
Overexpression of DUOX2 mediates doxorubicin resistance and predicts prognosis of pancreatic cancer
Introduction
Pancreatic cancer is one of the most lethal cancers worldwide (1). Although progress in diagnosis and treatment has improved clinical outcomes, the prognosis of pancreatic cancer patients remains poor, with a 5-year survival rate of 6% (2). Therefore, it is essential to develop new therapeutic targets to improve the prognosis of pancreatic cancer patients.
The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) family, which comprises 7 members NOX1–5 and dual oxidase 1/2 (DUOX1/2), is involved in the generation of reactive oxygen species (ROS) (3,4). The ROS are central factors in regulating oxidative stress, and the deregulation of oxidase signaling is associated with cancer initiation and development (5-7). Studies have shown that the expression and role of NOX family members are different in cancers. The gene NOX4 regulates the proliferation and apoptosis of gastric cancer cells by activating ROS formation through the GLI1 pathway (8); NOX4 mediates the migration of inflammatory cells and radiation-induced senescence by producing ROS (9); NOX1 plays a vital role in UV-associated keratinocyte responses and skin carcinogenesis (10); and NOX1 and NOX4 have shown different prognostic effects for liver cancer patients after hepatectomy (11). In pancreatic cancer, standard chemotherapy with gemcitabine promotes stemness and chemotherapy resistance through the NOX/ROS/NF-κB/STAT3 signaling pathway (12).
Studies have reported that NOXs play important roles in the carcinogenesis of pancreatic cancer. In pancreatic cancer, suppression of NOX4 initiates the apoptosis of Panc-1 cells via the AKT pathway (13). The pro-inflammatory cytokine interferon (IFN)-γ activates the reactive oxygen cascade by regulation of DUOX2 in human pancreatic cancer cells (14). In pancreatic cancer cells, NOX4 promotes ROS signaling and contributes to the epithelial-mesenchymal transition (EMT) induced by transforming growth factor-β (TGF-β) (15). In pancreatic cancer cells, overexpression of DUOX2 predicts poor prognosis and promotes cell invasion and growth (16). However, there is little systematic research on the expression, prognostic significance, and role of NOX family genes in pancreatic cancer. Furthermore, the effect of DUOX2 on chemosensitivity of pancreatic cancer cells still needs to be clarified.
In this study, we explored the expression levels and clinical significance of individual NOX family members in pancreatic cancer by mining Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) datasets and confirmed that DUOX2 might be a therapeutic target for pancreatic cancer patients. We present the following article in accordance with the MDAR reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-21-776/rc).
Methods
Pancreatic cancer tissues
Pancreatic cancer and adjacent nontumor tissues were collected between January 2015 and January 2016 in The First Affiliated Hospital of Zhengzhou University. A total of 44 patients were diagnosed with pancreatic ductal adenocarcinoma (PDAC) histologically. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of The First Affiliated Hospital of Zhengzhou University (No. 2018-KY-0137) and informed consent was taken from all the patients.
Cell culture and reagents
Pancreatic cancer cell lines and immortalized human pancreatic ductal epithelial cell lines (HPDE) were obtained from the Cell Repository, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, 11965092, Waltham, MA, USA) or Roswell Park Memorial Institute 1640 (RPMI-1640; Gibco, 21870076, USA) medium containing 10% inactivated fetal bovine serum (FBS; Gibco, 10099141C, USA). All cell lines were authenticated using short tandem repeat DNA profiling within 3 months and tested for mycoplasma contamination. Antibodies used in this study were DUOX2 (ab97266, Abcam, English) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Proteintech, 160004-1-Ig, China). Control and small interfering RNA (siRNA) sequences were constructed by GeneChem (Shanghai, China) and cell transfection was performed using Lipo 3000 (Invitrogen, L3000008, Carlsbad, CA, USA) according to the manufacturer’s instructions. The siRNA sequence for DUOX2 was CCCAACGTCTTTGTGAATGAT. Doxorubicin was purchased from Sigma-Aldrich (D1515, St. Louis, MO, USA). After transfection of siRNA for 48 h, pancreatic cancer cell lines AsPC-1 and BxPC-3 were treated with increasing concentrations of doxorubicin for 24 h.
