
Preoperative application of carbon nanoparticles in bilateral axillo-breast approach robotic thyroidectomy for papillary thyroid cancer
Introduction
In recent decades, the incidence of thyroid cancer has been increasing dramatically, especially in young individuals, and the majority (>90%) of these newly diagnosed thyroid cancers are papillary thyroid cancer (PTC) (1,2). Conventional open surgery and central neck dissection (CND) are known as the standard surgical treatment for PTC. However, a conspicuous cervical incision is unacceptable for many patients, especially for young women. With advanced endoscopic techniques and increasing demand for better appearance, the robotic surgical system has been rapidly developed and widely used in thyroid surgeries worldwide since 2009 (3,4). As the robotic system is equipped with three-dimensional vision, motion scaling, tremor elimination, and the articulated EndoWrist instruments, its clinical efficacy and safety are comparable to that of open surgery (5,6). Moreover, with significant superiority in cosmetic outcomes and potentially fewer postoperative complications, robotic thyroidectomy with or without neck dissection has become an accepted and preferred surgical option for selected patients with PTC (5-7).
Central lymph node metastasis (CLNM) was demonstrated to be an essential factor associated with regional recurrence in PTC (8,9), even occurring in up to 50% of patients when prophylactic central node dissection was performed (10). According to a recent meta-analysis (9), prophylactic CND significantly reduces locoregional recurrence. Therefore, it is widely believed in China that optimizing CND during surgery may decrease recurrence, and prophylactic CND is usually performed in PTC. However, expanding the extent of CND can increase the risk of postoperative hypoparathyroidism, which is the most common complication associated with injury or unintentional removal of parathyroid glands (9,11,12). In conclusion, it is a concerned and challenging issue to protect PGs in cases of improving the scope of CND to reduce regional recurrence.
Carbon nanoparticles (CNs) are a new lymphatic tracer that have been widely used for thyroid cancer surgery in China. With an average diameter of 150 nm, nanosized carbon particles are larger than blood capillaries (20–50 nm) but smaller than lymphatic vessels (120–500 nm). They can only enter lymphatic vessels where they are engulfed by macrophages and transported to the draining lymph node. The thyroid gland and lymph nodes are stained black while leaving the PGs unstained, allowing PGs to be easily identified from surrounding tissues (13). Previous studies have demonstrated the safety of CNs (13,14), and no apparent toxicity was observed even if CNs entered the blood circulation during the intratumoral injection. This was consistent with the clinical observations that only several cases in all the treated patients (over 500,000) showed very short hyperpyrexia after injection of CNs (15). Previous meta-analyses have reported that CNs could help improve CND and protect PGs in open thyroid surgery (16,17). However, inappropriate intraoperative injection of CNs can cause extravasation of CNs around the thyroid gland, making the identification of parathyroid glands and recurrent laryngeal nerve (RLN) challenging (18). Additionally, the intraoperative injection procedure of CNs prolonged the operative time (18-20). Moreover, the staining rate of the lymph node was not satisfactory (21-24). Although preoperative ultrasound-guided CNs injection became a promising feasible approach that seems to have fewer shortcomings and a favorable surgical outcome, only two studies have addressed the preoperative application of CNs (20,25). Meanwhile, little attention has been paid to the preoperative use of CNs in endoscopic thyroid surgery. To our best knowledge, there are no studies on CNs application in robotic thyroidectomy surgery. Thus, we conducted this cohort study to access the safety and feasibility of preoperative injection of CNs in BABA RT.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-671).
Methods
Patients
From March 2020 to March 2021, 114 consecutive adult patients with PTC treated with BABA RT and CND at the Department of Thyroid Surgery, Xiangya Hospital, were retrospectively enrolled. The inclusion criteria were as follows: patients with cosmetic requirements, postoperative pathologically confirmed PTC, maximum tumor diameter less than 40 mm. Exclusion criteria included the previous history of cervical surgery or radiotherapy, preoperative hypoparathyroidism or hypocalcemia, and patients less than 18 years old. Patients were divided into a CNs group (n=64) and a control group (n=50), based on whether they received a preoperative injection of CNs or not. The process of patient selection based on the inclusion and exclusion criteria is shown in Figure 1. All the robotic surgeries were conducted by a professional thyroid surgeon (Xinying Li). Preoperative injection of CNs was performed under ultrasound guidance by an experienced surgeon (Zhejia Zhang) the day before the surgery. The follow-up times were 6 to 18 months. Our study was approved by the Ethics Committee of Xiangya Hospital Central South University (No. 202108136), and written informed consent was obtained. All procedures involving human participants conducted in this study followed the Declaration of Helsinki (as revised in 2013).
