
Clinical observation of neoadjuvant chemotherapy with pyrotinib plus trastuzumab in HER2-positive breast cancer: a cohort study
Introduction
Breast cancer is the most prevailing malignancy in females. In recent years, the morbidity of breast cancer has increased annually, it now ranks the top in total female tumors, and correlates with the highest mortality (1). Among the 4 molecular subtypes of breast cancer, overexpression of human epidermal factor receptor 2 (HER2) and/or amplification of the HER2 gene occur in approximately 15–20% of breast cancers (2,3). Each subtype is specific in biological behaviors and treatment strategies. For the HER2-positive breast cancers, therapy targeting HER2 has become the most important and indispensable treatment (4). Owing to the development of trastuzumab, significant improvements have been obtained in the treatment of HER2-positive breast cancers as regards to its strong invasiveness, high recurrence risk, and poor prognosis, thus trastuzumab has become the first-line agent for such cancer types (5,6). Meanwhile, Neoadjuvant therapy (NT) plays a significant role in the treatment of HER2-positive breast cancers. Previous studies have shown that pCR obtained by neoadjuvant therapy can predict long-term survival, especially in the HER2-positive subgroup (7). A 5-year follow-up of NeoSphere confirmed that patients who achieved total pCR had longer disease-free survival (DFS) than those who did not (8). Results of a study on patients with HER2-positive breast cancer presented at the 2018 SAN Antonio Breast Cancer Conference (SABCS) showed that: After neoadjuvant therapy, the 5-year DFS and overall survival (OS) of pCR patients were 92.3% and 98.1%, respectively. The three-year follow-up of TRYPHAENA also confirmed the correlation between DFS and pCR (9). Thus, it has become a recognized alternative primary endpoint for long-term survival in the NT environment. However, drug resistance inevitably occurs in some patients, and even recurrence, metastases, or death may occur multiple years after the whole treatment completion (10,11). Thus, it is important to search for more effective treatment strategies with less adverse events. In recent years, multiple novel drugs targeting HER2 have emerged, including macromolecule monoclonal antibodies such as pertuzumab, small-molecule tyrosine kinase inhibitors (TKIs) such as lapatinib and pyrotinib, and trastuzumab-maytansine (T-DM1), which brings more treatment choices for people with HER2-positive breast cancers. Pyrotinib is a novel small-molecule TKI that can be given orally, and it is well tolerated and exhibits anti-tumor activity in HER2-positive advanced and metastatic breast cancers (12,13). However, evidence supporting the efficacy and safety of the new drug in neoadjuvant setting are lacking. Our study is an early observational study on the use of pyrotinib in neoadjuvant therapy for HER2-positive breast cancer, providing more data for the comparison of neoadjuvant double-target drugs for HER2-positive breast cancer, and providing guidance for the realization of more effective neoadjuvant therapy. Specifically, the efficacy and safety of pyrotinib in neoadjuvant setting was evaluated. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-794).
Methods
Objectives
Our primary objectives were to (I) to evaluate the efficacy and safety of pyrotinib plus trastuzumab in neoadjuvant setting for HER2-positive early or locally advanced breast cancers, and to make a comparison with pertuzumab plus trastuzumab. In this study, there were no adverse events such as recurrence, metastasis, or death during short-term follow-up (the median follow-up time was 8.5 months) of breast cancer patients treated with pyrotinib and completed surgery. Meanwhile, further long-term follow-up is needed to assess its long-term efficacy and safety. This step is ongoing. Therefore, the primary endpoint was total pathologicalal complete response (tpCR) and secondary endpoint was breast pathological complete response (bpCR) and objective response rate (ORR); (II) to observe whether the efficacy of pyrotinib plus trastuzumab combined with different neoadjuvant chemotherapy was different; (III) predictors of tpCR for HER2-positive breast cancer were analyzed based on baseline characteristics.
Study design
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (No. L2019-Y312). Our data was retrospectively and prospectively maintained.
Setting
The analysis in this study was performed on patients with positive HER-2 breast cancer who received neoadjuvant targeted therapy and completed surgery in the Department of Breast Surgery, The First Affiliated Hospital of Zhengzhou University from March 2019 to June 2021.
Participants
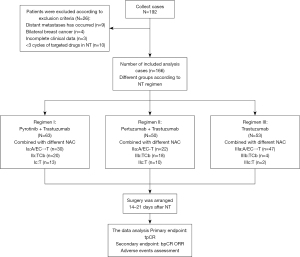
Of the 192 patients initially collected, 166 cases were included in the final analysis. Patients who met the following criteria were included: (I) Chinese females aged >18 years, with stage I–III HER2-positive invasive breast cancers confirmed by clinical pathology and histologic diagnosis (3+ in immunohistochemistry, or 2+ in immunohistochemistry but HER2 gene amplification in fluorescence in situ hybridization); (II) exclusion of distant metastases by adjuvant examinations prior to neoadjuvant chemotherapy (NAC), such as systemic bone scans, positron emission tomography-computed tomography (PET-CT), chest and/or abdominal CT; (III) Eastern Cooperative Oncology Group (ECOG) performance status score 0–2, normal organ function and normal treatment; (IV) presence of at least 1 measurable lesion according to response evaluation criteria in solid tumor (RECIST) version 1.1.
