Non-stereotactic method involving combination of ultrasound-guided wire localization and vacuum-assisted breast biopsy for microcalcification
Introduction
Pleomorphic or fine linear microcalcification has a high probability of malignancy, and clustered microcalcification may be an early sign of breast cancer (1,2). Such lesions are usually classified as more than category 4 using the Breast Imaging Reporting and Data System (BI-RADS) classification, which requires histologic confirmation (3). However, even for lesions classified as BI-RADS category 3, additional follow-up is usually recommended with imaging or biopsy (4).
Mammography is a very sensitive imaging modality for detection of microcalcification. However, microcalcification cannot be identified with ultrasound (US) because the inner region of the lesion contains echogenic glandular tissue, which is not readily discerned with US (5). Therefore, excisional biopsy with the mammography-guided needle localization or the use of stereotactic equipment is necessary for histologic confirmation. However, stereotactic equipment is expensive, and a radiologist with specific experience and skills is necessary to conduct the procedure. Furthermore, patients would be exposed to radiation more than once when undergoing this procedure.
Vacuum-assisted breast biopsy (VABB) was first introduced for biopsy of benign breast nodules in 1996 (6,7). The VABB subsequently developed as a new treatment tool for benign breast lesions. The main advantages of VABB are that it allows the operator to not only obtain a sufficient amount of specimen, but also to completely remove small, benign lesions (8). The indications for VABB include palpable or non-palpable breast lesions diagnosed as BI-RADS category 3 or 4a.
We previously described a combination method involving mammography-guided wire localization and US-guided VABB for clustered microcalcification (9). Herein, we devised a manual stereotactic (non-stereotactic) method based on conventional mammographic images that is performed using a combination technique involving US-guided wire localization and VABB.
Materials and methods
From January to December 2013, a total of 22 consecutive patients underwent our non-stereotactic combination method involving US-guided wire localization and VABB. The patients had clustered, diffuse, or scattered microcalcification on mammography, and all were categorized as BI-RADS 3 or 4a. The exclusion criteria were ipsilateral breast cancer and previous surgery. Breast lesions categorized as BI-RADS category 4b or higher were excluded from this study because of their higher potential for malignancy. Informed consent was obtained from all patients and the protocol used in this study was approved by the Institutional Review Board Committee of the Pusan National University Hospital.
Manual stereotaxis
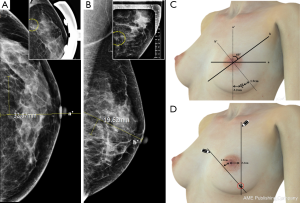
The microcalcifications were detected on cranio-caudal (CC) and medio-lateral oblique (MLO) views of mammography. On the CC view of mammography, we measured the diameter from the nipple to the core of the microcalcification using an electronic ruler and checked whether the microcalcification was located in the outer or inner portion. In the same way, the diameter from the vertical plane of the nipple to the clustered microcalcification was measured on an MLO view of mammography (Figure 1A,B). The patients lay on a plane table and raised both hands in the same position while undergoing mammography. The physician drew two straight lines from cranial to caudal and from medial to lateral along the 60 degrees of the oblique plane. The two straight lines met at the point at which the microcalcification was located (Figure 1C,D). A true lateral view of mammography was taken if necessary.
Ultrasound (US)-guided wire localization
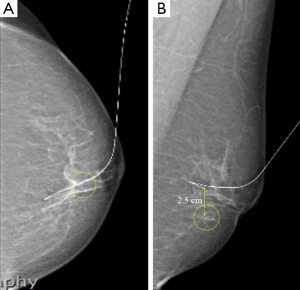
The US probe was placed on the marked location, and the US echogenicity was lowered to clearly visualize the microcalcification. A hook-shaped wire (Kopans spring-hook localization needle; Cook Inc., Bloomington, IN, USA) was then inserted into the glandular tissue. If the sonographer could not detect the microcalcification by US, the wire was inserted into the other structure, and the distance between the true lesion and needle tip was measured on the control mammograph (Figure 2).