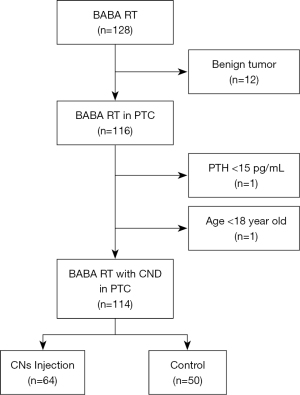
Ultrasound-guided preoperative injection of CNs
Carbon nanoparticles (0.5 mL: 25 mg, trade name: Kanalin, Chongqing LaiMei pharmaceutical Co., Ltd.,) were approved by the Chinese Food and Drug Administration (Permit No 2007204, registration number: National prescription H20041829). With a 1 mL syringe, 0.15 mL of CNs was injected into the tumor surrounding tissue. The special procedures are as follows: Firstly, we extracted 0.15 mL of CNs with a syringe, exchanged a new needle without staining, and simultaneously prepared a new syringe with 0.2 mL saline (Figure 2A). Secondly, the needle was inserted in a safe and short way under ultrasound guidance, followed by color flow Doppler to avoid the insertion into vessels and nodules (Figure 2B). Thirdly, after injections of CNs, a new syringe with 0.2 mL saline was altered to clean the needle track to prevent the subcutaneous tissue and skin from staining black (Figure 2C). Finally, the needle was gently withdrawn with negative pressure (Figure 2D), and the puncture site was pressed with gauze for about 5 min. A minimum of 1 hour of observation time was required, and side effects were recorded.
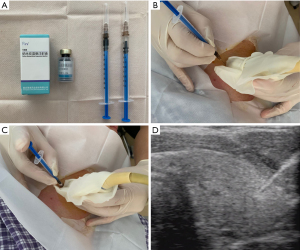
Surgical procedure
Surgeries were performed on the DaVinci Xi® systems (Intuitive Surgical, Sunnyvale, California, United States). All patients underwent total thyroidectomy or lobectomy with routine prophylactic or therapeutic unilateral CND on the side of the tumor, depending on disease features, the need for RAI therapy, and patient preference (26). When patients presented with bilateral PTC, bilateral CND was performed.
After general anesthesia, the patient was placed in a 15-degree supine position and drawn with the landmarks and trajectory lines on the chest and neck. Then a mixture of adrenaline and normal saline (1:200,000) was injected under the skin of the incision and working area to facilitate dissection, and areolae and axillary folds were incised bilaterally to allow insertion of 8 mm ports. Afterward, the working space (2 cm below the clavicle to the thyroid cartilage superiorly and beyond the medial border of the sternocleidomastoid muscle laterally) was made with electrocautery, and carbon dioxide insufflation was maintained at low pressure (6 mmHg) through the port of the left breast areolae. The robot was docked and arms were connected, equipped with energy devices or graspers (Prograsp and Maryland Forceps, Intuitive Surgical Inc., Sunnyvale, CA, USA). After separating the cervical midline, the thyroid isthmus was transected with the ultrasonic shears, followed by excision of the Delphian node and pyramidal lobe. The inferolateral side of the thyroid gland was exposed and coagulated away with ultrasonic shears. If possible, we would like firstly to handle the upper pole of the thyroid to allow better movement of the thyroid gland. Notably, we should dissect and divide close to the thyroid capsule to avoid injury to the external branch of the superior laryngeal nerve. In general, the superior parathyroid gland can be identified and preserved in situ frequently. With the thyroid retracted medially, the recurrent laryngeal nerve could be easily identified at the tracheoesophageal groove and carefully followed until it reached the laryngeal entry point. After thyroid removal, tumor-side central neck dissection was carefully performed. Once the dissection was completed, the specimen was placed into an endobag and taken out through the incision of the left breast areolae. After irrigating the operative field with saline and closing the cervical midline, we routinely inserted a drainage tube through axillary incisions on one side. The skin incisions were closed with absorbable sutures (7,27).