In order to achieve a more generally representative cohort, we established the following exclusion criteria: (I) pregnancy, lactation, or unwillingness to take effective contraceptive measures; (II) comorbidity of severe diseases of the circulatory, respiratory, digestive, or endocrine system, associated with an expected survival time of the above diseases less than 2 years; (III) other factors affecting drug absorption and metabolism (such as difficulty in swallowing, intestinal obstruction, influence on drug administration, and a history of drug absorption disorder or allergies); (IV) inflammatory breast cancer (IBC), bilateral breast cancer, and distant metastasis; (V) the use of HER2 blockade drugs in neoadjuvant therapy for less than 4 cycles. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013) and informed consent was taken from all individual participants.
Efficacy evaluation
The RECIST version 1.1 was referred to in the assessment of clinical efficacy. Complete response (CR): all target lesions disappeared; partial response (PR): the sum of the maximum diameter of target lesions decreased by ≥30%; progressive disease (PD): the sum of the maximum diameter of target lesions increased by at least 20%, or a new lesion occurred; stable disease (SD): changes of the sum of the maximum diameter of target lesions were between PR and PD; objective response rate (ORR): ORR = (CR + PR)/total lesions ×100%. Pathological efficacy was evaluated in samples before NAC versus after surgery, according to the Miller-Payne histological classification, as follows: G1—ineffectiveness: no noticeable changes in cancer cell morphology or number; G2—mild effectiveness: presence of degeneration and necrosis of cancer cells (<1/3), or the density of residual living cells was more than 2/3 of that before treatment; G3—moderate effectiveness: presence of necrosis and lysis of cancer cells (1/3–2/3), or the density of residual living cells was 1/3–2/3 of that before treatment; G4—high efficacy: presence of necrosis and lysis of cancer cells (>2/3), or the density of residual living cells was less than 1/3 of that before treatment; G5—pathological complete response (pCR): presence of necrosis or disappearance of all cancer cells, and tumors were replaced by granulation or fibrous tissue. Total pCR (tpCR, ypT0/isypN0) was defined by the absence of invasive lesions in the breast tissue and axillary lymph nodes, and possible presence of carcinoma in situ components. No pCR was defined by the presence of visible invasive cancer components in the breast tissue or axillary lymph nodes in the majority of surgical resections. Pathological complete response of the breast tumor (bpCR) was defined by the absence of invasive carcinoma in the primary breast tumor. Effective pathological efficacy was indicated by G3 + G4 + G5. The primary endpoint was tpCR and the secondary endpoint was bpCR and ORR. In addition, AEs during the NAC were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Event (NCI-CTCAE) version 5.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf). The main data were from laboratory results, and a few subjective indicators were collected from outpatient review and telephone follow-up.
Imaging examinations such as magnetic resonance imaging (MRI) were applied to monitor target lesions every 2 cycles before neoadjuvant therapy and after administration. Pathological efficacy was based on routine pathology and immunohistochemical (IHC) results of preoperative and postoperative samples from the department of pathology. All medical imaging diagnostic reports and histopathological results were doubly confirmed by 2 senior investigators.
Bias
In order to reduce the selection bias caused by the use cycle of anti-HER2 targeted agents during NAC, we excluded cases that had undergone less than 4 cycles. Regarding AEs, in addition to laboratory indicators, AEs also included a small number of indicators determined by patients’ subjective feelings, so recall bias was present to a certain extent.
Study size
The sample size of the case group was determined by the number of patients with early or locally advanced HER-2 positive breast cancer who received neoadjuvant pyrotinib or pertuzumab plus trastuzumab combined chemotherapy in the First Affiliated Hospital of Zhengzhou University during the study period. Simple random sampling was used to select the control group from the cases using trastuzumab single target combined with NAC, and the ratio of the control group to each case group was close to 1:1.
Study group
Patients’ baseline characteristics of primary tumor size were grouped according to tumor, node, metastasis (TNM) staging as promulgated by the American Joint Committee on Cancer (AJCC) at <2 cm, 2–5 cm, and >5 cm. According to the consensus of St. Gallen International Breast Cancer Conference, Ki-67≤14% was considered as low expression and >14% as high expression.
The main variables in this study were categorical variables, which were grouped according to different neoadjuvant therapy regimens. We designed 3 targeted regimens in this neoadjuvant program. Regimen I (dual-target): pyrotinib (400 mg/d, oral) + trastuzumab (I.V., initial dose 8 mg/kg followed by 6 mg/kg). Regimen II (dual-target): pertuzumab (I.V., initial dose 840 mg followed by 420 mg) + trastuzumab. Regimen III (single-target): trastuzumab (initial dose 8 mg/kg followed by 6 mg/kg). Details for specific NAC regimen, administration dosage, and mode are displayed in Figure 1. Prior to the beginning of each treatment cycle, patients were required to undergo blood routine and biochemistry tests to confirm no contraindications to chemotherapy. Granulocyte colony-stimulating factors (G-CSF) were used as appropriate after chemotherapy. Surgery was arranged 14–21 days after the final chemotherapy, followed by 1-year complete targeted therapy.