Vacuum-assisted breast biopsy (VABB)
The patient was placed in the supine position, and aseptic povidone-iodine dressing was applied to the ipsilateral breast. Local anesthesia was injected into the skin and subcutaneous tissues (2 mL of 1% lidocaine solution), and further local anesthesia was administered into the target lesion (20 mL of 1% lidocaine solution). A 5-mm skin incision was made for insertion of the VABB probe (Mammotome®; Breast Care Business of Johnson & Johnson, Devicor Medical Products, cincinnati, OH, USA).
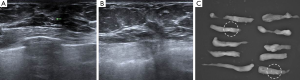
Once the location of the microcalcification was confirmed, the VABB probe was positioned underneath and parallel to the needle. If a distinction was present between the needle tip and the true lesion, the probe was moved to a more accurate position. Before performing the actual procedure, one or two samples were obtained to create a small hematoma or defect. This lesion was thus used to guide the VABB procedure. The H-wire was then gently pulled out from the breast parenchyma. Proper tissue sampling was performed about 10 to 20 times. The sampled tissues were arranged consecutively on an aseptic vinyl glove (Figure 3).

During the manual hematoma compression, specimen mammography was performed to verify the microcalcification. If a sufficient amount of microcalcification was shown by specimen mammography, the procedure was finished and compression dressing comprising crumpled gauze and an elastic bandage was applied (Figure 4). However, if significant microcalcification was not visible on specimen mammography, a further procedure or different biopsy method was considered. Compression with the elastic bandage was maintained for 24 hours after the procedure.
Postprocedural complications were assessed, and further management was determined according to the pathologic results.
Results
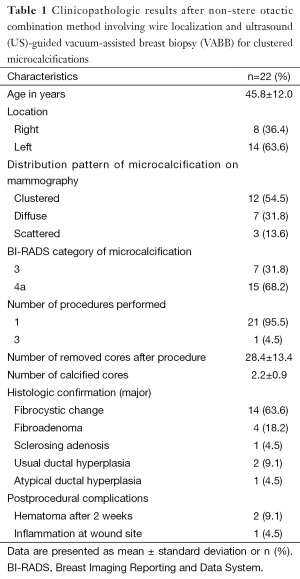
The mean ± standard deviation age of the patients was 45.8±12.0 years, and the microcalcifications were located on the right side in 5 patients (36.4%) and on the left side in 14 patients (63.6%). The distribution pattern of the microcalcifications on mammography was clustered in 12 patients (54.5%), diffuse in 7 (31.8%), and scattered in 3 (13.6%). The microcalcifications were classified as BI-RADS category 3 in 7 patients (31.8%) and 4a in 15 patients (68.2%).
In one case, the procedure was repeated 3 times. The mean ± standard deviation number of removed cores and calcified cores was 28.4±13.4 and 2.2±0.9, respectively. The major histologic type was fibrocystic change in 14 patients (63.6%), fibroadenoma in 4 (18.2%), sclerosing adenosis in 1 (4.5%), usual ductal hyperplasia in 2 (9.1%), and atypical ductal hyperplasia in 1 (4.5%). Three patients developed postprocedural complications. A significant hematoma remained 2 weeks after the procedure in two patients, and one patient developed mild inflammation at the incision site. All complications resolved with conservative treatment (Table 1).
Full table
Discussion
Breast microcalcification has become a common mammographic finding because of the widespread use of mammography as a screening modality (2). However, the clustered or amorphous microcalcifications on mammography classified as BI-RADS category 4a or higher should be evaluated with additional imaging modalities because such microcalcifications could be an early sign of breast cancer. Even if a breast lesion is classified as BI-RADS category 3, which has a <2% probability of malignancy, short-interval follow-up or tissue confirmation should be considered if the patient has a history of breast cancer or atypia (4,10). And when the patient has a history of contralateral breast cancer or high risk of breast cancer, the suspicious microcalcification should be confirmed histologically, even if it is category 3 lesion.