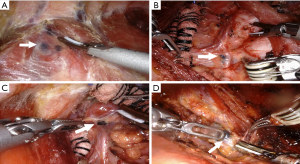
During dissection, special care should be taken to the inferior artery that needs ligation close to the thyroid to protect the artery blood supply for PGs. In addition, particular attention should be paid to ensure an appropriate and safe distance between energy instruments and the RLN or PGs. Furthermore, we would thoroughly dissect the black-stained nodes in the CNs group under the 15 times magnified three-dimension view, especially locating in the potential occult position, such as the prelaryngeal (Figure 3A), the paraesophageal (Figure 3B), and RLN laryngeal entry point (Figure 3C), and in the low central zone (Figure 3D). Moreover, an immediate PGs autotransplantation was performed when the PGs could not be retained in situ due to devascularization or accidental removal. The parathyroid glands were minced into 1 mm fragments and injected into the ipsilateral deltoid muscle with a 1 mL syringe.
Data collection and postoperative management
Data of general characteristics, complications of CNs (pain, hematoma, and CNs leakage), surgical parameters, pathological examinations, and PGs-related parameters (mean number of PGs in situ, mean number of PGs autotransplantation, and postoperative PTH levels) were collected and analyzed.
All patients are empirically intravenous calcium gluconate (0–2.0 g/day) as well as oral calcium (1.2–3.6 g/day) and calcitriol (0.25–0.75 µg/day) after surgery until discharge. The levels of PTH and calcium were measured on the first day and the first month after thyroid surgery to adjust doses of calcium and calcitriol. Hypoparathyroidism is defined as a decline in serum PTH below 15 pg/mL, regardless of hypocalcemic symptoms. Intact PTH was measured by an electrochemiluminescence immunoassay (Roche Elecsys System, Roche Diagnostics, Mannheim, Germany, reference values 15–65 pg/mL). If the serum intact PTH level at 6 months after surgery remains below normal, the patient was considered to have permanent hypoparathyroidism.
Statistical analysis
Continuous variables were presented as mean ± standard deviation and compared using independent samples t-tests. Chi-square tests were performed to analyze categorical data. Multivariate linear regression analyses were performed to exclude confounding factors and find the independent factors regarding the number of CLN and metastatic CLN (covariates including CNs, gender, age, BMI, tumor size, scope of CND, multifocal tumor, Hashimoto’s thyroiditis, and extrathyroid extension). The regression method was used to impute the missing values, including postoperative PTH levels on the first day (4.4%) and on the first month (8.8%). SPSS 26.0 and GraphPad Prism 9.0.2 were used to perform the statistical analysis. The value of P<0.05 was considered a statistically significant difference.
Results
Patient characteristics
A total of 128 consecutive patients underwent BABA RT. Among these patients, 114 (64 patients in the CNs group and 50 patients in the control group) were eligible and 14 were excluded. The follow-up times range from 6 to 18 months. Figure 1 shows the process of PTC patients selection according to inclusion and exclusion criteria. All robotic thyroidectomies were completed successfully without conversion to open surgery. The clinical characteristics of the patients enrolled in both groups are summarized in Table 1. Among the 114 PTC enrolled patients, 17were male and 97 were female. The mean age of the participants was 35.82±9.21 years old (range from 18 to 64 years old). No significant differences were found between the CNs and the control groups in terms of gender, age, BMI, surgical approach, the scope of CND, primary tumor size, tumor location, Hashimoto’s thyroiditis, extrathyroid extension, and tumor multifocality (P>0.05). The missing values in postoperative PTH levels on the first day (4.4%) and on the first month (8.8%) were imputed.
Table 1
| Variables | CNs group (n=64) | Control group (n=50) | P value |
|---|---|---|---|
| Gender (male/female) | 9/55 | 8/42 | 0.773 |
| Age (years) | 34.72±8.79 | 37.24±9.63 | 0.148 |
| BMI (kg/m2) | 22.57±3.01 | 22.48±2.67 | 0.857 |
| Tumor size (mm) | 9.90±5.24 | 11.80±13.56 | 0.307 |
| Surgical approach | 0.131 | ||
| LT | 41 | 25 | |
| TT | 23 | 25 | |
| Scope of CND | 0.836 | ||
| Unilateral | 57 | 46 | |
| Bilateral | 7 | 4 | |
| Tumor location | 0.109 | ||
| Left | 23 | 25 | |
| Right | 30 | 19 | |
| Bilateral | 9 | 2 | |
| Isthmus | 2 | 4 | |
| Hashimoto’s thyroiditis | 0.088 | ||
| No | 39 | 38 | |
| Yes | 25 | 12 | |
| Extrathyroid extension | 0.666 | ||
| No | 59 | 44 | |
| Yes | 5 | 6 | |
| Multifocal tumor | 0.142 | ||
| No | 39 | 37 | |
| Yes | 25 | 13 | |
| Operation time (min) | 63.75±18.20 | 69.10±26.59 | 0.227 |
| Intraoperative blood loss (mL) | 11.95±4.85 | 13.70±5.70 | 0.080 |
| Hoarseness | 0.666 | ||
| No | 61 | 47 | |
| Yes | 3 | 3 |
BMI, body mass index; LT, lobectomy; TT, total thyroidectomy, CND, central neck dissection.