Statistical analysis
All statistical analyses were completed on IBM SPSS 26.0 (IBM Corp., Chicago, IL, USA) and Excel (Windows Excel 2019, Microsoft, Redmond, WA, USA). Univariate logistic regression analysis was applied. A P value of <0.05 was considered statistically significant.
The chi-square test was used for comparison of disordered multi-classification data, and Kruskal-Wallis test was used for comparison of ordered multi-classification data. Baseline characteristics were included in univariate logistic regression analysis one by one, with the acquisition of tpCR as the dependent variable. The chi-square test was used to compare tpCR differences between the case group and the control group, and then the groups were included in univariate logistic regression analysis with tpCR as the dependent variable. Ennumeration data were processed by Fisher’s exact test.
Our missing data took the listwise deletion. There were 3 cases excluded from the 192 initially collected cases due to missing data.
We mainly used the chi-square test and univariate logistic regression analysis to compare efficacy differences of pyrotinib plus trastuzumab combined with NAC versus trastuzumab plus NAC alone, and analyzed factors that may affect tpCR patients with HER2-positive breast cancer.
Results
Participants
Based on the inclusion criteria, data were collected from 192 breast cancer patients who were admitted to the Department of Breast Surgery of the First Affiliated Hospital of Zhengzhou University from March 2019 to June 2021; among whom, 26 cases were excluded according to the exclusion criteria. According to inclusion and exclusion criteria, data of 166 patients were included in the final analysis (Figure 1). In the cohort, pyrotinib + trastuzumab was scheduled in 63 participants (37.95%, Group I), pertuzumab + trastuzumab in 50 participants (30.12%, Group II), and single trastuzumab in 53 participants (31.93%, Group III). Participant baseline characteristics are summarized in Table 1. The participants were all females who had a median age of 50 years (range, 26–71 years) and ECOG performance status of 0–2.
Table 1
| Characteristic | Total, n (%) | Patients, n (%) | ||
|---|---|---|---|---|
| Group I (n=63) | Group II (n=50) | Group III (n=53) | ||
| Age (years), median (range) | 50 (26–71) | 48 (26–71) | 47 (29–68) | 52 (33–65) |
| <60 | 146 (87.95) | 57 (90.48) | 44 (88.00) | 45 (84.91) |
| ≥60 | 20 (12.05) | 6 (9.52) | 6 (12.00) | 8 (15.09) |
| Menopause status | ||||
| Yes | 48 (28.92) | 16(25.40) | 11 (22.00) | 21 (39.62) |
| No | 118 (71.08) | 47 (74.60) | 39 (78.00) | 32 (60.38) |
| ECOG performance status | ||||
| 0 | 141 (84.94) | 55 (87.30) | 41 (82.00) | 45 (84.91) |
| 1 | 22 (13.25) | 7 (11.11) | 8 (16.00) | 7 (13.21) |
| 2 | 3 (1.81) | 1 (1.59) | 1 (2.00) | 1 (1.89) |
| Primary tumor size | ||||
| T1 | 4 (2.41) | 2 (3.17) | 1 (2.00) | 2 (3.77) |
| T2 | 103 (62.05) | 33 (52.38) | 30 (60.00) | 40 (75.47) |
| T3 | 59 (35.54) | 28 (44.44) | 19 (38.00) | 11 (20.75) |
| Primary lymph node status | ||||
| N0 | 42 (25.30) | 17 (26.98) | 13 (26.00) | 13 (24.53) |
| N1 | 95 (57.23) | 33 (52.38) | 30 (60.00) | 31 (58.49) |
| N2 | 20 (12.05) | 11 (17.46) | 4 (8.00) | 5 (9.43) |
| N3 | 9 (5.42) | 2 (3.17) | 3 (6.00) | 4 (7.55) |
| TNM stage | ||||
| II | 112 (67.47) | 38 (60.32) | 35 (70.00) | 39 (73.58) |
| III | 54 (32.53) | 25 (39.68) | 15 (30.00) | 14 (26.42) |
| Histologic classification | ||||
| II | 118 (71.08) | 49 (77.78) | 32 (64.00) | 37 (69.81) |
| III | 48 (28.92) | 14 (22.22) | 18 (36.00) | 16 (30.19) |
| Thrombus in the vasculature | ||||
| Yes | 30 (18.07) | 8 (12.70) | 9 (18.00) | 13 (24.53) |
| No | 136 (81.92) | 55 (87.30) | 41 (82.00) | 40 (75.47) |
| Hormone receptor | ||||
| HR+a | 88 (53.01) | 28 (44.44) | 30 (60.00) | 30 (56.60) |
| HR−b | 78 (46.99) | 35 (55.56) | 20 (40.00) | 23 (43.40) |
| Ki-67c levels | ||||
| ≤14% | 27 (16.27) | 10 (15.87) | 9 (18.00) | 8 (15.09) |
| >14% | 139 (83.73) | 36 (84.13) | 41 (82.00) | 45 (84.91) |
| Combination chemotherapy | ||||
| A/EC-T | 99 (59.64) | 30 (47.62) | 22 (44.00) | 47 (88.68) |
| TCb | 42 (25.30) | 20 (31.75) | 18 (36.00) | 4 (7.55) |
| T | 25 (15.06) | 13 (20.63) | 10 (20.00) | 2 (3.77) |
a, HR+ is ER or PR positive; b, HR− is ER and PR negative (less than 1% of the nuclei of tumor cells are stained; c, Ki-67 positive site was the nucleus, and 1,000 cells were counted under high magnification to calculate the proportion of positive cells. ECOG, Eastern Cooperative Oncology Group; TNM, tumor, node, metastasis; HR+, estrogen receptor and/or progesterone receptor–positive; HR-, estrogen receptor and progesterone receptor–negative; P, pyrotinib; A, doxorubicin; E, epirubicin; C, cyclophosphamide; T, paclitaxel/docetaxel; H, trastuzumab; Cb, carboplatin; ER, estrogen receptor; PR, progesterone receptor.