Because microcalcifications are not visible by US but are well visible by mammography, “microcalcification only on mammography” features are difficult to verify with the usual diagnostic tools. For several decades, the stereotactic biopsy method has been used to verify these suspicious microcalcifications (1). The stereotactic biopsy method is less invasive than excisional biopsy, induces minimal scarring, and has a short recovery time (11-15). However, stereotactic devices are expensive and can only be used by a skilled radiologist. If a non-palpable breast lesion is detected on mammography, the physician can estimate the location of the breast lesion; this is termed the non-stereotactic or manual stereotactic method.
The approximate location of the lesion can be measured by CC and MLO views of mammography. On a CC view of mammography, the location is determined to be either outer or inner based on a vertical line from the nipple. In the MLO view, the location is determined to be either upper outer or lower inner based on a vertical line from the nipple. The microcalcification would be located at the point at which the two straight lines meet. A true lateral mammograph is sometimes helpful to accurately locate a microcalcification (16). If the approximate location of the microcalcification is obtained, some experts can identify the microcalcification even using US. This would allow for wire localization under US guidance.
Wire-guided localization is the most commonly used intervention for non-palpable breast lesions (17,18). This technique uses a hook-shaped or J-shaped wire to localize the non-palpable breast lesion for excisional biopsy or lumpectomy. Although wire-guided localization is usually performed under US guidance, “microcalcification only on mammography” lesions can only be targeted under mammogram guidance. There are several disadvantages of mammography-guided wire localization. First, the dose of radiation exposure is higher than that associated with the US-guided method. Second, wire localization and excision of non-palpable breast lesions are usually performed by different physicians, such as a radiologist and surgeon, respectively. The radiologist and surgeon should discuss the localization status before the procedure to obtain good results. Third, movement among several rooms may be tiresome to the patient. Thus, US-guided wire localization should be performed if possible because it is easier and more effective for both the patient and physician.
Conventional wire localization was developed for open surgery of non-palpable breast lesions. However, this method can be combined with less invasive procedures, such as needle biopsy or VABB. When wire localization is performed with VABB, “microcalcification-only” lesions can be evaluated without a large incision or the parenchymal distortion that occurs after open surgery. Additionally, many advantages of VABB can be applied with the combination of wire localization and VABB (9,19-21).
There are several limitations in this technique. According to our previous study, more than 60% of category 4b lesions showed malignancy after biopsy (9). Thus, the combination technique described herein should be applied only to category 3 or 4a breast lesions. In addition, the breast physician should have a good understanding of mammographic findings and a high level of skill in both wire localization and VABB. And of course, it cannot be superior to conventional stereotactic biopsy for microcalcification. However, our technique would be helpful in some circumstances when the stereotactic method is impossible or failed.
In conclusion, the “microcalcification-only” breast lesions can be easily evaluated with a non-stereotactic method involving a combination of US-guided wire localization and VABB. This would be an effective diagnostic technique for breast lesion which reveals only microcalcification.
Acknowledgements
Funding: This work was supported by grants from the National Research Foundation of Korea funded by the Korean government (2014R1A5A2009242) and from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Korea (1420040). And this research was supported by Kyungpook National University Research Fund, 2012.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Atasoy MM, Tasali N, Çubuk R, et al. Vacuum-assisted stereotactic biopsy for isolated BI-RADS 4 microcalcifications: evaluation with histopathology and midterm follow-up results. Diagn Interv Radiol 2015;21:22-7. [Crossref] [PubMed]
- Gümüş H, Mills P, Fish D, et al. Breast microcalcification: diagnostic value of calcified and non-calcified cores on specimen radiographs. Breast J 2013;19:156-61. [Crossref] [PubMed]
- Burnside ES, Ochsner JE, Fowler KJ, et al. Use of microcalcification descriptors in BI-RADS 4th edition to stratify risk of malignancy. Radiology 2007;242:388-95.