Complications and surgical parameters of CNs
In general, no apparent complications occurred in the CNs group. The average injection time was approximately 2 min. No bleeding was observed at the injection area of the skin or thyroid. However, some patients felt slight swelling, which was tolerable and lasted for about 10 min. Since we just injected CNs without washing the needle at the early stage, there were two patients (2/10) with CNs leak out from the needle track, and the skin in the injection area became dark. Therefore, we optimized the injection method by replacing a new syringe to clean the needle. As a result, no further leakage occurred in the following 54 patients injected with CNs. In addition, CNs leaked out of the thyroid gland in three patients at the early stage, two patients with a local region of lateral thyroid darkened while the other one with the cricothyroid muscle stained black, which could be avoided by adjusting the needle into a proper depth and distance from the thyroid capsule when injecting CNs. Fortunately, these three patients did not have incidental parathyroidectomy and recurrent laryngeal nerve injury. Moreover, one patient showed flushing, palpitation, and nervousness after the injection, which resembled excitation of the visceral sympathetic nerves. This patient received immediate care and recovered within a few minutes. There was no significant difference between the two groups in terms of operation time, intraoperative blood loss, and hoarseness (P>0.05, Table 1).
Effects of CNs on CLN
As shown in Table 2, 607 CLN were dissected in the CNs group, with a mean number of 9.48±4.88 per patient. A total of 270 CLN were dissected in the control group, with a mean number of 5.40±2.67 per patient. The mean number of lymph nodes retrieved was significantly greater in the CNs group than the control group (P<0.001), as was the mean number of metastatic CLN (2.00±2.56 vs. 1.04±1.70, P=0.018). The CLNM positive rate was slightly higher in the CNs group (37/64, 57.81%) than in the control group (21/50, 42.00%), but not statistically significant (P=0.094). In addition, the detection rate of Delphian nodes was higher in the CNs group than in the control group (90% vs. 62%, P<0.001). Furthermore, CNs and Hashimoto’s thyroiditis were independently associated with the number of CLN in multivariate linear analyses (both P<0.001, Table 3). Regarding the number of metastatic CLN, CNs (P=0.041, Table 4) and tumor size (P=0.010, Table 4) were independent influence factors. Additionally, 584 (96.12%) of 607 dissected lymph nodes in the CNs Group were stained black, with 113 being metastatic (113/584, 19.35%). The remaining 23 nodes (3.88%) were unstained, of which 15 were metastatic (15/23, 65.22%).
Table 2
| Variables | CNs group (n=64) | Control group (n=50) | P value |
|---|---|---|---|
| CLN () | 9.48±4.88 | 5.40±2.67 | <0.001* |
| Metastatic CLN () | 2.00±2.56 | 1.04±1.70 | 0.018* |
| CLNM positive rate (positive/total) | 37/64 | 21/50 | 0.094 |
| Delphian node | <0.001* | ||
| No | 6 | 19 | |
| Yes | 58 | 31 |
*, P<0.05. CNs, carbon nanoparticles; CLN, central lymph nodes.
Table 3
| Factors | β (mean ± SD) | 95% CI | P value |
|---|---|---|---|
| CNs | 3.42±0.74 | 1.95 to 4.89 | <0.001* |
| Gender | 0.55±1.08 | −1.59 to 2.68 | 0.613 |
| Age | −0.03±0.04 | −0.11 to 0.05 | 0.476 |
| BMI | −0.03±0.14 | −0.31 to 0.24 | 0.807 |
| Tumor size | −0.06±0.07 | −0.2 to 0.09 | 0.417 |
| Scope of CND | 0.51±1.25 | −1.97 to 3.00 | 0.683 |
| Multifocal tumor | 0.25±0.82 | −1.37 to 1.87 | 0.757 |
| Hashimoto's thyroiditis | 3.57±0.84 | 1.91 to 5.23 | <0.001* |
| Extrathyroid extension | −0.27±1.24 | −2.73 to 2.19 | 0.827 |
*, P<0.05. CLN, central lymph nodes; CNs, carbon nanoparticles; BMI, body mass index; CND, central neck dissection.