Cell viability assay
Cells (3,000 per well) were cultured in 96-well plates and cell viability was measured using Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions after treatment. The absorbance value was measured at 450 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Transwell assay
After transfection for 48 h, Panc-1 cells were washed and collected. A total of 10,000 cells were resuspended in 100 μL DMEM and cultured in the top transwell chambers (Corning, USA), which were coated with Matrigel (BD Biosciences, CA, USA). The bottom chambers contained DMEM medium with 10% fetal bovine serum. After 24 h, cells on the bottom chambers were fixed, stained and counted manually by light microscopy.
Immunohistochemistry (IHC), western blot, and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
IHC staining was performed as described previously (8). Staining score was calculated based on the intensity and total area of the staining. The staining intensity was quantified as follows: 0 (none), 1 (weak), 2 (strong), or 3 (very strong). Distribution was scored as 0 (0%), 1 (1–50%), and 2 (51–100%). The IHC staining was performed as described previously (8). A TRIzol kit (Invitrogen, 15596026, USA) was used for total RNA extraction according to the manufacturer’s instructions. NanoDropND-2000 spectrophotometer (NanoDrop Tech., Wilmington, DE, USA) was used for RNA quality check. Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen, 28025013, USA) was used for cDNA synthesization according to the manufacturer’s instructions. Messenger RNA (mRNA) expression quantification was detected by a SYBR Green PCR Kit (Takara, RR420A, Beijing, China). We used GAPDH as the housekeeping gene. The primers used in this study were: DUOX2 forward: 5'-ACGCAGCTCTGTGTCAAAGGT-3', DUOX2 reverse: 5'-TGATGAACGAGACTCGACCAGGC-3'; GAPDH forward: 5'-AGAAGGCTGGGGCTCATTTG-3', GAPDH reverse: 5'-TGAGAGCTGTCCATTGGTAGAG-3'.
Analysis of mRNA microarray data
The gene expression profile datasets GSE16515, GSE28735, GSE62452, and GSE62165 were downloaded from GEO and analyzed utilizing the Bioconductor package Robust MultiArray Average (RMA) (R version 3.2.0; https://www.R-project.org/). The DEGs were defined by the Limma algorithm.
Statistical analyses
Overall survival (OS) and relapse-free survival (RFS) were identified as the time to occurrence of death or relapse after surgery, respectively. A two-sided Student’s t-test was used to compare the expression differences of NOX family genes between the tumor group and nontumor groups. Statistical analysis was performed using the SPSS software (version 17.0; Chicago, IL, USA). Images were plotted using GraphPad Prism software (version 6.0; San Diego, CA, USA). Publicly available TCGA data were downloaded from the TCGA data portal with clinical data available. The frequency of genomic alteration was analyzed according to the cBioPortal’s online instructions (https://www.cbioportal.org/). Expression levels of NOX family genes were determined by the log2[transcripts per million (TPM) +1] method. Kaplan-Meier method and the log-rank test were used for survival analysis. The independent prognostic value of DUOX2 was evaluated by a multivariate Cox proportional hazards regression model. The GSEA software package (version 3.0; Broad Institute; Cambridge, MA, USA) was used for gene set enrichment analysis (GSEA). All experiments were performed at least 3 times. A P value <0.05 was considered statistically significant; *P<0.05, **P<0.01, ***P<0.001.
Results
Expression of NOX family genes and hierarchical clustering analysis in pancreatic cancer
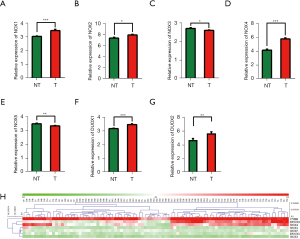
We compared the expression levels of NOX family genes in pancreatic cancer tissues (T) with those in matched nontumor tissues (NT) in GSE28735 dataset (n=45). The results showed that NOX1, NOX2 (CYBB), NOX4, DUOX1, and DUOX2 were significantly overexpressed in pancreatic cancer, while NOX3 and NOX5 were significantly downregulated (Figure 1A-1G). Unsupervised clustering analysis of 7 NOX family members was performed in GSE28735. Notably, part of the paired PDAC tissues and nontumor tissues could be grouped into clusters through hierarchical clustering (Figure 1H).