Efficacy of pyrotinib plus trastuzumab combined with chemotherapy
A total of 77 participants were treated with pyrotinib plus trastuzumab neoadjuvant therapy between March 2019 and June 2021. Among them, 10 patients were excluded on account of having received less than 4 cycles of pyrotinib, 3 patients were excluded because they had distant metastasis before treatment, and 1 had bilateral breast cancer. Eventually 63 patients in group I were included in the final analysis. There were no adverse events such as recurrence, metastasis, or death during short-term follow-up (the median follow-up time was 8.5 months) in group I. Of these 63 participants, 40 (63.49%) achieved tpCR, 48 (76.19%) achieved bpCR, and ORR reached 100% (Figure 2). In group I, the tpCR rate of group Ib combined with docetaxel/carboplatin (TCb) chemotherapy regimen was 75.00% (15/20), which was higher than that of group Ia (56.67%, 17/30) and group Ic (61.54%, 8/13) (Figure 3, Table 2).
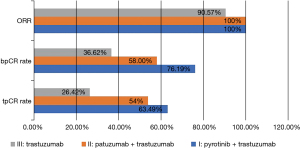
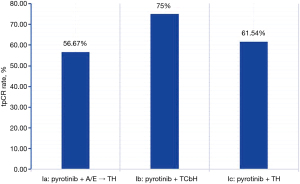
Table 2
| Groups | tpCR, n (%) | ORR, n (%) | CR, n (%) | PR, n (%) | SD, n (%) |
|---|---|---|---|---|---|
| Total | 81 (48.80) | 161 (96.99) | 54 (35.54) | 107 (64.46) | 5 (3.01) |
| Group I | 40 (63.49) | 63 (100.00) | 29 (46.03) | 34 (53.97) | 0 (0.00) |
| Ia | 17 (56.67) | 30 (100.00) | 11 (36.67) | 19 (63.33) | 0 (0.00) |
| Ib | 15 (75.00) | 20 (100.00) | 12 (60.00) | 8 (40.00) | 0 (0.00) |
| Ic | 8 (61.54) | 13 (100.00) | 6 (46.15) | 7 (53.85) | 0 (0.00) |
| Group II | 27 (54.00) | 50 (100.00) | 15 (30.00) | 35 (70.00) | 0 (0.00) |
| IIa | 9 (40.91) | 22 (100.00) | 1 (4.55) | 21 (95.45) | 0 (0.00) |
| IIb | 12 (66.67) | 18 (100.00) | 10 (55.56) | 8 (44.44) | 0 (0.00) |
| IIc | 6 (60.00) | 10 (100.00) | 4 (40.00) | 6 (60.00) | 0 (0.00) |
| Group III | 14 (26.42) | 48 (90.57) | 10 (18.87) | 38 (71.70) | 5 (9.43) |
| IIIa | 13 (27.66) | 43 (91.49) | 9 (19.15) | 34 (72.34) | 4 (8.51) |
| IIIb | 1 (25.00) | 3 (75.00) | 1 (25.00) | 2 (50.00) | 1 (25.00) |
| IIIc | 0 (0.00) | 2 (100.00) | 0 (0.00) | 2 (100.00) | 0 (0.00) |
Neoadjuvant treatment regimen: I, pyrotinib + trastuzumab; II, patuzumab + trastuzumab; III, trastuzumab; a, A/EC-T; b, TCb; c, T. tpCR, total pathologic complete response; ORR, objective remission rate. CR, complete response; PR, partial response; SD, stable disease.
TpCR predictive factors analysis of HER2-positive breast cancer
It was revealed that HR-negative patients were more likely to reach tpCR as compared to HR-positive patients (61.54% vs. 37.50%, P=0.002), and the tpCR rate of TNM stage III (37.04%, 20/54) was significantly lower than that of stage II (54.46%, 61/112), which was statistically significant (P=0.048) (Table 3). No associations of tpCR with age, menopause status, histological classification, and Ki-67 level were observed.