Table 4
| Factors | β (mean ± SD) | 95% CI | P value |
|---|---|---|---|
| CNs | 0.87±0.42 | 0.04 to 1.67 | 0.041* |
| Gender | 0.96±0.61 | −0.25 to 2.17 | 0.117 |
| Age | −0.04±0.02 | −0.09 to 0.01 | 0.113 |
| BMI | −0.05±0.08 | −0.21 to 0.11 | 0.529 |
| Tumor size | 0.12±0.04 | 0.03 to 0.19 | 0.010* |
| Scope of CND | 0.43±0.71 | −0.97 to 1.84 | 0.541 |
| Multifocal tumor | −0.05±0.46 | −0.96 to 0.87 | 0.917 |
| Hashimoto's thyroiditis | 0.20±0.47 | −0.74 to 1.14 | 0.670 |
| Extrathyroid extension | 0.17±0.70 | −1.22 to 1.56 | 0.811 |
*, P<0.05. CLN, central lymph nodes; CN, carbon nanoparticles; BMI, body mass index; CND, central neck dissection.
Effects of CNs on parathyroid
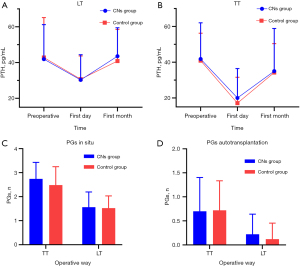
Details of parameters related to PGs are shown in Figure 4. Sixty-six patients underwent lobectomy (LT), and 48 patients underwent total thyroidectomy (TT). The baseline of preoperative PTH between the two groups was similar in LT (41.90±19.30 vs. 42.79±22.32, P=0.864) and TT (41.77±20.24 vs. 40.87±15.32, P=0.862). Although the postoperative PTH remarkably level dropped on the first day (Figure 4A,4B), no significant difference was found between the CNs group and the control group, both in LT (30.28±14.07 vs. 30.68±13.00, P=0.909) and TT (20.01±16.44 vs. 17.12±14.44, P=0.520). Additionally, postoperative PTH levels in the first month (Figure 4A,4B) were similar between the CN and the control groups (LT, 43.53±15.9 vs. 40.81±17.73, P=0.521; TT, 34.99±23.86 vs. 34.22±16.12, P=0.896). Moreover, there was no statistical difference in the mean number of PGs preserved in situ (Figure 4C) in either LT (1.56±0.63 vs. 1.52±0.51, P=0.785) or TT (2.74±0.69 vs. 2.48±0.77, P=0.227), and the mean number of PGs autotransplantation (Figure 4D) in LT (0.22±0.42 vs. 0.12±0.33, P=0.290) and TT (0.70±0.70 vs. 0.72±0.61, P=0.899) also had no statistical significance. The incidence of hypoparathyroidism in the CNs group was similar to that in the control group on the first postoperative day (5/23 vs. 6/25, P=0.852) and the first postoperative month (1/23 vs. 1/25, P=0.952). No patients in both groups developed permanent hypoparathyroidism.
Discussion
The robotic system has become used worldwide for thyroid surgeries (6,28,29), attracting the attention of both surgeons and patients. It is known that PTC is prone to early central lymph node metastasis (CLNM), an important risk factor for regional recurrence, which occurred in nearly half of clinical-negative lymph node patients (8-10). Therefore, prophylactic CND is routinely performed in China to reduce PTC recurrence, but improved CND may lead to an increase in postoperative hypoparathyroidism (11,12).
In recent years, intraoperative injection of CNs has been widely applied in clinical practice in China for its potential to improve CND and protect parathyroid in PTC surgeries (12,16,17). Unfortunately, there are some problems with the intraoperative injection of CNs (19-24), such as extravasation of CNs, prolonged operation time, and an unsatisfactory black-stained rate. In contrast, preoperative injection of CNs emerged as a promising approach (20,25). However, only little attention was paid to the preoperative CNs injection. Therefore, we designed this retrospective study to evaluate the safety and feasibility of preoperative injection of CNs in BABA RT.
Similar to two studies with the preoperative injection of injection (20,25), our study showed few complications, which is also consistent with the intraoperative injection of CNs in recent reports (18,21,23,24,30). Although leakage of CNs occurred in five patients during the early stage of our study, the risk of CNs leakage can be avoided by improving injection skills and increasing experience. Compared with previous studies (18,19), preoperative injection of CNs has less leakage and does not prolong the time of surgery. Several reasons might account for less chance of leakage in the preoperative injection of CNs. Firstly, the trap muscle still in contact with the thyroid capsule could play an important role in compressing the injection site. Secondly, we could ensure a proper injection depth in the preoperative injection of CNs under ultrasonic guidance. Thirdly, 0.2 mL saline administered to clean the needle track might also play a part. In brief, with a skillful and appropriate injection approach, preoperative injection of CNs could be a safe procedure.