- Obenauer S, Hermann KP, Grabbe E. Applications and literature review of the BI-RADS classification. Eur Radiol 2005;15:1027-36. [Crossref] [PubMed]
- Moon WK, Im JG, Koh YH, et al. US of mammographically detected clustered microcalcifications. Radiology 2000;217:849-54. [Crossref] [PubMed]
- Reynolds HE, Poon CM, Goulet RJ, et al. Biopsy of breast microcalcifications using an 11-gauge directional vacuum-assisted device. AJR Am J Roentgenol 1998;171:611-3. [Crossref] [PubMed]
- Pfarl G, Helbich TH, Riedl CC, et al. Stereotactic 11-gauge vacuum-assisted breast biopsy: a validation study. AJR Am J Roentgenol 2002;179:1503-7. [Crossref] [PubMed]
- Park HL, Kim LS. The current role of vacuum assisted breast biopsy system in breast disease. J Breast Cancer 2011;14:1-7. [Crossref] [PubMed]
- Choo KS, Kwak HS, Tae Bae Y, et al. The value of a combination of wire localization and ultrasound-guided vacuum-assisted breast biopsy for clustered microcalcifications. Breast 2008;17:611-6. [Crossref] [PubMed]
- Levy L, Suissa M, Chiche JF, et al. BIRADS ultrasonography. Eur J Radiol 2007;61:202-11. [Crossref] [PubMed]
- Becker W. Stereotactic localization of breast lesions. Radiology 1979;133:238-40. [Crossref] [PubMed]
- Parker SH, Burbank F. A practical approach to minimally invasive breast biopsy. Radiology 1996;200:11-20. [Crossref] [PubMed]
- Burbank F. Mammographic findings after 14-gauge automated needle and 14-gauge directional, vacuum-assisted stereotactic breast biopsies. Radiology 1997;204:153-6. [Crossref] [PubMed]
- Kaye MD, Vicinanza-Adami CA, Sullivan ML. Mammographic findings after stereotaxic biopsy of the breast performed with large-core needles. Radiology 1994;192:149-51. [Crossref] [PubMed]
- Burbank F, Parker SH, Fogarty TJ. Stereotactic breast biopsy: improved tissue harvesting with the Mammotome. Am Surg 1996;62:738-44. [PubMed]
- Jung YJ, Bae YT, Lee JY, et al. Lateral decubitus positioning stereotactic vacuum-assisted breast biopsy with true lateral mammography. J Breast Cancer 2011;14:64-8. [Crossref] [PubMed]
- van Esser S, Hobbelink MG, Peeters PH, et al. The efficacy of 'radio guided occult lesion localization' (ROLL) versus 'wire-guided localization' (WGL) in breast conserving surgery for non-palpable breast cancer: a randomized clinical trial - ROLL study. BMC Surg 2008;8:9. [Crossref] [PubMed]
- Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systematic review and meta-analysis. Breast Cancer Res Treat 2013;140:435-46. [Crossref] [PubMed]
- Su KL, Xu HB, Hu ZJ, et al. Vacuum-assisted biopsy and wire localization for the diagnosis of non-palpable breast lesions. Zhonghua Zhong Liu Za Zhi 2010;32:472-5. [PubMed]
- Kim HS, Kim MJ, Kim EK, et al. US-guided vacuum-assisted biopsy of microcalcifications in breast lesions and long-term follow-up results. Korean J Radiol 2008;9:503-9. [Crossref] [PubMed]
- Cangiarella J, Waisman J, Symmans WF, et al. Mammotome core biopsy for mammary microcalcification: analysis of 160 biopsies from 142 women with surgical and radiologic followup. Cancer 2001;91:173-7. [Crossref] [PubMed]