Genomic alterations of NOX family genes in pancreatic cancer
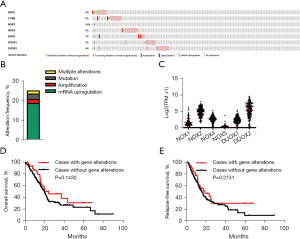
To investigate the regulation mechanism underlying the expression of NOX family genes in pancreatic cancer, genomic alterations, including copy number changes, somatic mutations, and mRNA upregulation, were analyzed using TCGA cBioPortal tool. The alterations of NOX family members occurring in 186 pancreatic cancer patients were shown (Figure 2A). Genetic aberrations were observed in 23.7% of 186 pancreatic cancer patients, mRNA upregulation was the most common alteration, with an alteration frequency of 18.2% (Figure 2B). The mRNA levels of DUOX2 were the highest among the NOX genes (Figure 2C), whereas NOX5 and NOX3 expression was downregulated (data not shown). Kaplan-Meier survival analysis revealed that there were no significant differences between OS and RFS in patients with NOX family genomic alterations and patients without (Figure 2D,2E). These findings collectively demonstrate that genomic alterations of NOX family genes are frequent and significantly contribute to their aberrant expression in pancreatic cancer.
Prognostic values of NOX family members in pancreatic cancer
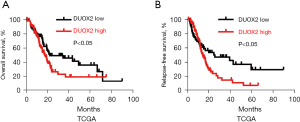
To determine the survival effect of the NOX family genes in pancreatic cancer patients, Kaplan-Meier analyses were performed. The results showed that only high DUOX2 mRNA expression was correlated with a significantly worse OS in pancreatic cancer patients (n=171; median: 17.9 vs. 22.47 months; log-rank test; Figure 3A). In addition, we found that only high DUOX2 mRNA expression was correlated with a far shorter RFS in pancreatic cancer patients (n=132; median: 14.75 vs. 25.89 months; log-rank test; Figure 3B). To explore whether DUOX2 is an independent prognostic factor for pancreatic cancer patients, a Cox multivariate regression analysis was performed. The results showed that DUOX2 maintained independence in predicting both the OS [hazard ratio (HR) =1.41, 95% confidence interval (CI): 1.131 to 1.723; Table S1] and RFS (HR =1.39, 95% CI: 1.231 to 1.896; Table S2) of patients with pancreatic cancer. Collectively, DUOX2 was revealed as an independent marker for outcome and recurrence in pancreatic cancer patients.
Expression and prognosis of DUOX2 in pancreatic cancer patients
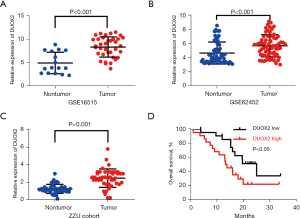
To further explore the expression level and survival value of DUOX2 in pancreatic cancer patients, DUOX2 expression levels were analyzed in independent cohorts of GSE16515 (n=36) and GSE62452 (n=69) datasets. It was shown that DUOX2 was significantly overexpressed in pancreatic cancer specimens compared with nontumor samples (Figure 4A,4B). Furthermore, DUOX2 expression was detected in fresh frozen pancreatic cancer specimens from The First Affiliated Hospital of Zhengzhou University (ZZU cohort; n=44; Table S3) with clinical data available by RT-qPCR. Results showed DUOX2 was significantly overexpressed in pancreatic cancer tissues compare with matched nontumor tissues (Figure 4C). Survival analysis showed that high DUOX2 mRNA expression was correlated with a significantly worse OS in pancreatic cancer patients (n=44; median: 15.9 vs. 23.32 months; log-rank test; Figure 4D). These results further suggest that DUOX2 might be a prognostic marker for pancreatic cancer patients.