Table 3
| Characteristics | TpCR, n (%) | χ2 | P value | Univariate logistic regression analysis | |
|---|---|---|---|---|---|
| OR | 95% CI | ||||
| Age (years) | 3.215 | 0.073 | |||
| <60 | 75 (51.37) | 0.406 | 0.148–1.114 | ||
| ≥60 | 6 (30.00) | 1.056 | |||
| Menopause status | 0.237 | 0.626 | |||
| Yes | 22 (45.83) | 0.846 | 0.432–1.658 | ||
| No | 59 (50.00) | 1 | |||
| Primary tumor size | 5.942 | 0.051 | |||
| T1 | 4 (100.00) | – | – | ||
| T2 | 48 (46.60) | 0.903 | 0.476–1.713 | ||
| T3 | 29 (49.15) | 1 | |||
| Primary lymph node status | 7.954 | 0.047 | |||
| N0 | 27 (64.29) | 1 | |||
| N1 | 45 (47.37) | 0.500 | 0.236–1.057 | ||
| N2 | 6 (30.00) | 0.238 | 0.076–0.749 | ||
| N3 | 3 (33.33) | 0.278 | 0.061–1.274 | ||
| TNM stage | 6.053 | 0.048 | |||
| II | 61(54.46) | 2.074 | 1.064–4.041 | ||
| III | 20(37.04) | 1 | |||
| Histologic classification | 0.021 | 0.885 | |||
| II | 58 (49.15) | 1.051 | 0.537–2.056 | ||
| III | 23 (47.92) | 1 | |||
| Thrombus in the vasculature | 4.344 | 0.037 | |||
| Yes | 11(36.67) | 1 | |||
| No | 70 (51.47) | 1.832 | 0.811–4.139 | ||
| Hormone receptor | 9.563 | 0.002 | |||
| HR+a | 33 (37.50) | 2.667 | 1.423–4.997 | ||
| HR−b | 48 (61.54) | 1 | |||
| Ki-67c levels | 0.244 | 0.621 | |||
| ≤14% | 12 (44.44) | 0.812 | 0.354–1.859 | ||
| >14% | 69 (49.64) | 1 | |||
a, HR+ is ER or PR (progesterone receptor) positive; b, HR− is ER and PR were negative (less than 1% of the nuclei of tumor cells are stained); c, Ki-67 positive site was the nucleus, and 1,000 cells were counted under high magnification to calculate the proportion of positive cells. ECOG, Eastern Cooperative Oncology Group; HR+, estrogen receptor and/or progesterone receptor-positive; HR−, estrogen receptor and progesterone receptor-negative; OR, odds ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; tpCR, total pathologic complete response.
Comparisons among 3 neoadjuvant regimens
There were 81 participants (48.80%) in total who reached tpCR, and the overall ORR was up to 96.99%. Specifically, 40 patients (40/63, 63.49%) reached tpCR in group I, 27 patients (27/50, 54.00%) in group II and 14 patients (14/53, 26.42%) in group III (Table 2, Figure 2). Further pair-wise comparisons were implemented to discuss the statistical significance of the difference in tpCR across the 3 groups (Table 4). Statistical significance was noted in regards tpCR in group I versus group III (P<0.001; 95% CI: 2.182 to 10.755) and in Group II versus group III (P=0.004; 95% CI: 1.432 to 7.469). The difference in tpCR in group I versus group II was not statistically significant. Subgroups were designed to identify the effect of diverse regimens on tpCR (Figure 3), and no significant differences were detected.
Table 4
| Groupa | tpCR | |||||
|---|---|---|---|---|---|---|
| Chi-squared test | Logistic regression analysis | |||||
| χ2 | P value | Group | OR | 95% CI | ||
| I/III | 15.904 | <0.001 | I | 4.845 | 2.182–10.755 | |
| II/III | 8.171 | 0.004 | II | 3.270 | 1.432–7.469 | |
| I/II | 1.041 | 0.308 | III | 1 | ||
a, Group I: pyrotinib + trastuzumab, Group II: patuzumab + trastuzumab, Group III: trastuzumab. OR, odds ratio; CI, confidence interval; tpCR, total pathologic complete response
Efficacy of dual anti-HER2 agents combined with different neoadjuvant chemotherapy regimens
The tpCR rate of group Ib combined with TCb was 75.00% (15/20), which was higher than the 56.67% (17/30) of group Ia and 61.54% (8/13) of group Ic. The tpCR rate of group IIb was 66.67% (12/18), which was higher than the 40.91% (9/22) in group IIa and 60.00% (6/10) in group IIc (Table 2).
Safety
This study mainly observed the AEs of pyrotinib in neoadjuvant therapy. Selection bias might have existed due to the retrospective nature of the study and some AEs by self-assessment in the follow-up by telephone interviews. We found that diarrhea (56/63, 88.89%) was the most frequent AE in group I with pyrotinib, including grade 3 diarrhea in 6 patients (7/63, 11.11%), followed by leukopenia (48/63, 76.19%) (Table 5). Besides, the incidence of diarrhea in group I was the highest among the 3 groups (88.89% vs. 54.00% vs. 22.64%). Diarrhea could be managed by montmorillonite powder or loperamide, and all AEs were tolerated.