As for improving CND, consistent with other studies (18,20-24,31-35), the mean number of CLN and metastatic CLN were greater in the CNs group than in the control group, while the CLNM positive rate was equivalent between the two groups. The results above largely correspond to the improvement in the extent of CND. With magnified three-dimensional vision and sophisticated manipulation in the robotic system, black-stained lymph nodes located in the potential occult position (Figure 3), such as the prelaryngeal, the right paraesophageal, RLN laryngeal entry point, and low central zone, could be visualized and dissected. While in the control group, some tissue might be preserved to protect PGs and their vascular supply or to avoid RLN injury. However, some studies argued that more tiny black-stained lymph nodes were identified during the surgery (22,24,31,32,34), but this effect might be weakened in the robotic surgery with 15 times magnified view. In addition, further supports for CNs facilitating CND were confirmed by multivariate analyses, demonstrating that CNs is an independent influence factor for the number of CLN and metastatic CLN.
Moreover, the black-stained rate of total LNs in the CNs group was 96.21%, which was higher than the previously reported rate (69.89–95.90%) (18,21-24). We speculate that the time effect may play a part in CNs diffusion and staining lymph nodes. Interestingly, the rate of metastatic lymph nodes was greater in unstained LNs than in black-stained lymph nodes (60% vs. 26.5%), similar to the previous study (18). It is considered that the metastatic tumor embolus blocks the lymphatic vessels and hinders the diffusion of CNs. Notably, vigilance is required for more aggressive tumors when many unstained LNs are present. In summary, more metastatic CLN could be retrieved by improving the extent of CND in the CNs group. Consequently, the subsequent treatment scheme and follow-up might be changed according to ATA guidelines (26), based on the accurate pathologic staging and potential decreased recurrence. Besides, further studies on the application of preoperative CNs at different times and under different surgical approaches are needed.
In terms of PGs protection, we found no significant difference in PGs-related factors between the two groups (Figure 4). The results of PGs protection in our study agreed with previous reports in terms of postoperative PTH levels (18) and PGs autotransplantation (36). It seems that CNs in robotic thyroid surgeries did not improve the protection of PGs, contrary to some evidence that CNs could protect PGs even their vascular in open surgery (20,21,33,35). There are two plausible reasons to explain our results. Firstly, as we know, in situ preservation of the PGs, professional thyroid surgeons, and meticulous capsular dissection were considered as the essential factors in protecting PGs (11,12,37). For skillful and experienced surgeons in tertiary hospitals, most parathyroid glands can be well protected even without the help of an imaging agent. In such cases, CNs may not provide practical help for parathyroid protection. Secondly, the artery supply and venous drainage of PGs are also critical to PGs’ function (38,39), and utilization of loupe magnification was found to be protective for PGs and their vessels (40). Thus, the effect of CNs on PGs protection may be decreased in robotic thyroid surgery with magnified three-dimensional vision, possibly due to the early identification of PGs and their vessels. There are data suggesting that higher postoperative PTH level is higher in BABA RT than in open thyroid surgery (7). overall, it appears that the preoperative application of CNs in robotic surgery did not produce any benefit in the protection of PGs, probably because of the extensive surgeon experience and robotic magnification.
Conclusions
To our knowledge, the present study is the first to evaluate preoperative injection of CNs in BABA RT. We find that preoperative injection of CNs is a safe procedure, and seems to have advantages over intraoperative injection of CNs, such as less CNs leakage, no surgical prolongation, and a higher black-stained rate of the CLN. Meanwhile, we demonstrate that preoperative CNs injection could dissect more potential occult CLN to improve the extent of CND. However, the value of CNs should not be exaggerated in BABA RT as it did not enhance the protection of PGs. There are still several limitations to our study. Firstly, the evidence provided by this retrospective study should be further validated by multicentric randomized controlled clinical trials with a large population. Secondly, the intelligence of robotic devices may diminish the protective effect of CNs on PGs, which needs further research and verification. Finally, considering the limited follow-up period (6 months), the potential long-term oncological safety awaits further elucidation.
Acknowledgments
We would like to thank our patients who allowed us to make this study possible.