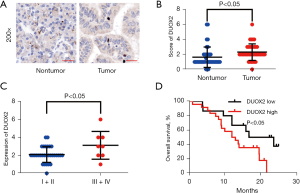
Overexpression of DUOX2 is associated with a poor prognosis in pancreatic cancer
To further validate the expression level of DUOX2 in pancreatic cancer patients, the expression of DUOX2 was detected by IHC. It was found that DUOX2 was significantly overexpressed in pancreatic cancer tissues compared with nontumor tissues (Figure 5A,5B). Late-stage pancreatic cancer patients exhibited higher expression levels of DUOX2 compared with early-stage patients (Figure 5C). Patients were stratified into two groups based on the median staining score. Kaplan-Meier analysis showed that high DUOX2 expression group participants exhibited shorter survival time compared to the DUOX2 low expression group (median: 12.5 vs. 20.22 months; log-rank test; Figure 5D). Thus, DUOX2 expression may serve as a valuable prognostic factor for pancreatic cancer patients.
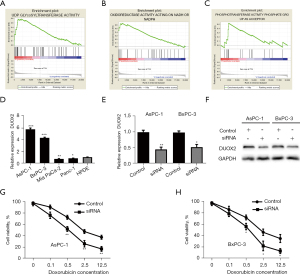
Knockdown of DUOX2 increases chemosensitivity to doxorubicin
To understand the role of DUOX2 in pancreatic cancer, GSEA was performed in GSE62165 dataset (n=118). In the DUOX2 overexpression group, genes related to uridine 5’-diphospho-glucuronosyltansferase (UDP) glycosyltransferase activity, oxidoreductase activity acting on nicotinamide adenine dinucleotide hydrogen (NADH) or NADPH and phosphotransferase activity were significantly enriched compared with the DUOX2 downregulation group (Figure 6A-6C). Next, we detected DUOX2 expression in pancreatic cancer cell lines. Compared with HPDE, DUOX2 was upregulated in four pancreatic cancer cell lines (Figure 6D). We knocked down the expression of DUOX2 by transfection of siRNA and the transfection effect was validated by RT-qPCR and western blot (Figure 6E,6F). The CCK-8 assays were performed to measure the cell viability after treatment with doxorubicin, which is a DNA damage-inducing first-line antineoplastic drug. The CCK-8 assays showed that inhibition of DUOX2 suppressed the proliferation of both AsPC-1 and BxPC-3 cells compared with control group (Figure 6G,6H). Collectively, our results suggest that upregulation of DUOX2 regulates doxorubicin resistance of pancreatic cancer cells.
Discussion
Recent studies have shown that NOX family genes could serve as valuable diagnostic and prognostic markers for many cancers (17). In our study, we found that the expression levels of NOX1, NOX2, NOX4, DUOX1, and DUOX2 were increased and NOX3 and NOX5 were decreased in pancreatic cancer tissue samples compared with adjacent nontumor tissues, which could be partially explained by genomic alterations. Among them, DUOX2 was a good outcome predictor and recurrence biomarker. Therefore, DUOX2 may represent a potential biomarker and be the subject of new therapeutic studies in the future.
Previous studies have shown that NOX family genes play vital roles in the development and progression of human cancers, although studies about the function of NOX3 in cancer are still rare. For example, NOX1 supports the growth of colon cancer cells by activating ROS-dependent signal transduction (18). In gallbladder cancer, NOX1 mediates cell chemoresistance via the HIF1α/MDR1 signaling pathway (19). Natural killer cells regulate murine melanoma metastasis by NOX2-derived ROS (20). In rectal cancer, the NOX2-ROS-HIF-1α signaling pathway plays a vital role in the growth inhibitory effect of oleanolic acid (21). It has also been shown that NOX4 regulates drug resistance of cancer cells by functioning as a mitochondrial energetic sensor that assists cancer metabolic reprogramming (22). Additionally, NOX4 inhibits growth of hepatocyte proliferation and progression of liver cancer (23). In vitro, NOX5 protects LK-positive anaplastic large-cell lymphoma cell lines against apoptosis (24). In lung cancer, knockdown of DUOX1 promotes invasion, EMT, and cancer stem cell characteristics (25). Epigenetic silencing of DUOX1 by promoter hypermethylation has been observed in human hepatocellular carcinoma (26). The DUOX2-ROS pathway promotes the elimination of the Klebsiella pneumoniae strain K5 from T24 cells (27). In rectal cancer, overexpression of DUOX2 is associated with poor response to preoperative chemoradiation therapy and clinical prognosis (28). In pancreatic cancer, DUOX2 is upregulated and associated with poor survival of pancreatic cancer patients, and regulates the invasion and proliferation of pancreatic cancer cells, which is also confirmed in our study (Figure S1). However, the relationship between chemosensitivity and dysregulation of DUOX2 was unclear.