Table 5
| AEs | Group I, n (%) | Group II, n (%) | Group III, n (%) |
|---|---|---|---|
| Diarrhea | |||
| Any gradea | 56 (88.89) | 27 (54.00) | 12 (22.64) |
| ≥3 | 7 (11.11) | 1 (2.00) | 0 (0.00) |
| Leukopenia | |||
| Any gradea | 48 (76.19) | 39 (78.00) | 40 (75.47) |
| ≥3 | 2 (3.17) | 2 (4.00) | 3 (5.66) |
| Neutropenia | |||
| Any gradea | 24 (38.10) | 16 (32.00) | 15 (28.30) |
| ≥3 | 1 (1.59) | 0 (0.00) | 0 (0.00) |
| Hemoglobin decreased | |||
| Any gradea | 28 (44.44) | 20 (40.00) | 20 (37.74) |
| ≥3 | 3 (4.76) | 1 (2.00) | 1 (1.89) |
| Thrombocytopenia | |||
| Any gradea | 12 (19.05) | 11 (22.00) | 11 (20.75) |
| ≥3 | 1 (1.59) | 0 (0.00) | 0 (0.00) |
| Nausea/vomiting | |||
| Any gradea | 29 (46.03) | 28 (56.00) | 30 (56.60) |
| ≥3 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Transaminase increased | |||
| Any gradea | 19 (30.12) | 18 (36.00) | 17 (32.08) |
| ≥3 | 1 (1.59) | 1 (2.00) | 0 (0.00) |
| Cough | |||
| Any gradea | 6(9.52) | 7 (14.00) | 6 (11.32) |
| ≥3 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| K+ decreased | |||
| Any gradea | 12 (19.05) | 8 (16.00) | 6 (11.32) |
| ≥3 | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Hand-foot syndrome | |||
| Any gradea | 8 (12.70) | 9 (18.00) | 8 (15.09) |
| ≥3 | 1 (1.59) | 2 (4.00) | 1 (1.89) |
| Peripheral neuritis | |||
| Any gradea | 5 (7.94) | 6 (12.00) | 4 (7.55) |
| ≥3 | 1 (1.59) | 0 (0.00) | 0 (0.00) |
a, most common adverse events (any grade) detected in more than 5% of patients. Percentage are calculated over total patients who received each combination. One patient experienced grade IV thrombocytopenia. AEs, adverse events.
Discussion
Chemotherapy combined with targeted therapy has become the standard of neoadjuvant therapy for HER2-positive breast cancer. Four cycles doxorubicin (A) plus cyclophosphamide (C) followed by four cycles paclitaxel (T) and trastuzumab (H), known as AC-TH, was first brought forward in NAC and developed from an AC-T scheme (14). Epirubicin (E) was subsequently introduced as an anthracycline to ameliorate the cardiotoxicity of A during chemotherapy. Referred to as EC-TH. Regimens were selected for patient characteristics. Reasons for selection of 6 cycles T plus carboplatin (Cb) and H, the necessity of anthracycline avoidance due to cardiac issues, and lower-risk patients who met qualifications for NAC but had smaller tumors or fewer nodes involved. In addition, the targeted drugs anti-HER2 consist of large-molecule monoclonal antibodies and small-molecule TKIs. Currently, frequently used novel monoclonal antibody drugs include pertuzumab, and small-molecule TKIs such as lapatinib and pyrotinib. The various combinations of these drugs provide more choices for HER2 positive breast cancer patients. Pyrotinib is a novel TKI and was officially approved for HER2-positive advanced breast cancers as a second-line treatment by the Food and Drug Administration (FDA) in August 2018 (15). Although there is evidence showing the favorable anti-tumor effect and good tolerance of pyrotinib in HER2-positive recurrent or metastatic breast cancers when combined with capecitabine (16,17), its efficacy and safety in neoadjuvant setting of this particular cancer type remain to be explored. Research has shown that patients with HER2-positive advanced breast cancers could attain pCR after neoadjuvant therapy, and they could gain more benefit in event-free survival (EFS) and OS (18). Inspired by this, this study focused on tpCR as the main outcome.
We found that in neoadjuvant therapy for HER2-positive early or locally advanced breast cancers, pyrotinib plus trastuzumab contributed to 63.49% tpCR, which was significantly higher than the 26.42% by single trastuzumab. Besides, the bpCR rate of the combination of pyrotinib plus trastuzumab was as high as 76.19%, and the ORR could reach 100% after NAC completion. Subsequently, when exploring whether there was a significant difference in the efficacy of 3 different chemotherapy regimes combined with pyrotinib for neoadjuvant therapy, it was found that 15 out of 20 participants in group Ib who received pyrotinib combined with TCbH attained tpCR (75.00%), which was higher than the 56.67% of group Ia and 61.54% of group Ic. This was significantly different from the 73.7% (14/19) tpCR rate obtained in a phase II clinical trial of neoadjuvant pyrotinib plus EC sequential TH (19). The reason for this difference is that pyrotinib was used for 8 cycles in the above clinical trials; while in our study, pyrotinib was mostly used for 4–6 cycles. The pCR rate of 51.6% was reported in another phase II clinical trial of neoadjuvant pyrotinib plus trastuzumab in combination with docetaxel and carboplatin (20), compared to 73.33% in this study using the same regimen. Therefore, it may be related to the cycle of use of dual-target drugs in neoadjuvant therapy, in the meantime, sample size could also be a factor. More samples need to be collected in order to verify this viewpoint. Thus, it is not yet clear which chemotherapy regimen is more effective in combination with the 2-target combination of pyrotinib and trastuzumab.
