) | |
| Radiation | 1090 (75.28) | 166 (75.11) | |
| Unknown | 179 (12.36) | 30 (13.57) | |
| Chemotherapy | 0.235 | ||
| Non-chemotherapy | 431 (29.77) | 78 (35.29) | |
| Chemotherapy | 886 (61.19) | 123 (55.66) | |
| Unknown | 131 (9.05) | 20 (9.05) | |
| T stage | 0.024 | ||
| T1 | 896 (61.88) | 125 (56.56) | |
| T2 | 314 (21.69) | 66 (29.86) | |
| T3 | 238 (16.44) | 30 (13.57) | |
| N stage | 0.063 | ||
| N0 | 1044 (72.10) | 165 (74.66) | |
| N1 | 304 (20.99) | 38 (17.19) | |
| N2 | 66 (4.56) | 7 (3.17) | |
| N3 | 34 (2.35) | 11 (4.98) | |
| Molecular subtype | 0.221 | ||
| Luminal A | 934 (64.50) | 144 (65.16) | |
| Luminal B | 151 (10.43) | 29 (13.12) | |
| HER2 enriched | 92 (6.35) | 12 (5.43) | |
| TNBC | 257 (17.75) | 36 (16.29) | |
| Unknown | 14 (0.97) | 0 | |
| HR | 0.094 | ||
| Negative | 349 (24.10) | 48 (21.72) | |
| Positive | 1085 (74.93) | 173 (78.28) | |
| Unknown | 14 (0.97) | 0 | |
| HER2 | 0.114 | ||
| Negative | 1191 (82.25) | 180 (81.45) | |
| Positive | 243 (16.78) | 41 (18.55) | |
| Unknown | 14 (0.97) | 0 |
BCS, breast-conserving surgery; HR, hormone receptor.
Discussion
Our study found that patients who underwent BCS in the central region and non-central region had relatively the same DSS and OS. Radiation, chemotherapy, T stage, N stage, molecular subtype, ER, and PR were independent prognostic factors for patients. Radiation, chemotherapy, lower T stage, lower N stage, ER+, and PR+ indicated a better DSS and OS. In the subsequent verification, we also found that there was no significant difference in OS of patients undergone BCS in different regions.
In recent years, a few consensuses, which are based on low-level evidence, have concluded that BCS in the central region is safe and it has been included in some guidelines; however, most have lacked long-term follow-up data. With an average follow up of 111 months, Haffty et al. performed BCS on 98 cases of early breast cancer patients with NAC <2.0 cm, among which 88 patients retained NAC. Only 6 patients had local recurrence and there was no significant difference in 10-year survival rate and RFS rate (8). Nevertheless, Haffty et al.’s study only included a small number of patients and it was performed in 1995 which cannot provide enough evidence for today’s guideline. Another study (13) also concluded BCS is feasible for breast cancer in the central region, which does not affect the patient’s prognosis and can obtain better cosmetic results. This study only included 45 patients with a follow-up of 51 months. These studies all indicate that patients with subareolar breast cancer that occur within 2 cm of the NAC are suitable for BCS, meaning their NAC did not need to be removed and could be safely included in the radiotherapy with acceptable complications and cosmetic effects. However, these trials were all between 1970 and 1990 and cannot give guidance for today’s BCS in centrally located breast cancer patients.
As a result, there is still controversy over BCS in the central region of breast. Nowadays, due to the controversy and low-level evidence, BCS is conducted in less than 10% of patients with breast cancer in the central region in our hospital. So many patients have failed to reserve a satisfactory postoperative breast appearance. It is necessary to conduct a study assessing the oncological safety of BCS in patients with cancer in the central region. This study was the first to examine the oncological safety of BCS in centrally located breast cancer patients based on the data from the SEER database and our own hospital’s database. In our study, when evaluating patients who had BCS, there were no statistically significant differences in OS and DSS between the centrally located and noncentrally located breast cancer patients. These data show that from the aspect of survival, patients who received breast-conserving surgery in the central region were as safe as those with breast-conservation in other areas of breast. With a large sample of real-world statistics and a good clinical reference value, we have more confidence in recommending BCS to patients with tumors in the central region, which will also improve the quality of life for more women with breast cancer. Here we suggest that for patients with cancer in the central region, BCS is first preferred as long as there is no other contraindications and appropriate adjuvant therapy or systemic therapy is administered to create opportunities for patients not suitable for BCS.
The study had several limitations. Since all the data we used to analyze were from the SEER database, we cannot include other factors, such as race, breast size, margin, and cosmetic results. In addition, local recurrence data are unavailable from the SEER database, the primary outcome of our study was DSS and OS. There is a lack of information on the surgical methods of BCS in the central region, including whether to remove NAC or whether to use oncoplastic technology. These may influence the risk of recurrence and survival outcome. As a result, we plan to conduct a multicenter study in China to further verify and find the same results.
To conclude, after retrospectively analyzing the oncological safety of BCS in patients with cancer in the central breast region, we conclude that breast cancer in the central region should not be contraindicated for breast conserving surgery which means that BCS can benefit a wider range of patients.
Acknowledgments
The authors would like to thank the SEER program for providing open access to their database.
Funding: This study was funded by grants from the National Natural Science Foundation of China (81001179); Xiangya-Peking University Weiming Fund Project (xywm2015I08); and Clinical Medical Technology Innovation and Technology Project of Hunan Provincial Department of Science and Technology (2018SK52611). The funding was used in the study of SEER database and data processing.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-21-914/rc
Data Sharing Statement: Available athttps://gs.amegroups.com/article/view/10.21037/gs-21-914/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-21-914/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for ethical approval and informed consent was waived by ethics committee of Xiangya Hospital, Central South University, because of the retrospective nature of the study. The researchers will do their best to protect the information provided by the patient from revealing personal privacy.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002;347:567-75. [Crossref] [PubMed]
- Blichert-Toft M, Nielsen M, Düring M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 2008;47:672-81. [Crossref] [PubMed]
- van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158-70. [Crossref] [PubMed]
- Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412-9. [Crossref] [PubMed]
- Black DM, Hunt KK, Mittendorf EA. Long term outcomes reporting the safety of breast conserving therapy compared to mastectomy: 20-year results of EORTC 10801. Gland Surg 2013;2:120-3. [PubMed]
- Fisher ER, Gregorio RM, Fisher B, et al. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer 1975;36:1-85. [Crossref] [PubMed]
- Eberl MM, Fox CH, Edge SB, et al. BI-RADS classification for management of abnormal mammograms. J Am Board Fam Med 2006;19:161-4. [Crossref] [PubMed]
- Haffty BG, Wilson LD, Smith R, et al. Subareolar breast cancer: long-term results with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 1995;33:53-7. [Crossref] [PubMed]
- Zhang M, Wu K, Zhang P, et al. Breast-Conserving Surgery is Oncologically Safe for Well-Selected, Centrally Located Breast Cancer. Ann Surg Oncol 2021;28:330-9. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Qi WX, Cao L, Xu C, et al. Adjuvant regional nodal irradiation did not improve outcomes in T1-2N1 breast cancer after breast-conserving surgery: A propensity score matching analysis of BIG02/98 and BCIRG005 trials. Breast 2020;49:165-70. [Crossref] [PubMed]
- Tausch C, Hintringer T, Kugler F, et al. Breast-conserving surgery with resection of the nipple-areola complex for subareolar breast carcinoma. Br J Surg 2005;92:1368-71. [Crossref] [PubMed]
(English Language Editor: J. Jones)