We performed GSEA on DUOX2 which predicted prognosis and relapse of pancreatic cancer patients. In patients with high DUOX2 expression, the GO terms response to organic substance, actin cytoskeleton organization and biogenesis, protein transport, cell junction, Golgi apparatus, intercellular junction, cation binding, oxidoreductase activity acting on NADH or NADPH, and UDP glycosyltransferase activity were significantly enriched. Chemoresistance is a major issue for pancreatic cancer. Thus, screening biomarkers and targets to overcome resistance are significant for cancer therapy. Herein, it was our observation that increased DUOX2 expression is associated with chemotherapy resistance and may predict the chemo-therapeutic sensitivity for pancreatic cancer.
We provided a comprehensive bioinformatic analysis of NOX family genes involved in pancreatic cancer using online databases. Importantly, overexpression of DUOX2 was associated with worse prognosis of pancreatic cancer and contributed to chemoresistance to doxorubicin. However, further molecular biological experiments need to be conducted in the future in order to determine the vital role of DUOX2 and the underlying molecular mechanism in pancreatic cancer.
Acknowledgments
Funding: This study was supported by grants from the Medical Science and Technology Program of Henan Province to PWL (2018020084) and XDX (LHGJ20190145).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-21-776/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-776/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-21-776/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of The First Affiliated Hospital of Zhengzhou University (No. 2018-KY-0137) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004;4:181-9. [Crossref] [PubMed]
- Diaz de Barboza G, Guizzardi S, Moine L, et al. Oxidative stress, antioxidants and intestinal calcium absorption. World J Gastroenterol 2017;23:2841-53. [Crossref] [PubMed]
- He N, Jia JJ, Li JH, et al. Remote ischemic perconditioning prevents liver transplantation-induced ischemia/reperfusion injury in rats: role of ROS/RNS and eNOS. World J Gastroenterol 2017;23:830-41. [Crossref] [PubMed]
- Gao X, Sun J, Huang C, et al. RNAi-mediated silencing of NOX4 inhibited the invasion of gastric cancer cells through JAK2/STAT3 signaling. Am J Transl Res 2017;9:4440-9. [PubMed]
- You X, Ma M, Hou G, et al. Gene expression and prognosis of NOX family members in gastric cancer. Onco Targets Ther 2018;11:3065-74. [Crossref] [PubMed]
- Tang CT, Lin XL, Wu S, et al. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell Signal 2018;46:52-63. [Crossref] [PubMed]
- Sakai Y, Yamamori T, Yoshikawa Y, et al. NADPH oxidase 4 mediates ROS production in radiation-induced senescent cells and promotes migration of inflammatory cells. Free Radic Res 2018;52:92-102. [Crossref] [PubMed]
- Raad H, Serrano-Sanchez M, Harfouche G, et al. NADPH oxidase-1 plays a key role in keratinocyte responses to UV radiation and UVB-induced skin carcinogenesis. J Invest Dermatol 2017;137:1311-21. [Crossref] [PubMed]
- Zhang Z, Duan Q, Zhao H, et al. Gemcitabine treatment promotes pancreatic cancer stemness through the Nox/ROS/NF-κB/STAT3 signaling cascade. Cancer Lett 2016;382:53-63. [Crossref] [PubMed]