Trastuzumab is a landmark monoclonal antibody drug for HER2-positive breast cancers, but it cannot be denied that nearly 25–30% of patients still experience recurrence or metastasis (21,22). Besides, mechanisms behind the drug resistance are multiple and complex, which have not yet been clarified (23,24). Pertuzumab is a macromolecular monoclonal antibody drug, as is trastuzumab. Both drugs exhibit an anti-tumor effect in HER2-positive breast cancers, while the former is via binding to the extracellular subdomains I/II/III of HER2 to suppress the production of HER2 homodimer and HER2/HER3 heterodimers (25), and the latter is via binding to the extracellular subdomain IV of HER2 (26). Single use of the 2 drugs may exert nonsignificant anti-tumor effects, which will be enhanced upon coordination. In a phase II NeoSphere study (27), dual-target chemotherapy contributed to a higher tpCR than single-target chemotherapy, where the use of pertuzumab plus trastuzumab and docetaxel reached a pCR rate of 45.8% in HER2+ early beast cancers, from 29% by the use of trastuzumab alone. Additionally, in a phase III PEONY study in an Asian cohort (28), patients with HER2-positive early or advanced breast cancers gained more benefits when pertuzumab and trastuzumab were combined in neoadjuvant setting (39.3% vs. 29.8%). Currently, pertuzumab plus trastuzumab combined with taxus drugs is used as the first-line treatment for HER2+ advanced breast cancers. A series of large-scale research reported that tpCR was 39.3–63.6% when pertuzumab + trastuzumab was used in neoadjuvant therapy. In this study, pertuzumab+trastuzumab reached a tpCR of 54%, which was within the abovementioned range. In subgroups, 66.67% of participants (12/18) in subgroup IIb with pertuzumab + trastuzumab combined with TCb achieved tpCR. Different from macromolecules, TKIs suppress the activity of intercellular tyrosine kinase domains after crossing the cell membrane (16). Pyrotinib as one of the TKIs which irreversibly binds to the intercellular tyrosine kinase domains of HER1 (EGFR), HER2, and HER4, in turn inhibiting the proliferative activity of breast cancer cells (29). Lapatinib is another TKI that can permanently bind to adenosine triphosphate (ATP) with conjugated double bonds, which is irreversible and contributes to a higher bioavailability and stronger efficacy. In a phase III NeoALTTO study (30), the combination of trastuzumab and lapatinib reached a higher pCR rate as compared to trastuzumab and lapatinib single agents (51.3% vs. 29.5% vs. 24.7%), while there was a significant increase in the incidence of ≥ grade 3 diarrhea and the increase of transaminase. In another phase II randomized controlled trial (31) involving trastuzumab, lapatinib, and paclitaxel in neoadjuvant setting, there was no difference in the pCR between the lapatinib group and the sham group. However, there have been few studies on the efficacy of pyrotinib in neoadjuvant therapy, so this study provides good data support for the application of pyrotinib in neoadjuvant therapy. In addition, it was further analyzed whether there was a significant difference in the efficacy of pyrotinib plus trastuzumab and pertuzumab plus trastuzumab combined with NAC in the treatment of HER2-positive breast cancer. It was found that the tpCR rate (63.49%) of the combination of pyrotinib plus trastuzumab, a micromolecule TKI and a macromolecular monoclonal antibody drug, was higher than that of the combination of trastuzumab and pertuzumab, 2 macromolecular monoclonal antibody drugs (54.00%); however, the difference was not statistically significant (P=0.308). Therefore, it cannot be determined which combination of TKIs plus macromolecular monoclonal antibody drugs and 2 monoclonal antibody drugs is superior in the neoadjuvant therapy of breast cancer patients with positive HER2.
In addition, our data revealed that HR-negative patients were more likely to reach tpCR as compared to HR-positive patients (61.54% vs. 37.50%, P=0.002). Shen et al.’s (32) in vitro test showed that ER-negative tumor cell lines and tumors were more sensitive to chemotherapy, and their sensitivity to chemotherapy also increased with the enhancement of their amplification ability. The author believed that this phenomenon might be related to the poor differentiation and strong proliferation ability of ER-negative tumor cells. The other hypothesis is that HER2-positive, HR-negative tumors are highly dependent on the HER2 gene, so treatment against HER2 has shown a favorable response. Studies have shown that with the increase of the use of NAC and targeted drugs, the expression status and level of HR and HER2 may change, reported discordances in HR status range from 2.5% to 37% (33,34). Our study found that The HR expression level was altered in 12.65% (21/166) patients with HER2-positive breast cancer who received the targeted drug in combination with NAC. The HR modified its expression from positive to negative in 1 patient and from negative to positive in 5 cases. HER2 status did not show a remarkable change before or after NAC. Since this is a relatively new treatment modality and the number of studies is low, more studies are needed to confirm these results. No unanimous conclusion has been reached on such changes at present.
The question remains as to whether our observations suggest that pyrotinib is as viable as pertuzumab in neoadjuvant therapy for HER2-positive breast cancer. Our data were from a single-center study, and most of the patients came from central China; despite this, the main exposure categories of patients were well represented. Therefore, this study provides real-world data to support the use of pyrotinib plus trastuzumab in combination with NAC in patients with early or locally advanced HER2-positive breast cancer.