Funding: This work was supported by the National Natural Science Foundation (NSFC) of China (No. 82073262).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-671
Data Sharing Statement: Available at https://dx.doi.org/10.21037/gs-21-671
Peer Review File: Available at https://dx.doi.org/10.21037/gs-21-671
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-671). XL serves as an unpaid Associate Editor of Gland Surgery from March 2018 to February 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xiangya Hospital Central South University (No. 202108136) and written informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li M, Brito JP, Vaccarella S. Long-Term Declines of Thyroid Cancer Mortality: An International Age-Period-Cohort Analysis. Thyroid 2020;30:838-46. [Crossref] [PubMed]
- Lim H, Devesa SS, Sosa JA, et al. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017;317:1338-48. [Crossref] [PubMed]
- Lee KE, Rao J, Youn YK. Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: our initial experience. Surg Laparosc Endosc Percutan Tech 2009;19:e71-5. [Crossref] [PubMed]
- Kang SW, Jeong JJ, Yun JS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc 2009;23:2399-406. [Crossref] [PubMed]
- Liu H, Wang Y, Wu C, et al. Robotic surgery versus open surgery for thyroid neoplasms: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2020;146:3297-312. [Crossref] [PubMed]
- Lee J, Chung WY. Robotic surgery for thyroid disease. Eur Thyroid J 2013;2:93-101. [PubMed]
- Paek SH, Kang KH, Park SJ. A Comparison of Robotic Versus Open Thyroidectomy for Papillary Thyroid Cancer. Surg Laparosc Endosc Percutan Tech 2018;28:170-3. [Crossref] [PubMed]
- Zhou Y, Sun Z, Zhou Y, et al. Thyroid antibody status exerts insignificant effect on lymph node metastasis of thyroid cancer. Transl Cancer Res 2020;9:6423-30. [Crossref]
- Zhao WJ, Luo H, Zhou YM, et al. Evaluating the effectiveness of prophylactic central neck dissection with total thyroidectomy for cN0 papillary thyroid carcinoma: An updated meta-analysis. Eur J Surg Oncol 2017;43:1989-2000. [Crossref] [PubMed]
- Park CH, Song CM, Ji YB, et al. Significance of the Extracapsular Spread of Metastatic Lymph Nodes in Papillary Thyroid Carcinoma. Clin Exp Otorhinolaryngol 2015;8:289-94. [Crossref] [PubMed]
- Orloff LA, Wiseman SM, Bernet VJ, et al. American Thyroid Association Statement on Postoperative Hypoparathyroidism: Diagnosis, Prevention, and Management in Adults. Thyroid 2018;28:830-41. [Crossref] [PubMed]
- Zhu J, Tian W, Xu Z, et al. Expert consensus statement on parathyroid protection in thyroidectomy. Ann Transl Med 2015;3:230. [PubMed]
- Hagiwara A, Takahashi T, Sawai K, et al. Lymph nodal vital staining with newer carbon particle suspensions compared with India ink: experimental and clinical observations. Lymphology 1992;25:84-9. [PubMed]
- Xie P, Yang ST, He T, et al. Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice. Int J Mol Sci 2017;18:2562. [Crossref] [PubMed]
- Chongqing Lummy Pharmaceutical Co. L, Chongqing, China. Internal data of carbon nanoparticles suspension injection. 2017. Available online: https://db.pharnexcloud.com/database/13/table/245?q=carbon%20nanoparticles
- Wang L, Yang D, Lv JY, et al. Application of carbon nanoparticles in lymph node dissection and parathyroid protection during thyroid cancer surgeries: a systematic review and meta-analysis. Onco Targets Ther 2017;10:1247-60. [Crossref] [PubMed]
- Li Y, Jian WH, Guo ZM, et al. A Meta-analysis of Carbon Nanoparticles for Identifying Lymph Nodes and Protecting Parathyroid Glands during Surgery. Otolaryngol Head Neck Surg 2015;152:1007-16. [Crossref] [PubMed]
- Zhang D, Fu Y, Dionigi G, et al. A Randomized Comparison of Carbon Nanoparticles in Endoscopic Lymph Node Dissection Via the Bilateral Areola Approach for Papillary Thyroid Cancer. Surg Laparosc Endosc Percutan Tech 2020;30:291-9. [Crossref] [PubMed]
- Liu X, Chang S, Jiang X, et al. Identifying Parathyroid Glands With Carbon Nanoparticle Suspension Does Not Help Protect Parathyroid Function in Thyroid Surgery: A Prospective, Randomized Control Clinical Study. Surg Innov 2016;23:381-9. [Crossref] [PubMed]