- Ha SY, Paik YH, Yang JW, et al. NADPH oxidase 1 and NADPH oxidase 4 have opposite prognostic effects for patients with hepatocellular carcinoma after hepatectomy. Gut Liver 2016;10:826-35. [Crossref] [PubMed]
- Mochizuki T, Furuta S, Mitsushita J, et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 2006;25:3699-707. [Crossref] [PubMed]
- Wu Y, Antony S, Juhasz A, et al. Up-regulation and sustained activation of Stat1 are essential for interferon-gamma (IFN-gamma)-induced dual oxidase 2 (Duox2) and dual oxidase A2 (DuoxA2) expression in human pancreatic cancer cell lines. J Biol Chem 2011;286:12245-56. [Crossref] [PubMed]
- Hiraga R, Kato M, Miyagawa S, et al. Nox4-derived ROS signaling contributes to TGF-β-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res 2013;33:4431-8. [PubMed]
- Cao M, Zhang PB, Wu PF, et al. DUOX2 As a Potential Prognostic Marker which Promotes Cell Motility and Proliferation in Pancreatic Cancer. Biomed Res Int 2021;2021:6530298. [Crossref] [PubMed]
- Cho SY, Kim JS, Eun HS, et al. Expression of NOX family genes and their clinical significance in colorectal cancer. Dig Dis Sci 2018;63:2332-40. [Crossref] [PubMed]
- Juhasz A, Markel S, Gaur S, et al. NADPH oxidase 1 supports proliferation of colon cancer cells by modulating reactive oxygen species-dependent signal transduction. J Biol Chem 2017;292:7866-87. [Crossref] [PubMed]
- Zhan M, Wang H, Chen T, et al. NOX1 mediates chemoresistance via HIF1α/MDR1 pathway in gallbladder cancer. Biochem Biophys Res Commun 2015;468:79-85. [Crossref] [PubMed]
- Aydin E, Johansson J, Nazir FH, et al. Role of NOX2-derived reactive oxygen species in NK cell-mediated control of murine melanoma metastasis. Cancer Immunol Res 2017;5:804-11. [Crossref] [PubMed]
- Guo Y, Han B, Luo K, et al. NOX2-ROS-HIF-1α signaling is critical for the inhibitory effect of oleanolic acid on rectal cancer cell proliferation. Biomed Pharmacother 2017;85:733-9. [Crossref] [PubMed]
- Shanmugasundaram K, Nayak BK, Friedrichs WE, et al. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat Commun 2017;8:997. [Crossref] [PubMed]
- Crosas-Molist E, Bertran E, Sancho P, et al. The NADPH oxidase NOX4 inhibits hepatocyte proliferation and liver cancer progression. Free Radic Biol Med 2014;69:338-47. [Crossref] [PubMed]
- Antony S, Jiang G, Wu Y, et al. NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates normoxic HIF-1α and p27Kip1 expression in malignant melanoma and other human tumors. Mol Carcinog 2017;56:2643-62. [Crossref] [PubMed]
- Little AC, Sham D, Hristova M, et al. DUOX1 silencing in lung cancer promotes EMT, cancer stem cell characteristics and invasive properties. Oncogenesis 2016;5:e261. [Crossref] [PubMed]
- Ling Q, Shi W, Huang C, et al. Epigenetic silencing of dual oxidase 1 by promoter hypermethylation in human hepatocellular carcinoma. Am J Cancer Res 2014;4:508-17. [PubMed]
- Lu H, Wu Q, Yang H. DUOX2 promotes the elimination of the Klebsiella pneumoniae strain K5 from T24 cells through the reactive oxygen species pathway. Int J Mol Med 2015;36:551-8. [Crossref] [PubMed]
- Lin SC, Chang IW, Hsieh PL, et al. High immunoreactivity of DUOX2 is associated with poor response to preoperative chemoradiation therapy and worse prognosis in rectal cancers. J Cancer 2017;8:2756-64. [Crossref] [PubMed]
(English Language Editor: J. Jones)