In conclusion, the pCR rate of pyrotinib plus trastuzumab combined with NAC in the treatment of early or advanced HER2-positive breast cancer is high, showing a good anti-tumor activity, and the adverse events are controllable and well tolerated by most patients. What we could learn from this is that our study further strengthens the evidence of the efficacy of pyrotinib combined with trastuzumab and other chemotherapy agents in patients with HER2-positive breast cancer in the neoadjuvant setting. However, the main limitation of this study lies in the small sample size. The optimal combination of dual target drugs and pyrotinib combined with NAC regimen for better efficacy and the optimal cycle of pyrotinib use in the neoadjuvant therapy of HER2-positive breast cancer are still problems requiring urgent resolution.
Acknowledgments
Funding: This study was supported by the 2021 Young Talent Promotion Project of Henan Province, China (2021HYTP050).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-794
Data Sharing Statement: Available at https://dx.doi.org/10.21037/gs-21-794
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-794). All authors report funding from 2021 Young Talent Promotion Project of Henan Province, China (No. 2021HYTP050). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Committee on Scientific Research of The First Affiliated Hospital of Zhengzhou University (No. L2019-Y312) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 2014;138:241-56. [Crossref] [PubMed]
- Gong DH, Ge JY, Chen YY, et al. HER2 overexpression in ductal carcinoma in situ is associated with ipsilateral breast cancer recurrence after conservative surgery. Transl Cancer Res 2020;9:3787-93. [Crossref]
- Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009;14:320-68. [Crossref] [PubMed]
- Escrivá-de-Romaní S, Arumí M, Bellet M, et al. HER2-positive breast cancer: Current and new therapeutic strategies. Breast 2018;39:80-8. [Crossref] [PubMed]
- Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 2019;11:1758835919833519. [Crossref] [PubMed]
- Spring LM, Fell G, Arfe A, et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin Cancer Res 2020;26:2838-48. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791-800. [Crossref] [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer 2018;89:27-35. [Crossref] [PubMed]
- Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 2014;15:640-7. [Crossref] [PubMed]
- Puglisi F, Minisini AM, De Angelis C, et al. Overcoming treatment resistance in HER2-positive breast cancer: potential strategies. Drugs 2012;72:1175-93. [Crossref] [PubMed]
- Ma F, Ouyang Q, Li W, et al. Pyrotinib or Lapatinib Combined With Capecitabine in HER2-Positive Metastatic Breast Cancer With Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study. J Clin Oncol 2019;37:2610-9. [Crossref] [PubMed]
- Li X, Yang C, Wan H, et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci 2017;110:51-61. [Crossref] [PubMed]
- Slamon DJ, Eiermann W, Robert NJ, et al. Abstract S5-04: Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. San Antonio, TX: Oral presentation at San Antonio breast Cancer Symposium, 2015.
- Blair HA. Pyrotinib: First Global Approval. Drugs 2018;78:1751-5. [Crossref] [PubMed]
- Ma F, Li Q, Chen S, et al. Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol 2017;35:3105-12. [Crossref] [PubMed]
- Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Xuhong J, Qi X, Tang P, et al. Neoadjuvant Pyrotinib plus Trastuzumab and Chemotherapy for Stage I-III HER2-Positive Breast Cancer: A Phase II Clinical Trial. Oncologist 2020;25:e1909-20. [Crossref] [PubMed]
- Liu Z, Zhu J, Wang C, et al. Neoadjuvant pyrotinib plus trastuzumab, docetaxel, and carboplatin in patients with HER2-positive early breast cancer: A single-group, multicenter, phase II study. Ann Oncol 2020;31:S331. [Crossref]
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744-52. [Crossref] [PubMed]
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013;382:1021-8. [Crossref] [PubMed]
- Nahta R, Yu D, Hung MC, et al. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 2006;3:269-80. [Crossref] [PubMed]
- Ahmad A. Current Updates on Trastuzumab Resistance in HER2 Overexpressing Breast Cancers. Adv Exp Med Biol 2019;1152:217-28. [Crossref] [PubMed]
- Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009;69:9330-6. [Crossref] [PubMed]
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 2009;9:463-75. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Shao Z, Pang D, Yang H, et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:e193692. [Crossref] [PubMed]
- Butti R, Das S, Gunasekaran VP, et al. Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol Cancer 2018;17:34. [Crossref] [PubMed]
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633-40. [Crossref] [PubMed]
- Masuda N, Toi M, Yamamoto N, et al. Efficacy and safety of trastuzumab, lapatinib, and paclitaxel neoadjuvant treatment with or without prolonged exposure to anti-HER2 therapy, and with or without hormone therapy for HER2-positive primary breast cancer: a randomised, five-arm, multicentre, open-label phase II trial. Breast Cancer 2018;25:407-15. [Crossref] [PubMed]
- Shen K, Rice SD, Gingrich DA, et al. Distinct genes related to drug response identified in ER positive and ER negative breast cancer cell lines. PLoS One 2012;7:e40900. [Crossref] [PubMed]
- Burcombe RJ, Makris A, Richman PI, et al. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer 2005;92:147-55. [Crossref] [PubMed]
- Rossi L, Verrico M, Tomao S, et al. Expression of ER, PgR, HER-2, and Ki-67 in core biopsies and in definitive histological specimens in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy. Cancer Chemother Pharmacol 2020;85:105-11. [Crossref] [PubMed]
(English Language Editor: J. Jones)