- Yan S, Zhao WX, Wang B, et al. Preoperative injection of carbon nanoparticles is beneficial to the patients with thyroid papillary carcinoma From a prospective study of 102 cases. Medicine (Baltimore) 2018;97:e11364. [Crossref] [PubMed]
- Zhu Y, Chen X, Zhang H, et al. Carbon nanoparticle-guided central lymph node dissection in clinically node-negative patients with papillary thyroid carcinoma. Head Neck 2016;38:840-5. [Crossref] [PubMed]
- Sun SP, Zhang Y, Cui ZQ, et al. Clinical application of carbon nanoparticle lymph node tracer in the VI region lymph node dissection of differentiated thyroid cancer. Genet Mol Res 2014;13:3432-7. [Crossref] [PubMed]
- Hao RT, Chen J, Zhao LH, et al. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur J Surg Oncol 2012;38:718-24. [Crossref] [PubMed]
- Yu W, Xu G, Sun J, et al. Carbon nanoparticles guide contralateral central neck dissection in patients with papillary thyroid cancer. Oncol Lett 2018;16:447-52. [Crossref] [PubMed]
- Zhao WJ, Luo H, Zhou YM, et al. Preoperative ultrasound-guided carbon nanoparticles localization for metastatic lymph nodes in papillary thyroid carcinoma during reoperation A retrospective cohort study. Medicine (Baltimore) 2017;96:e6285. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- He QQ, Zhu J, Zhuang DY, et al. Comparative Study between Robotic Total Thyroidectomy with Central Lymph Node Dissection via Bilateral Axillo-breast Approach and Conventional Open Procedure for Papillary Thyroid Microcarcinoma. Chin Med J (Engl) 2016;129:2160-6. [Crossref] [PubMed]
- Tae K. Transoral robotic thyroidectomy using the da Vinci single-port surgical system. Gland Surg 2020;9:614-6. [Crossref] [PubMed]
- Berber E, Bernet V, Fahey TJ 3rd, et al. American Thyroid Association Statement on Remote-Access Thyroid Surgery. Thyroid 2016;26:331-7. [Crossref] [PubMed]
- Liu J, Xu C, Wang R, et al. Do carbon nanoparticles really improve thyroid cancer surgery? A retrospective analysis of real-world data. World J Surg Oncol 2020;18:84. [Crossref] [PubMed]
- Xu ZL, Li ZY, Wu Q, et al. Potential Role of Carbon Nanoparticles in Guiding Central Neck Dissection and Protecting Parathyroid Glands in Patients with Papillary Thyroid Cancer. Current Nanoscience 2019;15:254-9. [Crossref]
- Tang J, Chen ZQ, Chu YQ, et al. Application value of carbon nanoparticles in multifocal papillary thyroid carcinoma. Acta Medica Mediterranea 2019;35:2721-8.
- Xue S, Ren P, Wang P, et al. Short and Long-Term Potential Role of Carbon Nanoparticles in Total Thyroidectomy with Central Lymph Node Dissection. Sci Rep 2018;8:11936. [Crossref] [PubMed]
- Yu W, Cao X, Xu G, et al. Potential role for carbon nanoparticles to guide central neck dissection in patients with papillary thyroid cancer. Surgery 2016;160:755-61. [Crossref] [PubMed]
- Wang B, Su AP, Xing TF, et al. The function of carbon nanoparticles to improve lymph node dissection and identification of parathyroid glands during thyroid reoperation for carcinoma. Medicine (Baltimore) 2018;97:e11778. [Crossref] [PubMed]
- Xu Z, Meng Y, Song J, et al. The role of carbon nanoparticles in guiding central neck dissection and protecting the parathyroid in transoral vestibular endoscopic thyroidectomy for thyroid cancer. Wideochir Inne Tech Maloinwazyjne 2020;15:455-61. [Crossref] [PubMed]
- Reeve T, Thompson NW. Complications of thyroid surgery: how to avoid them, how to manage them, and observations on their possible effect on the whole patient. World J Surg 2000;24:971-5. [Crossref] [PubMed]
- Kong DD, Wang W, Wang MH. Superior parathyroid blood supply safety in thyroid cancer surgery: A randomized controlled trial. Int J Surg 2019;64:33-9. [Crossref] [PubMed]
- Park I, Rhu J, Woo JW, et al. Preserving Parathyroid Gland Vasculature to Reduce Post-thyroidectomy Hypocalcemia. World J Surg 2016;40:1382-9. [Crossref] [PubMed]
- Pata G, Casella C, Mittempergher F, et al. Loupe magnification reduces postoperative hypocalcemia after total thyroidectomy. Am Surg 2010;76:1345-50. [Crossref] [PubMed]


